Abstract
Nucleotide-activated P2X channels and P2Y metabotropic receptors participate in nociceptive signaling. Agonist availability is regulated by nucleoside triphosphate diphosphohydrolase-1 (NTPDase) -1, -2, -3 and -8, a family of enzymes that hydrolyze extracellular ATP to generate adenosine diphosphate (ADP, a P2Y agonist) and monophosphate (AMP). They provide a major source of extracellular AMP, the substrate for adenosine production by ecto-5′-nucleotidase (NT5E), and thereby regulate adenosine (P1) receptor signaling. NTPDases vary in their efficiency of tri- and di-phosphate hydrolysis; therefore which family members are expressed impacts nucleotide availability and half-life. This study employed enzyme activity histochemistry to examine the distribution of ATPase activity and immunohistochemistry for NTPDase1, -2, -3 and -8 in dorsal root ganglion (DRG) and spinal cord. Nucleotidase activity was robust in spinal dorsal horn, confirming that nociceptive pathways are a major site of nucleotide transmission. In DRG, extensive staining revealed ATPase activity in a subset of neurons and in non-neuronal cells. mRNA for NTPDase1-3, but not NTPDase8, was detected in lumbar DRG and spinal cord. Immunoreactivity for NTPDase3 closely matched the distribution of ATPase activity, labeling DRG central projections in the dorsal root and superficial dorsal horn, as well as intrinsic spinal neurons concentrated in lamina II. In DRG, NTPDase3 co-localized with markers of nociceptors and with NT5E. In addition, labeling of a subset of larger-diameter neurons in DRG was consistent with intense staining of Meissner corpuscle afferents in glabrous skin. Merkel cells and terminal Schwann cells of hair follicle afferents were also labeled, but the axons themselves were negative. We propose that NTPDase3 is a key regulator of nociceptive signaling that also makes an unexpected contribution to innocuous tactile sensation.
Keywords: Ecto-nucleotidases, ATP, Adenosine, P2Y receptors, Pain, Analgesic
1. Delete
Extracellular nucleotides signal through the P2X family of ATP-gated ion channels and the P2Y family of G protein-coupled receptors, which respond to a variety of nucleotide agonists (Burnstock, 2007b, a). P2Y2 and (mouse) P2Y4 show equipotent activation by uridine triphosphate (UTP) and ATP, whereas P2Y6 is selectively activated by UDP. While the human recombinant P2Y6 is preferentially activated by UDP (100 fold) over UTP (Communi et al., 1996), mouse (Vial and Evans, 2002; Kauffenstein et al., 2010) and rat (Hartley et al., 1998) forms show equipotent activation by these uridine nucleotides. ADP is a selective agonist for P2Y1, P2Y12 and P2Y13. The recently characterized P2Y14, thought to be solely activated by UDP-glucose (UDP-G) and other UDP sugars, has recently been shown to respond also to UDP (Harden et al., 2010). P2Y1,2,4,6 are coupled to Gq/11 and P2Y12,13,14 are coupled to Gi/o G proteins. The ATP-selective receptor P2Y11 is coupled to both Gq/11 and Gs, but is not expressed in rodents (Ralevic and Burnstock, 1998; Communi et al., 1999). Therefore, nucleotides can have diverse physiological effects depending on which receptors are expressed and the availability of different nucleotide species.
P2 receptors expressed in dorsal root ganglion (DRG) and spinal cord dorsal horn are implicated in the acute transduction of noxious thermal and mechanical sensory stimuli, and also in the modulation of nociceptor excitability in models of persistent pain (Burnstock, 2006; Inoue, 2007). Most ionotropic pro-nociceptive responses appear to be mediated by channels consisting of P2X3 homomers or P2X2/P2X3 heteromers (Wirkner et al., 2007). Substantial evidence implicates both P2Y1 and P2Y2 in pro-nociceptive signaling (Moriyama et al., 2003; Chen et al., 2010; Malin and Molliver, 2010; Molliver et al., 2011). In contrast, we recently reported that the three Gi-coupled P2Y receptors exert anti-nociceptive actions in sensory neurons (Malin and Molliver, 2010). Together, these findings support a model in which ATP signaling (through both P2X and P2Y receptors) is pro-nociceptive, whereas ADP signaling may be pro-nociceptive (P2Y1) or anti-nociceptive (P2Y12,13), depending on which receptors are present.
The availability of extracellular nucleotides is regulated by membrane-bound members of the ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) family, including NTPDase1, NTPDase2, NTPDase3 and NTPDase8. These isoenzymes terminate ATP signaling by hydrolyzing ATP to ADP or AMP (Robson et al., 2006). NTPDases show regional differences in expression (Vorhoff et al., 2005) and vary in their efficiency in hydrolyzing nucleoside triphosphates versus diphosphates (Kukulski et al., 2005). For instance, NTPDase1 rapidly converts ATP to ADP and then AMP, favoring production of AMP over ADP. NTPDase2 on the other hand acts preferentially as a triphosphonucleotidase, rapidly generating ADP, but only slowly degrading ADP to AMP. NTPDase3 and NTPDase8 also show a preference for hydrolysis of triphosphates but with greater efficiency of ADP hydrolysis than NTPDase2, resulting in transient accumulation of ADP (Kukulski et al., 2005). Hydrolysis of extracellular ATP is the principal source of extracellular ADP, the endogenous agonist of P2Y1, 12, 13 receptors. Thus, NTPDases act to terminate triphosphate (both ATP and UTP) signaling, and produce agonist for activation of diphosphate receptors, then hydrolyze diphosphates to produce AMP. These characteristics indicate that the local distribution of individual NTPDase isoenzymes may significantly impact the kinetics of nucleotide receptor signaling (Table 1).
Table 1.
Pharmacological Properties of Ectonucleotidases
| Enzyme | a Substrate Preference | Products | Optimal pH Range |
|---|---|---|---|
| NTPDase1 | ATP ≅ UTP ≅ ADP ≥ UDP (ATP/ADP = 1–1.5:1) Most efficient enzyme for ADP hydrolysis | Preferentially AMP (and UMP) |
pH 7–10 (Kukulski et al., 2005) |
| NTPDase2 | ATP = UTP > ADP = UDP (ATP > ADP, 10–40:1) | Preferentially ADP, little AMP |
pH 4.5 – 8.5 (Kukulski et al., 2005) |
| NTPDase3 | ATP = UTP > ADP = UDP (ATP > ADP, 3–4:1) | AMP with transient ADP |
pH 4.5 – 11 (Kukulski et al., 2005) |
| NTPDase8 | ATP = UTP > ADP > UDP (ATP > ADP, 2:1) | AMP with transient ADP |
pH 4.5 – 8.5 (Kukulski et al., 2005) |
| NT5E | AMP (and other 5′ monophosphates) | Major source of Adenosine |
pH 7 – 8 (Zimmermann, 1992) |
| PAP | AMP ≫ ADP > ATP |
|
pH 3 – 8 (Van Etten, 1982). |
species differences exist in efficiency of substrate hydrolysis
AMP is the preferred substrate at pH 7.0 while ADP and ATP are also substrates at pH 5.6
Although AMP generated by these enzymes lacks signaling receptors, removal of the final phosphate from AMP by ecto-5′-nucleotidases generates adenosine, which contributes to both sensory and motor transmission in the spinal cord through the P1 family of G protein-coupled receptors. In the DRG and dorsal horn, adenosine production is mediated by transmembrane prostatic acid phosphatase (TM-PAP) and NT5E (also known as CD73), which are selectively expressed by nociceptive DRG neurons (Zylka, 2011). Adenosine provides a tonic anti-nociceptive signal by signaling through A1 receptors expressed on peripheral sensory and spinal cord neurons (Sawynok, 2007). Therefore, NTPDases may indirectly modulate adenosine (P1) receptor signaling by generating AMP, the precursor for adenosine production.
This study examines the distribution of enzyme activity and NTPDase1-3 and 8 expression in mouse DRG and spinal cord to determine which isoenzymes are involved in the regulation of nociceptive signaling. AMPase activity was also visualized to compare the distribution of adenosine production to that of ATPase activity.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
All studies were conducted in strict accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Pittsburgh and guidelines of the Committee for Research and Ethical Issues of IASP. Adult male C57BL/6 mice were purchased from Jackson Laboratories and housed in group cages, maintained on a 12:12 hr light-dark cycle in a temperature-controlled environment. They were provided with food and water ad libitum. All efforts were made to ensure the suffering of animals as well as the number employed in all experiments was kept to the minimum. Perfusion-fixed (4% paraformaldehyde) rat tissue generated for other projects was kindly provided by Dr. Steve Prescott and Dr. Alan Sved and was treated identically to the mouse tissue.
2.2 Tissue preparation
Mice were administered an overdose of avertin (2,2,2-tribromoethanol and tert-amyl alcohol) anesthetic, euthanized by transcardial perfusion with phosphate buffered saline (PBS) at 4°C and then 4 % paraformaldehyde. Tissues of interest (DRG, spinal cord and paw skin) were rapidly dissected and cryoprotected in 30 % sucrose overnight at 4°C. Tissue was subsequently frozen in OCT mounting medium and sections cut on a cryostat at 20 μm for spinal cord and skin and 12 μm for DRG. Sections were collected on Superfrost Plus microscope slides (Fisher) and stored at −20°C until required.
2.3 DRG neuron dissociation
Previously described protocols were employed in the dissociation of DRG neurons (Malin et al., 2007). Mice were administered an overdose of avertin anesthetic and euthanized by transcardial perfusion with Hanks buffered salt solution (HBSS) maintained at 4°C. All DRGs were rapidly dissected, enzyme-treated and dissociated by trituration. Neurons were then plated on poly-D lysine/laminin-coated cover slips in 6 well culture plates, incubated at 37°C for 90 min and then maintained in 2 ml complete medium containing F12 (Gibco BRL), 10% fetal bovine serum (Invitrogen,) and 1 % penicillin/streptomycin (Gibco BRL). Cells were allowed to grow for 2 days, and then assayed by enzyme histochemistry.
2.4 Enzyme histochemistry
For localization of NTPDase and 5′-nucleotidase activity, a modification of the Gomori lead nitrate (Pb(NO3)2) method was employed (Braun et al., 2004). Frozen tissue sections were thawed to room temperature and pre-incubated in Trizma-Maleate sucrose buffer (TMSB; 40 mM trizma-maleate, 8 % (w/v) sucrose) at pH 7.4 or 5.6 for 30 min. Next, the enzyme reaction was performed at room temperature in separate TMSB nucleotide substrate solutions containing 2 mM Pb(NO3)2 and one of the following substrates: thiamine monophosphate (TMP), AMP, ADP (3 mM), ATP or UTP (1 mM) at pH 7.4. In some experiments, levamisole (10 mM) and ouabain (5 mM) were included to inhibit alkaline phosphatase and sodium/potassium (Na+/K+)-ATPases, respectively (Langer et al., 2008). For staining of dissociated DRG neurons, cells were fixed for 10 min in ice cold 3 % paraformaldehyde followed by cold methanol for 10 min, rinsed several times with TMBS, pre-incubated in TMSB (pH 7.4) as for tissue sections and the enzyme reaction was performed with TMP (3 mM) and ATP (1 mM). Reactions were allowed to run for 3 hr for TMP, AMP and ADP while ATP and UTP ran for 1.5 hr. Visualization of the reaction product was achieved by developing for 30 s in ammonium sulfide solution (0.5%) followed by 3 washes in TMSB buffer for 3 min each. Slides were then cover slipped with mounting medium (Dako). Images were acquired using a Leica DMRX microscope, photographed with a Retiga 1300 digital camera and collected using Q Capture software and Adobe Photoshop.
2.5 Immunohistochemistry
For immunohistochemical analysis of frozen tissue sections, slides were thawed to room temperature and placed in blocking buffer containing 2.0 % normal horse serum and 0.2 % Triton X-100 in PBS for 30 min. They were then incubated overnight in the same blocking buffer containing one of the following antibodies, which have been previously validated; guinea-pig mN1-2C (Martin-Satue et al., 2009) to mouse NTPDase1 (1:2000), rabbit mN2-36LL (Bartel et al., 2006) to mouse NTPDase2 (1:1000), guinea-pig mN3-3C (Martin-Satue et al., 2009) to mouse NTPDase3 (1:1000), rabbit rN3-3L (Vekaria et al., 2006) to rat NTPDase3 (1:500), guinea pig rN8-8C (Fausther et al., 2007) to rat NTPDase8 (1:1000) and rabbit rNU-9L (Fausther M, 2008) to rat ecto-5′-Nucleotidase (NT5E; 1:1000). Some slides were double- or triple-labeled with isolectin B4 (IB4) conjugated to Alexa fluoro 488 (Invitrogen, CA, USA, 1:100), transient receptor potential vanilloid receptor (TRPV1, Neuromics, MN, USA, 1:2000) and protein kinase C gamma (PKCγ, Santa Cruz Biotechnology, CA, USA 1:2000). Slides were then washed 3 times in PBS for 3 min each and incubated for 60 min in blocking buffer containing appropriate secondary antibody: donkey anti-guinea pig conjugated to CY3 (Jackson Immunoresearch, PA, USA, 1:500) for NTPDase1, NTPDase3 and NTPDase8, donkey anti-rabbit conjugated to CY3 (Jackson Immunoresearch, PA, USA, 1:500) for NTPDase2 and NT5E and donkey anti-rabbit conjugated to CY2 (Jackson Immunoresearch, PA, USA, 1:500) for TRPV1 and PKCγ. Control slides were labeled with secondary antibodies only, at the same concentrations used in test experiments. Pre-immune sera were used in place of the primary antibodies as a negative control for the NTPDase antibodies. Next, slides were washed 3 times in PBS and analyzed as above. For staining of dissociated DRG neurons, cells were fixed as above, rinsed with PBS and incubated with appropriate primary and secondary antibodies as with tissue sections. Slides were coverslipped and imaged using a Leica DMRX microscope and photographed with a QImaging Retiga 1300 digital camera. Images were collected using Q Capture software and Adobe Photoshop.
2.6 Quantification of co-localization
The co-localization of NTPDase3 with TRPV1 and IB4 was analyzed in L4 DRG using systematic random sampling (Pakkenberg and Gundersen, 1988). Six evenly-spaced sections (every nth section, where n = total number of sections/6) from L4 DRG were chosen, starting with a randomly-chosen section between 1 and n. Sections were photographed for NTPDase3 and the alternate label, and the percentages of labeled neurons with clearly defined nuclei was determined and the results presented as the mean ± SEM from three mice.
2.7 PCR
RT-PCR analysis was carried out as previously described (Molliver et al., 2005). In summary, mice (n = 5) were administered an overdose of avertin, euthanized by transcardial perfusion with PBS at 4°C and L2-5 DRGs as well as lumbar spinal cord sections were dissected and collected on dry ice. To isolate RNA, frozen tissue samples were homogenized in lysate buffer RLT (RNeasy mini kit, Qiagen, USA) and supernatants treated with 70% ethanol to facilitate binding of total RNA to the RNeasy elution column. Contaminants were washed out using buffers provided. RNA was finally eluted in RNase-free water. RNA quality was determined applying the 260/280 nm absorbance ratio using a Biochrom WPA Biowave DNA Life Science Spectrophotometer (Cambridge, UK) according to the manufacturer’s instructions and quantity was determined using the 260 nm absorbance recorded by the spectrophotometer. Extracted RNA was treated with DNase (Invitrogen) to remove genomic DNA (1 μl DNase, 2 μl 10x DNase buffer, 0.25 μl RNaseout/5 μg RNA in H2O, 10 μl total/reaction). Reverse transcription of RNA was carried out using Invitrogen Superscript II reverse transcriptase according to the manufacturer’s instructions. Absence of contamination was confirmed by running negative control reactions without RNA. PCR primers for NTPDases1, 2, 3 and 8 were designed using MacVector software and tested for specificity by NCBI Primer-BLAST. Products were analyzed on 1.5% agarose gels containing ethidium bromide (EtBr, 5 μg/ml) with 1× TAE buffer and photographed under UV on a Cell Biosystems gel imaging workstation.
3. RESULTS
3.1 Distribution of nucleotidase activity
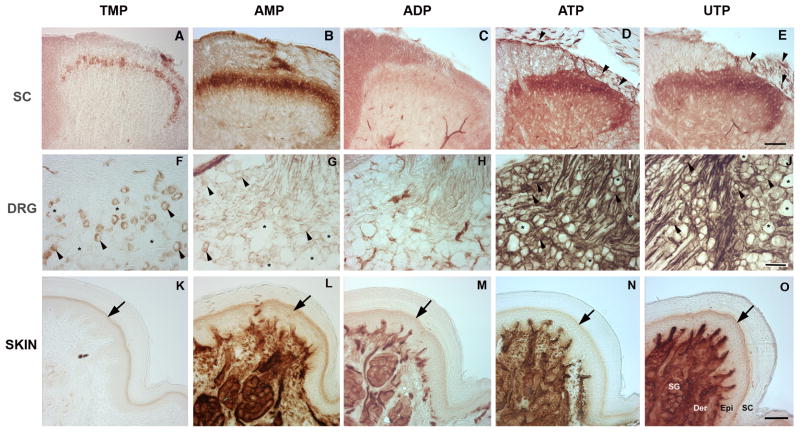
Enzyme histochemical staining is traditionally used at pH 5.6 to detect TMP staining (Waters and Butcher, 1980; Silverman and Kruger, 1988; Shields et al., 2003). Our experiments were conducted at physiological pH (7.4) with TMP as the substrate and revealed the same pattern consistently reported with this technique: a thin band of staining in the superficial dorsal horn restricted to lamina IIi (Fig. 1A) (Knyihar-Csillik et al., 1986; Zylka et al., 2008). Compared to TMP staining, AMP showed a broader distribution, with intense staining throughout lamina II and faint staining in lamina I (Fig. 1B). ADP showed only a faint band of staining in lamina II (Fig. 1C). We were surprised to find that staining with ATP and UTP was also restricted to the superficial dorsal horn, with the most intense staining in lamina II (Fig. 1D–E), similar to AMP but with diffuse staining throughout the grey matter. Nucleotidase staining with ATP or UTP strongly labeled primary afferent axons in the dorsal root and their central projections entering the superficial dorsal horn, as well as a subset of intrinsic spinal neurons concentrated in lamina IIi. In addition to the neuronal staining, vascular staining in the spinal cord at pH 7.4 was particularly strong compared with that previously observed at pH 5.6. Previous studies have identified NTPDase1 as the principal ectonucleotidase in vascular endothelium (Braun et al., 2000; Braun et al., 2004) and this enzyme is maximally active at physiological pH (Kukulski et al., 2005).
Fig. 1. Nucleotidase enzyme histochemistry.
Nucleotidase histochemical staining of spinal cord, DRG and glabrous paw from mice using TMP, AMP, ADP (3 mM), ATP and UTP (1 mM) as substrates. (A, B) In spinal cord, histochemistry with TMP shows a narrow band of staining restricted to lamina II as widely reported, while AMP staining is broader, suggesting that TMP may be a selective substrate for one of the two known AMPases. (C) Only limited staining is seen with ADP. (D, E) Strong ATPase activity (ATP, UTP) is restricted to the superficial dorsal horn, similar to AMP but with low-intensity diffuse staining in the grey matter that is not evident with AMP. Staining includes axons coursing through the dorsal root and entering the spinal cord (arrowheads). (F, G) In DRG, AMPase activity (TMP, AMP) is evident in small neurons (arrowheads, asterisks show negative cells). (H) As in spinal cord, ADP shows substantial staining only in blood vessels. (I, J), Extensive non-neuronal as well as neuronal staining is evident with ATP and UTP (arrowheads show positive cell, asterisks show negative cells). (K–O) In the foot pad, epidermal staining (arrows) is restricted to keratinocytes of the stratum granulosum for all nucleotides except ADP, which does not produce staining in epidermis. Intense staining is also evident in sweat glands, ducts and blood vessels for all nucleotides except TMP (suggesting that TMP may be a selective substrate for some but not all AMPases). SC, stratum corneum; Epi, epidermis; Der, dermis; SG, sweat gland. Scale bars: A–E, K–O, 100 uM; F–J, 50 uM.
In the DRG, AMPase activity (TMP/AMP staining; Fig. 1F–G) clearly identified a subset of small-diameter neurons, as previously reported, while ADP staining (Fig. 1H) was undetectable. ATPase activity (ATP/UTP staining; Fig. 1I–J) in the DRG was more widespread and intense. Labeled neuronal cell bodies were clearly identifiable (arrow heads, negative cells shown by asterisks); however, extensive staining of axon tracts and around cell bodies indicated labeling of non-neuronal cells as well.
In glabrous skin from the hindpaw, enzyme staining was robust in sweat glands, ducts and blood vessels for all the nucleotides except TMP, which showed only faint traces in the sweat glands (Fig. 1K). Epidermal staining was restricted to a band of keratinocytes in the stratum granulosum for all the nucleotides tested, except for ADP, which provided very limited staining in the epidermis (Fig. 1M). The limited ADP staining indicates the absence of nucleotidases with efficient ADPase activity (e.g., NTPDase1) in DRG and spinal cord neurons.
In order to rule out a significant contribution of Na+/K+-ATPases (transmembrane electrogenic ATPases that hydrolyze ATP to ADP) and alkaline phosphatases (non-specific phosphomonoesterases that hydrolyze nucleoside 5′-tri-, -di- and mono-phosphates with equal efficiency) to the distribution of triphosphate staining, the effects of specific inhibitors on nucleotidase histochemistry were investigated. High concentrations of both ouabain (5 mM) and levamisole (10 mM), Na+/K+-ATPase and alkaline phosphatase inhibitors, respectively, failed to alter the pattern of ATP and UTP staining in the spinal cord, although there was some reduction of diffuse background staining throughout the grey matter (data not shown). In addition, 20 mM sodium azide, an inhibitor of mouse NTPDase1 (Bigonnesse et al., 2004; Leal et al., 2005), but not NTPDase2 (Shi and Knowles, 1994) or NTPDase8 (Sawynok, 2006; Fausther et al., 2007), failed to alter the intensity of neuronal ATP staining (data not shown).
3.2 Expression of NTPDase family members in DRG and spinal cord
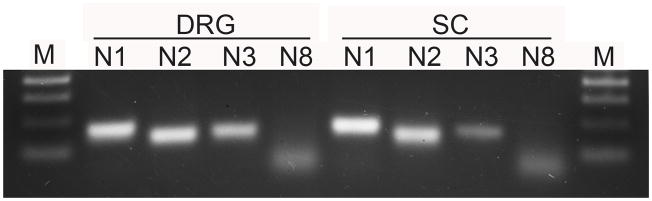
Given the intense staining for nucleotidase activity in the spinal cord and DRG, we tested for expression of the different NTPDase family members in lumbar DRG and spinal cord by RT-PCR. Fig. 2 illustrates expression of mRNA for NTPDase1-3 but not 8 in both DRG and spinal cord. We then used antibodies against NTPDase1-3, 8 to examine the distribution of these family members.
Fig. 2. NTPDase1 - 3 are expressed in DRG and spinal cord.
RT-PCR reveals single bands for NTPDase1, NTPDase2 and NTPDase3 at the expected size (130 – 170 bp), while NTPDase8 is not expressed. The low molecular weight diffuse band corresponds to unpolymerized oligomers. The same primers were used for real-time and conventional PCR. M, 100 bp ladder.
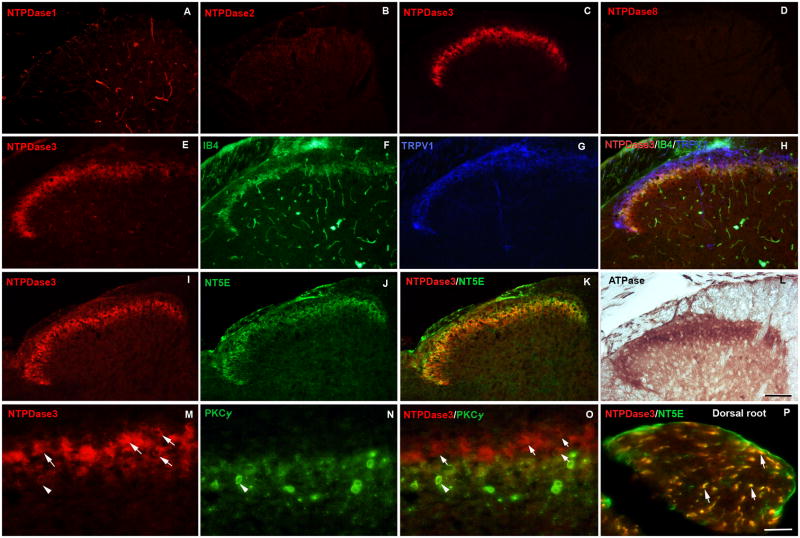
Immunohistochemical staining of spinal cord sections revealed that NTPDase3 was the only isoform that showed significant expression in the superficial dorsal horn; staining for NTPDase1 was not visible in the dorsal horn although blood vessels were copiously labeled, while staining for NTPDase2 was very faint (Fig. 3A–C). To confirm the absence of NTPDase8 and because our NTPDase8 antibody recognizes rat but not mouse protein, we stained rat tissue sections for the presence of NTPDase8. The absence of immunoreactivity in the dorsal horn (Fig. 3D) confirmed our PCR results. Double- and triple-labeling for NTPDase3 and markers for subsets of nociceptive sensory neurons revealed that NTPDase3-positive axons co-localized extensively with IB4 binding (a marker for nociceptive axons terminating in dorsal horn lamina IIi), and partially with staining for TRPV1 (a heat-gated channel preferentially expressed in IB4-negative nociceptors) superficial to the band of IB4-positive axon terminals (Fig. 3E–H). NTPDase3 staining also labeled a narrow band of intrinsic spinal cord neurons in lamina II (Fig. 3M–O). Staining for NTPDase3 and PKCγ (used to identify a discrete zone of spinal cord interneurons at the lamina II-III border (Polgar et al., 1999) that receive non-nociceptive low threshold mechanoreceptive input (Braz and Basbaum, 2009)) showed only very limited colocalization; most NTPDase3-positive spinal neurons were superficial to the neurons identified by PKCγ staining. In the dorsal horn and dorsal root, NTPDase3 was widely co-localized with NT5E (Fig 3I–K, P). NT5E is expressed in DRG neurons, but not in dorsal horn neurons (Sowa et al., 2010). The co-localization of NTPDase3 and NT5E immunoreactivity was consistent with the similar distribution of enzyme histochemistry for ATPase activity (ADP and AMP production) and AMPase activity (adenosine production).
Fig. 3. NTPDase3 is localized in axon terminals and intrinsic neurons of the spinal dorsal horn.
(A–D) Immunohistochemical staining for NTPDase1, -2, -3 and -8 in spinal cord. (A) NTPDase1 staining is restricted to blood vessels, whereas NTPDase3 (C) but not the other family members displays immunoreactivity in a narrow band within the superficial dorsal horn. (E–H) The distribution of NTPDase3 (red) in the spinal cord is compared to IB4 (green), a marker of non-peptidergic C-fiber nociceptors, and TRPV1 (blue), a marker for a subset of peptidergic C-fiber nociceptors. NTPDase3 co-localizes extensively with IB4 (yellow) and partially overlaps with TRPV1 (pink). (I–K) NTPDase3 and the ecto-5-nucleotidase (NT5E) are co-localized. (L) The distribution of ATPase staining in the dorsal horn is similar to that of NTPDase3. (M O) High power magnification of a dorsal horn section labeled for NTPDase3 (red) and PKCγ (green), a marker for a population of excitatory interneurons at the lamina II/III border, shows only rare neurons are double labeled (arrowhead). Neurons positive for NTPDase3 only are largely superficial to the PKCγ positive neurons, (arrows). (P) Double labeling of a section of dorsal root for NTPDase3 and NT5E (expressed in DRG neurons but not spinal cord) shows colocalization in individual axons in the dorsal root entering the spinal cord (arrows). Scale bars: A–L, 100 uM; M–P, 50 uM.
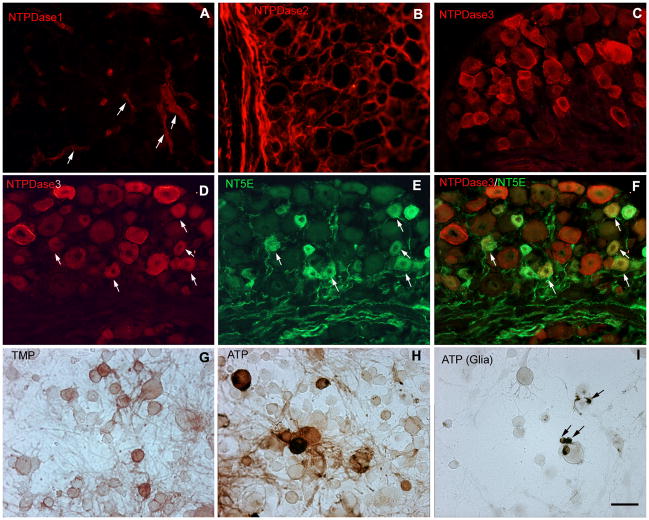
Immunohistochemical staining for NTPDase1 in lumbar DRG was strong in blood vessels but not detected in neurons (Fig. 4A). Immunoreactivity for NTPDase2 in the DRG was mainly localized in glia cells (Fig. 4B), as previously reported (Braun et al., 2004). NTPDase3 staining was primarily seen in a subset of neurons of all sizes (Fig. 4C). Double labeling of DRG sections for NTPDase3 and either IB4 or TRPV1 supports the conclusion that NTPDase3-positive neurons include nociceptors. NTPDase3 was expressed in almost all (97.23 ± 0.72%) IB4-positive neurons, while 74.45 ± 4.69% of TRPV1-positive neurons were also positive for NTPDase3. NTPDase3 was found in a wider range of neurons than NT5E, which labeled principally small diameter neurons (Fig. 4D–F). Most NT5E-positive neurons were also labeled for NTPDase3, consistent with the overlap of ATPase and AMPase histochemical staining in the superficial dorsal horn.
Fig. 4. Restricted distribution of NTPDase immunoreactivity in DRG.
(A–B) Immunohistochemical staining of lumbar DRG for NTPDases shows only vascular staining for NTPDase1 (arrows in A), while NTPDase2 staining is limited to glia. (C) NTPDase3 labeled a substantial subset of neurons including both small (likely unmyelinated) and large (likely myelinated) neurons. (D–F) Double labeling of lumbar DRG indicates most NT5E-positive cells (green) are also labeled for NTPDase3 (red). Arrows show positive cells in (D) and (E), arrows show double labeled cells in (F). (G– H) Histochemical staining of dissociated mouse DRG neurons using TMP (3 mM) and ATP (1 mM) as substrates to confirm neuronal labeling. Neurons labeled for AMPase activity were principally small, whereas ATPase activity labeled both small and large cells. (I) Glial cells were also labeled in the presence of ATP (arrows). Scale bars: A–I, 50 uM.
The extensive labeling of glia with enzyme histochemistry in lumbar DRG made it difficult to definitively identify labeled neurons. We therefore analyzed AMPase and ATPase activity in dissociated DRG cultures. Histochemical staining with TMP and ATP in cultures confirmed that a subset of small neurons was positive for AMPase activity (Fig. 4G) while ATPase activity was evident in a wider subpopulation of small and medium-large neurons (Fig. 4H). Non-neuronal cells tentatively identified as satellite cells were intensely labeled (Fig. 4I). Immunohistochemistry in dissociated DRG neurons revealed NTPDase2 immunoreactivity in non-neuronal cells as well as in a few neurons, whereas NTPDase3 staining was seen primarily in neurons (not shown).
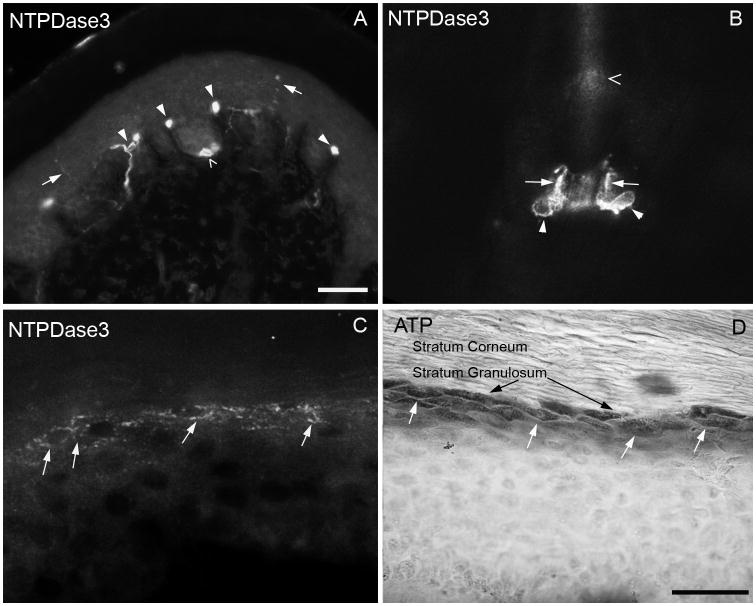
To examine the distribution of NTPDase3 at the site of sensory stimulus transduction, we examined the distribution of immunoreactivity in peripherally-projecting axons in paw skin. NTPDase3 staining (Fig. 5A) was evident in fine axons penetrating the glabrous epidermis, consistent with expression in cutaneous nociceptors. Staining for NT5E also labeled axons in the epidermis, as previously described (Sowa et al., 2010). In addition, large caliber axons were clearly visible passing through the dermis into the dermal pegs and associating with Meissner corpuscles at the dermal-epidermal border. Merkel cells were also strongly labeled, but axons associated with the Merkel cells were never visible. Finally, intense NTPDase3 immunoreactivity was seen in terminal Schwann cells surrounding the bases of hair follicles, clearly identifiable by their unique morphology and bulb-like cell body (Fig. 5B, see also (Kaidoh and Inoue, 2008). These Schwann cells envelop the lanceolate endings of myelinated hair follicle afferents, however the axons themselves were not labeled.
Fig. 5. NTPDase3 is localized in peripheral cutaneous nociceptors.
(A) Immunoreactivity for NTPDase3 in a cross section of mouse paw labels unmyelinated axons in the glabrous epidermis (arrows), Meissner corpuscles and innervating axons (filled arrowheads) and Merkel cells (empty arrowheads). (B) Intense NTPDase3 immunoreactivity was evident in terminal Schwann cells surrounding hair follicles (arrowheads show bulbous cell bodies, arrows show processes. Open arrowhead shows hair shaft). (C) A band of keratinocytes in the stratum granulosum of glabrous paw skin is labeled for NTPDase3 (white arrows), coinciding with ATP histochemical staining of keratinocytes (D, white arrows; black arrows point to stratum granulosum). Scale bars: A, 100 uM; B–D, 50 uM.
NTPDase3 clearly labeled a band of keratinocytes in the stratum granulosum (Fig. 5C), similar to ATPase nucleotidase staining of keratinocytes. (Fig. 5D). This superficial band of keratinocytes is the principal zone of termination of unmyelinated sensory afferents that express P2X3, which are the most superficially-projecting cutaneous afferents (Denda et al., 2002; Inoue et al., 2005). Keratinocyte staining for NTPDase3 was not evenly distributed throughout the footpad, and was commonly reduced or absent in the weight-bearing volar foot pads (Fig. 5A), where the dermal pegs and their innervation are most pronounced, compared to non weight-bearing regions (Fig. 5C, D). Interestingly, discontinuous epidermal staining was also reported for NT5E (Sowa et al., 2010), although they saw increased staining in volar footpads, and prominent staining for NT5E was restricted to the stratum basalis, in contrast to NTPDase3 in the stratum granulosum. Differences in regional epidermal expression of NTPDase3 may reflect different levels of mechanical stimulation in different portions of the skin.
4. Discussion
ATP, ADP, UTP and UDP are extracellular signaling molecules that act at P2 receptors in the central and peripheral nervous system (Ralevic and Burnstock, 1998). These nucleotides are endogenous agonists of metabotropic P2Y receptors and (in the case of ATP) ionotropic P2X receptors (Burnstock, 2007b, a). Despite the considerable body of evidence supporting an important role for nucleotides in nociceptive signaling, there is a dearth of information regarding the mechanisms that regulate nucleotide transmission in somatosensation. Studies in diverse tissues indicate that P2 signaling is tightly regulated by extracellular nucleotidases that control the local concentration and half-life of nucleotide agonists (Zimmermann et al., 1998; Langer et al., 2008; Yegutkin, 2008).
Of the eight enzymes that constitute the NTPDase family of ectonucleotidases, four (NTPDase1, NTPDase2, NTPDase3 and NTPDase8) are transmembrane proteins with their active zone on the extracellular surface that hydrolyze nucleotides at concentrations known to activate P2 receptors (Picher et al., 1996; Mateo et al., 1999; Smith and Kirley, 1999; Bigonnesse et al., 2004; Lavoie et al., 2004). These enzymes regulate P2 receptor activation by converting nucleotide triphosphates into diphosphates and monophosphates. Because the various NTPDase isoforms degrade triphosphates and diphosphates with differing efficiency, the relative abundance and half-life of ATP and ADP is determined by which ectonucleotidase family members are expressed in a given location. Extracellular AMP generated by these enzymes is the substrate for the ecto-5′-nucleotidases, which produce extracellular adenosine; inhibition of ecto-5′-nucleotidase by ADP may provide an additional level of temporal regulation over the transition from P2 signaling to adenosine receptor (P1) signaling (Zimmermann, 1992; Kukulski et al., 2005).
To identify which NTPDases are important for somatosensory signaling, we compared the distribution of ATPase activity and NTPDase immunoreactivity in DRG and spinal cord. Given the ubiquitous nature of ATP, we were somewhat surprised to discover the restricted localization of ATPase activity in both central spinal and peripheral cutaneous target fields of DRG neurons. In the spinal cord, robust ATPase histochemical staining was limited to the superficial dorsal horn, the principal region for termination of unmyelinated sensory neurons and nociceptors. In contrast, ADPase staining was very limited except in blood vessels, ruling out NTPDase1 as a major contributor to nucleotide catabolism in neurons and glia due to its efficient hydrolysis of ADP. The pattern of ATPase and AMPase activity in both DRG and spinal cord was quite similar, although ATPase staining was more intense than that for AMPase in all tissue investigated. In fact, while not a quantitative assay, our enzyme histochemical studies required the use of considerably lower concentrations of substrates (3 mM for monophosphates and 1 mM for triphosphates) and incubation periods (3 hr for monophosphates and 1.5 hr for triphosphates) to visualize the ATPase activity in the various tissues than AMPase, indicating substantially more efficient metabolism of triphosphates than of AMP under these conditions and suggesting a possible accumulation of AMP. Consistent with this idea, several studies indicate that transmembrane-prostatic acid phosphatase (TM-PAP) and NT5E provide the rate-limiting step in the production of adenosine from extracellular ATP, rather than the production of AMP (Goldman et al., 2010; Sowa et al., 2010).
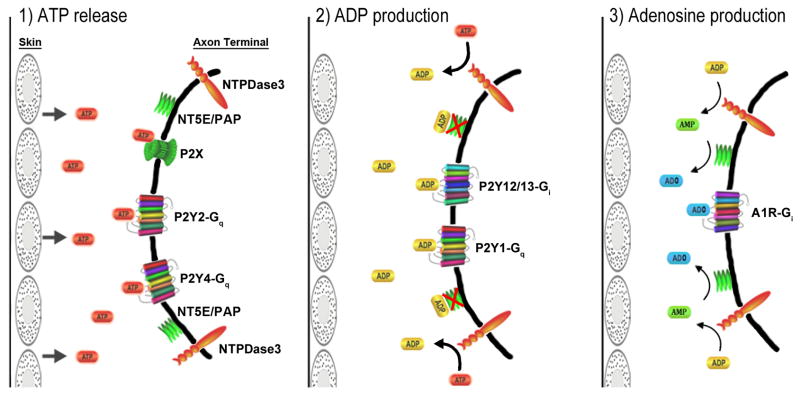
The intense staining for ATPase activity throughout the superficial dorsal horn supports an important role for nucleotide/P2 transmission at nociceptive synapses. Evidence has been reported for the release of ATP by primary afferent terminals, as well as for actions of presynaptic, postsynaptic and glial P2 receptors in the dorsal horn (White et al., 1985; Gibbins et al., 1987; Sawynok et al., 1993). Under “normal” conditions of limited, spontaneous ATP release, NTPDase3 would be expected to limit the activity of ATP receptors and promote the activity of ADP receptors by preferentially hydrolyzing ATP over ADP (Kukulski et al., 2005). We propose that NTPDase3 is a key regulator of nociceptive nucleotide transmission that regulates agonist availability for both the ATP and ADP receptors, and that ultimately produces AMP as a substrate for analgesic adenosinergic signaling (Fig. 6). Figure 6 illustrates the signaling machinery for nucleotide signaling at the peripheral sensory axon terminal, however a similar organization is likely at the central presynaptic terminal in the dorsal horn.
Fig. 6. Model of nucleotide signaling in sensory neurons.
1) In response to a painful stimulus to the skin (or other peripheral tissue), ATP is released to activate ionotropic and metabotropic ATP receptors. 2) ATP signaling is terminated by degradation of ATP to ADP by NTPDase3, resulting in a more prolonged activation of ADP receptors (due to the lower efficiency of NTPDase3 for ADP hydrolysis over ATP), as well as inhibition by ADP of adenosine production by 5′-ectonucleotidases. Whereas ATP receptors (P2X, P2Y2, P2Y4) are either ionotropic or Gq-coupled and thus likely to be excitatory, ADP receptors in sensory neurons include both the pro-nociceptive Gq-coupled P2Y1 and the Gi-coupled P2Y12 and P2Y13 receptors, which are likely to exert an anti-nociceptive influence (Malin and Molliver, 2010). 3) Finally, hydrolysis of ADP provides a pool of extracellular AMP and removes inhibition of 5′-ectonucleotidases, resulting in production of adenosine (ADO) by NT5E and TM-PAP (PAP). Arrow sizes indicate differences in efficiency for hydrolysis of ATP versus ADP and AMP. The G protein coupling of each receptor is indicated. Zylka and colleagues have demonstrated a powerful antinociceptive effect of adenosine in the dorsal horn mediated by the A1 receptor (Zylka et al. 2008, Sowa et al., 2009, Sowa et al., 2010). This figure illustrates the signaling machinery for nucleotide signaling at the peripheral sensory axon terminal, however a similar organization is likely at the central presynaptic terminal in the dorsal horn. Note that NTPDase3 expression was also found in the superficial epidermis (not shown), and presumably regulates levels of ATP released in this region. This figure was constructed in part using the pathway builder tool on www.proteinlounge.com.
Although this study has focused on the NTPDase family of enzymes, other ecto-enzymes may also affect nucleotide availability in somatosensory circuits, including adenylate kinases, which catalyze transphosphorylation of ADP into ATP and AMP, and nucleoside diphosphate (NDP) kinases, which coexist with NTPDases in some mammalian tissues (Yegutkin, 2008). Both classes of enzymes can regenerate ATP from extracellular metabolites. It is not currently known whether these kinases are expressed in sensory neurons; this possibility will require further investigation.
Immunohistochemical analysis demonstrated that NTPDase3, but not NTPDase1, 2 or 8, was highly expressed in DRG neurons and their central projections. NTPDase3 was localized in both IB4-binding and TRPV1-expressing sensory neurons and their axon terminals in lamina II of the dorsal horn. IB4 and TRPV1 identify two separate populations of unmyelinated nociceptors in the mouse that project to adjacent zones of lamina II in the spinal dorsal horn. We also found extensive co-localization of NTPDase3 and NT5E, suggesting that these two enzymes act in concert in nociceptive circuits to produce adenosine from extracellular ATP (see Sowa et al. 2010). An alternate enzyme that can also dephosphorylate AMP, TM-PAP, is also highly colocalized with NT5E and both enzymes contribute to the production of adenosine (Sowa et al., 2010). The intense staining for ATPase activity as well as immunoreactivity for NTPDase2 and to a lesser extent NTPDase3 in peripheral glia supports a role for nucleotide signaling in communication between sensory neuron cell bodies and satellite cells, as has been previously reported (Zhang et al., 2007).
In addition to the NTPDase3 staining in DRG neurons, staining was also seen in a narrow band of intrinsic neurons in lamina II, a region comprised mainly of interneurons. Excitatory interneurons expressing PKCγ found at the border of laminae II and III (Polgar et al., 1999) receive input from myelinated, non-nociceptive afferents, whereas more superficial lamina II receives primarily unmyelinated nociceptive input (Braz and Basbaum, 2009). NTPDase3-positive neurons formed a diffuse band directly superficial to the PKCγ band, and colocalization of the two markers was rare. This distribution indicates the presence of extracellular ATPase activity in nociceptive circuits and supports our conclusion that NTPDase3 is one of the principal enzymes regulating a zone of intense nucleotide transmission in the superficial dorsal horn. Intriguingly, although NTPDase3 was widely colocalized with NT5E in DRG neurons and their central projections, NT5E and TM-PAP do not appear to be expressed in intrinsic spinal neurons (Sowa et al., 2010). Further investigation will be required to determine the functional significance of postsynaptic NTPDase3 expression; it will be interesting to determine whether these spinal neurons also express P2 receptors.
In contrast to NTDPase3, NTPDase1 immunoreactivity was seen almost exclusively in blood vessels, rather than in neuronal structures. This distribution is consistent with the lack of ADPase staining in DRG and spinal cord outside of blood vessels. The distribution of NTPDase2 staining in DRG was equivalent to a previous report that its expression is restricted to satellite cells and non-myelinating Schwann cells (Braun et al., 2004). NTPDase2 presumably contributed to the ATP histochemical staining of satellite cells we observed in DRG sections and cultures. Together with the absence of NTPDase8 mRNA and immunoreactivity in DRG and spinal cord, these findings provide evidence that NTPDase3 is the major NTPDase isoform in DRG and dorsal horn neurons. We cannot exclude the possibility that additional, possibly novel nucleotidases participate in extracellular nucleotide metabolism in the DRG and dorsal horn. However, there is unlikely to be a significant contribution from alkaline phosphatases or Na/K-ATPases in sensory neurons, because antagonists failed to alter the distribution of ATPase histochemical staining.
The identification of ATPase activity and NTPDase3 immunoreactivity in the superficial epidermis is intriguing given that this is the principal termination zone of sensory axons containing P2X3, which are also likely to express one or more P2Y receptors (Molliver et al., 2002; Greig et al., 2003; Zylka et al., 2005). These axons are likely to be the first-line sensors of external noxious stimuli. Several laboratories have demonstrated stimulation-dependent release of ATP from keratinocytes, supporting the hypothesis that keratinocytes may convey information to sensory axons through P2X and/or P2Y receptors (Malin et al., 2008; Zhao et al., 2008; Dussor et al., 2009; Mandadi et al., 2009; Molliver et al., 2011). It remains to be determined how the disparate P2 receptor signaling pathways are integrated, both temporally and spatially, to affect nociceptor excitability.
Investigations of purinergic mechanisms in somatosensation have focused on pain, with few exceptions (Nakamura and Strittmatter, 1996). Sensory neurons innervating the skin include fast-conducting, myelinated afferents that transduce a wide array of stimuli, including pressure and vibration (Fradette et al., 2003), through specialized end organs including Merkel cells and Meissner, Ruffini and Pacininian corpuscles (Holbrook, 1987). Intriguingly, we found that NTPDase3 is localized not only in unmyelinated nociceptive afferents but also in Merkel cells and in large-caliber axons innervating Meissner’s corpuscles. The Merkel cell-axon complex is tactically positioned at the dermal-epidermal junction, functioning as a slowly-adapting type 1 mechanoreceptor (Tachibana, 1995). Meissner corpuscles are innervated by rapidly adapting low threshold mechanoreceptors with large or intermediate-sized cell bodies, which presumably include some of the large-diameter neurons labeled for NTPDase3 in the DRG (Perl, 1992; Zelena, 1994; Johnson, 2001).
A distinctive feature in hairy skin was the localization of NTPDase3 in terminal Schwann cells on hair follicles. Terminal Schwann cells wrap around the longitudinal lanceolate endings, which are fast-conducting myelinated axons that detect hair movement (Dykes, 1975; Halata and Munger, 1980). ATP causes increases in extracellular calcium in terminal Schwann cells that accompany cutaneous mechanoreceptors in rat vibrissae, a phenomenon reportedly mediated by P2Y2 receptors (Takahashi-Iwanaga and Habara, 2002). Although the hair follicle afferent axons were not labeled, the presence of NTPDase3 in these specialized Schwann cells suggests that nucleotide signaling contributes to low threshold mechanical transduction at the hair follicle. An important question is whether hair follicle afferents express P2Y receptors, and whether they can detect ATP released by the terminal Schwann cells. (Nakamura and Strittmatter, 1996) reported evidence that P2Y1 is expressed in large-diameter DRG neurons and contributes to fine touch sensation. Furthermore, Molliver et al. (2002) reported widespread expression of P2Y2 in large-diameter DRG neurons as well as small neurons, and Stucky et al. (2004) found that a subset of large myelinated low threshold axons were activated by the P2Y2/P2Y4 agonist UTP. Our findings here suggest that NTPDase3 is a key regulator of nociceptive nucleotide signaling and also point to roles for nucleotide signaling in innocuous mechanosensation.
5. Conclusion
We provide evidence that NTPDase3 is a major ectonucleotidase present in nociceptive circuits and likely to be one of the principal enzymes regulating somatosensory purinergic transmission mediated by P2X and P2Y receptors. Immunoreactivity for NTPDase3, but not for other family members, coincided with ATPase enzyme histochemistry in DRG neurons, spinal cord dorsal and epidermal keratinocytes, suggesting that this enzyme is largely responsible for nucleotide hydrolysis in these regions. The colocalization of NTPDase3 with NT5E and AMPase enzyme activity indicates that NTPDase3 is positioned to regulate the production of adenosine (which is anti-nociceptive in the dorsal horn) by NT5E through the progressive generation of both its inhibitor, ADP, and its substrate, AMP. Finally, the discovery that NTPDase3 is present in large myelinated cutaneous axons and in specialized end organs that participate in tactile sensation indicates that nucleotide signaling may contribute to low threshold mechanotransduction.
Highlights.
NTPDase3 is a major ectonucleotidase regulating purinergic transmission in nociceptive circuits.
Immunoreactivity for NTPDase3 matched ATPase enzyme histochemistry in DRG neurons and dorsal horn.
NTPDase3 co-localized with markers of nociceptors and the adenosine-generating enzyme NT5E as well as with its associated AMPase activity.
NTPDase3 was observed in some large myelinated axons and specialized end organs that participate in tactile sensation.
Acknowledgments
This work was supported by NIH grant NS056122 (DCM) and by a grant from the Samuel and Emma Winters Foundation (DCM). The authors thank Mansi Shah and Robert Friedman for technical assistance.
ABBREVIATIONS
- ADO
adenosine
- ADP
adenosine 5′-diphosphate
- AMP
adenosine 5′-monophosphate
- ATP
adenosine 5′-triphosphate
- CFA
complete Freund’s adjuvant
- DRG
dorsal root ganglion
- GAPDH
p53-glyceraldehyde-3-phosphate dehydrogenase
- HBSS
Hanks buffered salt solution
- IB4
Isolectin B4
- PBS
phosphate buffered saline
- PAP
prostatic acid phosphatase
- PKCγ
protein kinase C gamma
- Na+/K+-ATPases
sodium/potassium ATPases
- NT5E
ecto-5′-nucleotidase
- NTPDase
Nucleoside triphosphate diphosphohydrolase
- Pb(NO3)2
lead nitrate
- TMP
thiamine 5′-monophosphate
- TMSB
trizma-maleate sucrose buffer
- TRPV1
transient receptor potential vanilloid
- TM-PAP
transmembrane prostatic acid phosphatase
- UDP
uridine diphosphate
- UTP
uridine triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigonnesse F, Levesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, Sevigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia. 2004;45:124–132. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci. 2000;12:4357–4366. [PubMed] [Google Scholar]
- Braz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience. 2009;163:1220–1232. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007a;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007b;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Chen X, Molliver DC, Gebhart GF. The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. J Neurosci. 2010;30:2365–2372. doi: 10.1523/JNEUROSCI.5462-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Parmentier M, Boeynaems JM. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda M, Inoue K, Fuziwara S, Denda S. P2X purinergic receptor antagonist accelerates skin barrier repair and prevents epidermal hyperplasia induced by skin barrier disruption. J Invest Dermatol. 2002;119:1034–1040. doi: 10.1046/j.1523-1747.2002.19505.x. [DOI] [PubMed] [Google Scholar]
- Dussor G, Koerber HR, Oaklander AL, Rice FL, Molliver DC. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res Rev. 2009;60:24–35. doi: 10.1016/j.brainresrev.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes RW. Afferent fibers from mystacial vibrissae of cats and seals. J Neurophysiol. 1975;38:650–662. doi: 10.1152/jn.1975.38.3.650. [DOI] [PubMed] [Google Scholar]
- Fausther M, Lecka J, Kukulski F, Levesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sevigny J. Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2007;292:G785–795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausther MLJ, Zimmermann H, Sévigny J. Co-expression of ecto-5′-nucleotidase/CD73 with NTPDases in the liver distinctly regulates adenosine formation. Purinergic Signal. 2008;4(4):S105. [Google Scholar]
- Fradette J, Larouche D, Fugere C, Guignard R, Beauparlant A, Couture V, Caouette-Laberge L, Roy A, Germain L. Normal human Merkel cells are present in epidermal cell populations isolated and cultured from glabrous and hairy skin sites. J Invest Dermatol. 2003;120:313–317. doi: 10.1046/j.1523-1747.2003.12024.x. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Wattchow D, Coventry B. Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Res. 1987;414:143–148. doi: 10.1016/0006-8993(87)91335-7. [DOI] [PubMed] [Google Scholar]
- Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol. 2003;120:1007–1015. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]
- Halata Z, Munger BL. Sensory nerve endings in rhesus monkey sinus hairs. J Comp Neurol. 1980;192:645–663. doi: 10.1002/cne.901920403. [DOI] [PubMed] [Google Scholar]
- Harden TK, Sesma JI, Fricks IP, Lazarowski ER. Signalling and pharmacological properties of the P2Y receptor. Acta Physiol (Oxf) 2010;199:149–160. doi: 10.1111/j.1748-1716.2010.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SA, Kato K, Salter KJ, Kozlowski RZ. Functional evidence for a novel suramin-insensitive pyrimidine receptor in rat small pulmonary arteries. Circulation Research. 1998;83:940–946. doi: 10.1161/01.res.83.9.940. [DOI] [PubMed] [Google Scholar]
- Holbrook K, Wolff K, editors. The structure and development of skin. NewYork: McGraw-Hill; 1987. [Google Scholar]
- Inoue K. P2 receptors and chronic pain. Purinergic Signal. 2007;3:135–144. doi: 10.1007/s11302-006-9045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Denda M, Tozaki H, Fujishita K, Koizumi S. Characterization of multiple P2X receptors in cultured normal human epidermal keratinocytes. J Invest Dermatol. 2005;124:756–763. doi: 10.1111/j.0022-202X.2005.23683.x. [DOI] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Kaidoh T, Inoue T. N-cadherin expression in palisade nerve endings of rat vellus hairs. J Comp Neurol. 2008;506:525–534. doi: 10.1002/cne.21550. [DOI] [PubMed] [Google Scholar]
- Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D’Orleans-Juste P, Marceau F, Thorin E, Sevigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyihar-Csillik E, Bezzegh A, Boti S, Csillik B. Thiamine monophosphatase: a genuine marker for transganglionic regulation of primary sensory neurons. J Histochem Cytochem. 1986;34:363–371. doi: 10.1177/34.3.3005391. [DOI] [PubMed] [Google Scholar]
- Kukulski F, Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sevigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res. 2008;334:199–217. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- Lavoie EG, Kukulski F, Levesque SA, Lecka J, Sevigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-3. Biochem Pharmacol. 2004;67:1917–1926. doi: 10.1016/j.bcp.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Leal DB, Streher CA, Neu TN, Bittencourt FP, Leal CA, da Silva JE, Morsch VM, Schetinger MR. Characterization of NTPDase (NTPDase1; ecto-apyrase; ecto-diphosphohydrolase; CD39; EC 3.6.1.5) activity in human lymphocytes. Biochim Biophys Acta. 2005;1721:9–15. doi: 10.1016/j.bbagen.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Satue M, Lavoie EG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, Robson SC, Sevigny J. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem Cell Biol. 2009;131:615–628. doi: 10.1007/s00418-008-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo J, Harden TK, Boyer JL. Functional expression of a cDNA encoding a human ecto-ATPase. Br J Pharmacol. 1999;128:396–402. doi: 10.1038/sj.bjp.0702805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Lindsay J, Albers KM, Davis BM. Overexpression of NGF or GDNF alters transcriptional plasticity evoked by inflammation. Pain. 2005;113:277–284. doi: 10.1016/j.pain.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16:1850–1860. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Rau KK, McIlwrath SL, Jankowski MP, Koerber HR. The ADP receptor P2Y1 is necessary for normal thermal sensitivity in cutaneous polymodal nociceptors. Mol Pain. 2011;7:13. doi: 10.1186/1744-8069-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci U S A. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150:1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- Perl ER, editor. Function of dorsal root ganglion neurons: an overview. New York: Oxford University Press; 1992. [Google Scholar]
- Picher M, Sevigny J, D’Orleans-Juste P, Beaudoin AR. Hydrolysis of P2-purinoceptor agonists by a purified ectonucleotidase from the bovine aorta, the ATP-diphosphohydrolase. Biochem Pharmacol. 1996;51:1453–1460. doi: 10.1016/0006-2952(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J. Adenosine and ATP receptors. Handbook of Experimental Pharmacology. 2006;177:309–328. doi: 10.1007/978-3-540-33823-9_11. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Adenosine and ATP receptors. Handb Exp Pharmacol. 2007:309–328. doi: 10.1007/978-3-540-33823-9_11. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Downie JW, Reid AR, Cahill CM, White TD. ATP release from dorsal spinal cord synaptosomes: characterization and neuronal origin. Brain Res. 1993;610:32–38. doi: 10.1016/0006-8993(93)91213-c. [DOI] [PubMed] [Google Scholar]
- Shi XJ, Knowles AF. Prevalence of the mercurial-sensitive EctoATPase in human small cell lung carcinoma: characterization and partial purification. Arch Biochem Biophys. 1994;315:177–184. doi: 10.1006/abbi.1994.1487. [DOI] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Acid phosphatase as a selective marker for a class of small sensory ganglion cells in several mammals: spinal cord distribution, histochemical properties, and relation to fluoride-resistant acid phosphatase (FRAP) of rodents. Somatosens Res. 1988;5:219–246. doi: 10.3109/07367228809144628. [DOI] [PubMed] [Google Scholar]
- Smith TM, Kirley TL. Site-directed mutagenesis of a human brain ecto-apyrase: evidence that the E-type ATPases are related to the actin/heat shock 70/sugar kinase superfamily. Biochemistry. 1999;38:321–328. doi: 10.1021/bi9820457. [DOI] [PubMed] [Google Scholar]
- Sowa NA, Taylor-Blake B, Zylka MJ. Ecto-5′-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits. J Neurosci. 2010;30:2235–2244. doi: 10.1523/JNEUROSCI.5324-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T. The Merkel cell: recent findings and unresolved problems. Arch Histol Cytol. 1995;58:379–396. doi: 10.1679/aohc.58.379. [DOI] [PubMed] [Google Scholar]
- Van Etten RL. Human prostatic acid phosphatase: a histidine phosphatase. Ann N Y Acad Sci. 1982;390:27–51. doi: 10.1111/j.1749-6632.1982.tb40302.x. [DOI] [PubMed] [Google Scholar]
- Vekaria RM, Shirley DG, Sevigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol. 2006;290:F550–560. doi: 10.1152/ajprenal.00151.2005. [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- Vorhoff T, Zimmermann H, Pelletier J, Sevigny J, Braun N. Cloning and characterization of the ecto-nucleotidase NTPDase3 from rat brain: Predicted secondary structure and relation to other members of the E-NTPDase family and actin. Purinergic Signal. 2005;1:259–270. doi: 10.1007/s11302-005-6314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SE, Butcher RG. Studies on the Gomori acid phosphatase reaction: the preparation of the incubation medium. Histochem J. 1980;12:191–200. doi: 10.1007/BF01024549. [DOI] [PubMed] [Google Scholar]
- White TD, Downie JW, Leslie RA. Characteristics of K+- and veratridine-induced release of ATP from synaptosomes prepared from dorsal and ventral spinal cord. Brain Res. 1985;334:372–374. doi: 10.1016/0006-8993(85)90235-5. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol. 2007;36:165–183. doi: 10.1007/s12035-007-0033-y. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Zelena J. Nerves and Mechanoreceptors. London: Chapman and Hall; 1994. [Google Scholar]
- Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Barr TP, Hou Q, Dib-Hajj SD, Black JA, Albrecht PJ, Petersen K, Eisenberg E, Wymer JP, Rice FL, Waxman SG. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain. 2008;139:90–105. doi: 10.1016/j.pain.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285 (Pt 2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Braun N, Kegel B, Heine P. New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochem Int. 1998;32:421–425. doi: 10.1016/s0197-0186(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]