Abstract
Adolescence is a critical transition period, during which fundamental changes prepare the adolescent for becoming an adult. Heuristic models of the neurobiology of adolescent behavior have emerged, promoting the central role of reward and motivation, coupled with cognitive immaturities. Here, we bring focus to two basic sets of processes, attention and conditioning, which are essential for adaptive behavior. Using the dual-attention model developed by Corbetta and Shulman (2002), which identifies a stimulus-driven and a goal-driven attention network, we propose a balance that favors stimulus-driven attention over goal-driven attention in youth. Regarding conditioning, we hypothesize that stronger associations tend to be made between environmental cues and appetitive stimuli, and weaker associations with aversive stimuli, in youth relative to adults. An attention system geared to prioritize stimulus-driven attention, together with more powerful associative learning with appetitive incentives, contribute to shape patterns of adolescent motivated behavior. This proposed bias in attention and conditioning function could facilitate the impulsive, novelty-seeking and risk-taking behavior that is typical of many adolescents.
Keywords: Adolescent, Goal-directed attention, Stimulus-driven, Appetitive conditioning, Saccadic eye movement, Development
1. Introduction
Adolescence is a transition period that has recently attracted widespread interest among neuroscientists. The age boundaries of adolescence are not easy to define, given the complex biological and psychosocial developmental processes it entails, as well as the lengthy transition from childhood to adulthood in Western cultures. Even definitions of the onset of adolescence vary widely by institution or research field. For example, adolescence is defined as the period spanning age 10–24 by the Centers for Disease Control (CDC), 11–21 by The Maternal Child Health Bureau (MCHB), or 12–24 by The World Health Organization. Yet the oncology field considers that adolescence starts at age 15 years (Geiger and Castellino, 2011). Here, we define adolescence conservatively as the period covering 12–17 years of age.
Three main reasons account for the explosion of interest among neuroscientists in the adolescent period. First, increasing evidence of fundamental brain changes across a variety of domains (molecular, cellular, anatomical and anatomical–functional) has motivated scientists to gain a better understanding of the mechanisms underlying these ontogenic changes during adolescence (Casey et al., 2010, Ernst et al., 2009). Second, a paradigm shift in psychiatry recognizes most psychiatric disorders as being neurodevelopmental (Rutter et al., 2006), a position that underscores the importance of tracking normative maturational trajectories through adolescence to identify atypical processing during this period (Pine et al., 1998). Third, the well-established notion that adolescence represents a vulnerable time for the onset of psychiatric illnesses (Kessler et al., 2005, Kim-Cohen et al., 2003), and for the disastrous consequences of risky behaviors (Arnett, 1992, Dahl, 2004), is largely responsible for the neuroscience push in research on adolescence.
A number of heuristic neural systems models have been proposed to help guide research on the neural underpinnings of adolescent behavior (Casey et al., 2008, Ernst et al., 2006, Spear, 2000a, Steinberg, 2005). Reward systems and motivation processes figure prominently in these models, as they help explain the propensity for risk-taking and sensation-seeking that typifies adolescent behavior. These models have been described recently (Ernst and Fudge, 2009), and will not be reviewed presently. Instead, we propose to add new considerations to these models, bringing into focus the processes of attention and conditioning. These two processes of attention and conditioning determine how individuals apprehend the world, and, in turn, respond to the world (Fig. 1). They interact with motivation, the fuel of goal-directed behavior. They are thus fundamental to adaptive behavior, and may be particularly critical to adolescent-typical behaviors, such as potentially problematic decision-making and risk-taking, as well as adolescent-onset of psychopathologies, such as anxiety or substance use disorders.
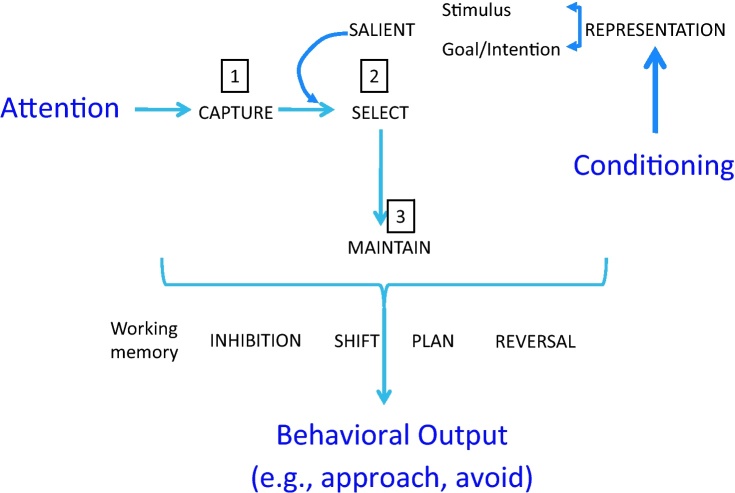
Fig. 1.
Articulation among attention, conditioning, and motivated behavior. This schematic describes how attention, conditioning and decision-making are tightly interdependent and significantly contribute to behavioral output. These operations can be organized along 3 sequential steps: (1) First, attention captures the context as a whole (e.g., visual scene of a crowd; hubbub). (2) It then selects a stimulus (e.g., the girl with a red sweater; song in the background). The critical notion here is that attention will select the most salient stimulus. Salience is determined either by the physical features of the stimulus (e.g., color red; favorite song), or by a preset goal or intention (e.g., looking for your friend who has a red coat). Schematically, the selective orienting by physical/perceptual characteristics is mediated by “stimulus-driven attention” mechanisms, whereas the selective orienting by internal rules/intentions is mediated by “goal-driven attention”. Stimuli can be endowed with affective value if they are systematically associated with other affectively-laden stimuli (e.g., song repeatedly heard during summer vacation becomes the favorite song). This is at this juncture that conditioning comes to play a critical role. A neutral stimulus conditioned to threat or to reward will acquire a unique salience that will determine the focus of selective attention. (3) Once attention orients selectively to an object, it maintains the focus on the object to permit, or prime, other cognitive processes to manipulate the information. These cognitive processes pertain mostly to executive function, e.g., working memory, inhibition, shift, plan, reversal, and they ultimately generate a course of action. The course of action is the behavioral output, which can be generalized as an approach or an avoidant response (Ernst et al., 2006). This latter hypothesis requires to be further qualified. The proposed valence bias in associative conditioning may be different for cue- and context-conditioning. Furthermore, the direction of the bias in cue-conditioning may depend on the context in which learning occurs. As a first iteration of this theory, we will make the case for a positive bias, with the understanding that this is only a first approximation awaiting systematic testing. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Attention orients individuals towards stimuli, or draws focus to behavioral rules in order to guide appropriate behavior (Fig. 1). A number of attention models have been described (Posner and Dehaene, 1994, Shallice, 1988, Ungerleider and Mishkin, 1982), each emphasizing specific aspects of attention, such as attention orienting for the Perceptual Filtering Theory (Broadbent, 1958, Treisman, 1960) and Feature Integration Theory (Treisman and Gelade, 1980); divided attention for the Resource Theory (Kahneman, 1973) and the Biased Competition Theory (Desimone and Duncan, 1995); or orienting and sustained attention for the Cognitive Neuroanatomical Model (Posner and Petersen, 1990). These and other models have recognized separate roles of attention, including orienting, selective, sustained, discriminative, dividing, or shifting attention. A special place has been given to engagement and disengagement of attention in the studies of attention bias in anxiety. Here, we will focus yet on a different type of model, the dual-attention model formulated by Corbetta and Shulman (2002). This model identifies stimulus-driven attention, i.e., attention grabbed by environmental stimuli, and goal-driven attention, i.e., attention directed to endogenous information. The former is dominant in orienting/re-orienting attention, whereas the latter might contribute strongly to sustained and divided attention. Theoretically, engagement and disengagement can occur in both attention modes.
Conditioning, also termed associative learning, entails changes in behavior based on experience. In other words, stimuli that occur repeatedly together eventually influence behavior in similar ways, presumably because neural associations between the stimuli are established (e.g., classical conditioning). Similarly, a behavior that is consistently followed by certain outcomes can be increased or decreased by that outcome, depending on whether the outcome is generally ‘good’ or ‘bad’, respectively (e.g., operant conditioning). Evaluative conditioning is yet another form of conditioning that is defined as a change in the “liking” or “disliking” of a stimulus following the pairing of that stimulus with another positive or negative stimulus (De Houwer, 2007, Hofmann et al., 2010). All of these forms of conditioning can essentially ‘tag’ stimuli with emotional valence, which creates the subjective significance (salience) of these stimuli. Of note, increased salience leads to increased attention, thereby linking the processes of attention and conditioning. For example, an aficionado of Starbucks coffee (i.e., this brand carries a positive emotional tag), will first notice Starbucks products when walking in a supermarket, rather than other brands of coffee beans. Conversely, conditioning requires attention, such that a stimulus has to receive a minimum of attention to be conditioned (Kruschke, 2001).
The leading thread of the present work is that adolescent behavior can be shaped by the unique patterns of functioning of three basic processes: attention, conditioning, and motivation. Motivation is defined as the amount of effort that an individual is ready to expend to achieve a goal (Dehaene et al., 2006, Robbins and Everitt, 1996, Schultz, 2006). Motivation is fueled by reward, or positive (appetitive) reinforcement for approach behavior, and by punishment, or negative (aversive) reinforcement for avoidance behavior. Motivation has been linked to dopamine activity and the reward neurocircuitry (Berridge and Robinson, 1998, Di Chiara, 2002, Wise, 1980), which has been shown in most studies to be hyper-responsive in adolescents vs. adults (Ernst and Spear, 2009). The ideal conditions for the expression of impulsivity and risk-taking behavior would entail the combination of (1) stronger motivation towards reward, (2) dominant stimulus-driven attention, and (3) facilitation of appetitive conditioning. As indicated earlier, we focus this paper on the changes in processes of attention and conditioning across adolescence into adulthood, with the goal of bringing to light their potential importance in the control of adolescent behavior. We will not address motivated behavior per se, which has been widely covered in the recent years (Ernst and Fudge, 2009). Similarly, we will not review conditioning studies in adults (Mazurski et al., 1996, Sehlmeyer et al., 2009), infants or children (Craske et al., 2008, Field, 2006, Gao et al., 2010b, Liberman et al., 2006, Neumann et al., 2008, Waters et al., 2009). The goal of these pediatric studies aimed to demonstrate the presence and the role of conditioning in young age groups, particularly in relation to the formation of anxiety. Their goal was not to examine how associative learning in early life could differ from that in more mature individuals. Instead, we will provide suggestive evidence for the two hypotheses delineated below, by highlighting some of the literature that is most relevant and enlightening to these ideas.
First, regarding attention, we propose that brain function during adolescence favors the engagement of stimulus-driven attention over goal-driven attention. This weighted emphasis on stimulus-driven attention could contribute to the propensity of adolescents to be impulsive and more likely to orient towards external rather than internal cues. Because the power of stimuli to attract attention increases with their salience (e.g., novelty, affective intensity), the purported reward hypersensitivity or social hypersensitivity in adolescents, which makes appetitive stimuli even more salient (Ernst and Fudge, 2009, Nelson et al., 2005), could further tip this balance towards stimulus-driven behavior. In this review, we will focus the attention section on behavioral and neuroimaging human studies of eye movements, because of the relevance of eye movements to orienting.
Second, regarding conditioning, we propose that adolescent characteristics, i.e., novelty- and sensation-seeking, risk-taking, emotional intensity, and vulnerability for substance abuse and anxiety disorders, could all be facilitated by enhanced appetitive or aversive conditioning. Appetitive or aversive conditioning is the process that imbues neutral stimuli (cue-conditioning), environment (context-conditioning), or actions (operant conditioning) with the positive (appetitive) or negative (aversive) value of a repeatedly paired stimulus, context or action (Martin-Soelch et al., 2007). Enhanced appetitive conditioning fits well with the reward hypersensitivity described in adolescents (Ernst and Fudge, 2009). Similarly, heightened aversive conditioning might underlie the propensity for anxiety in adolescence. However, weaker aversive conditioning might also facilitate risk-taking behavior. Notwithstanding, conditioning is not a unitary process. It comprises many facets with potentially different maturational progressions. For example, cue- vs. context-conditioning are two different modes of conditioning that have distinct behavioral and neural correlates (fear vs. anxiety states), as well as psychopathological significances (e.g., phobia vs. generalized anxiety disorder) (Davis et al., 2010, Grillon, 2008). In contrast to the vast body of research on the multiple facets of conditioning in mature animals and human adults (Sehlmeyer et al., 2009), as well as the existing literature in infants and young children (Craske et al., 2008, Field, 2006, Gao et al., 2010b, Liberman et al., 2006, Neumann et al., 2008, Waters et al., 2009), there is a conspicuous dearth of developmental studies of both aversive and appetitive conditioning during the adolescence period, particularly in human research. Therefore, we will turn to studies of animal models of adolescence to examine unique characteristics of conditioning in adolescents.
This review will first consider the attention hypothesis, using behavioral and neuroimaging human work of saccadic eye movements; and then the conditioning hypothesis, using some human work, but mostly animal work. As such, quite different sources of literature will be used to examine these two hypotheses: the attention hypothesis rests not only on behavioral accounts, but also on neural systems function (see Fig. 2), whereas the conditioning hypothesis is not anchored in neural mechanisms, but mainly in neurobehavioral studies of animal models of adolescence. The goal of this review is to stimulate research in both basic and clinical neuroscience to help test these hypotheses, and refine (or refute) this model.
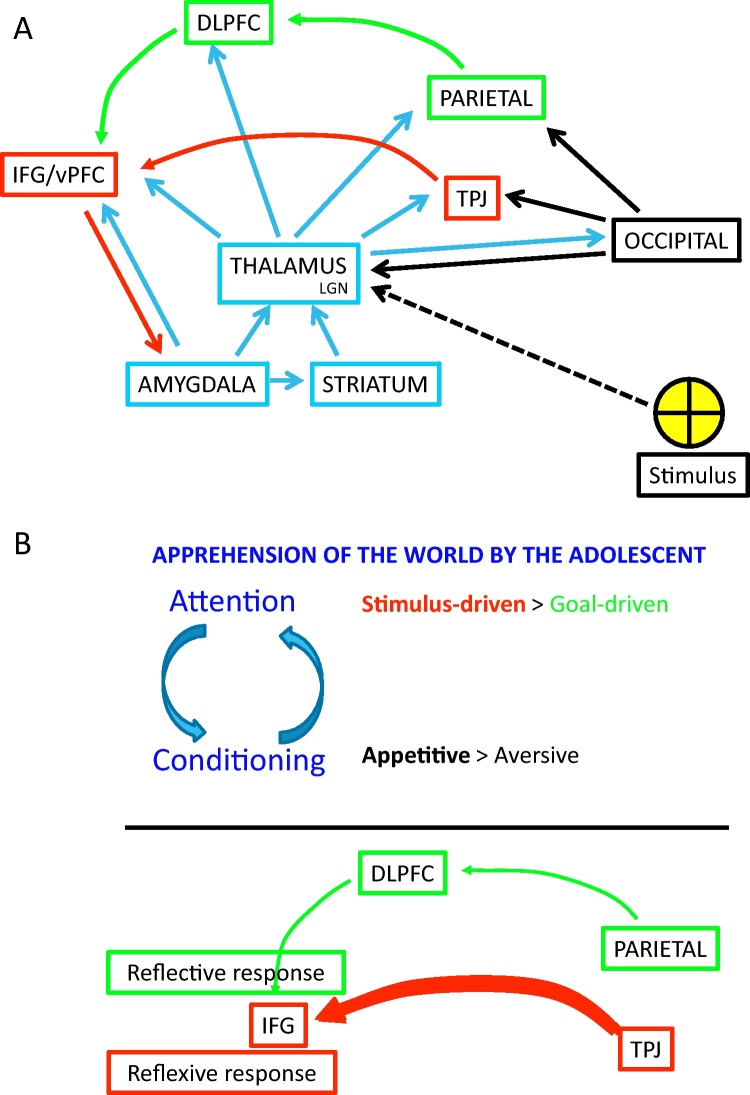
Fig. 2.
Model of the dual attention system (Corbetta and Shulman, 2002). (A) Information from an external stimulus can be processed along the following path. (1) Information from the retina about a stimulus (yellow sign) is first sent to the thalamus (through the LGN) (hatched black lines). (2) The thalamus dispatches the filtered information to cortex, as well as amygdala and striatum (these arrows are not shown here). (3) The occipital cortex carries this information to both the ventral (red lines) and dorsal attention (green lines) pathways. The ventral pathway will generate prepotent actions. The dorsal pathway will evaluate the information; activate rule representations that will engage networks to deal with the stimulus appropriately. The dorsal pathway can also influence prepotent responses through the modulation of the ventral pathway, here represented as the arrow from the DLPFC to the IFG/vPFC. (4) The amygdala and striatum receive, in addition to thalamic information, cortical information (arrows from thalamus to striatum and amygdala not represented here), and code the emotional/motivational value of the stimulus. This information is then shared with other brain areas, directly or through thalamic connections. Depending on the conditions of presentation of the stimuli (e.g., repeated pairing of stimuli), these subcortical structures may engage conditioning processes. DLPFC: dorsolateral prefrontal cortex; IFG: Inferior frontal gyrus; LGN: lateral geniculate nucleus; TPJ: temporoparietal junction; vPFC: ventral prefrontal cortex. (B) The upper panel schematizes the articulation between conditioning and attention in adolescents. The lower panel represents the simplified dual-attention system in adolescents. These functional patterns are likely to apply to specific conditions (e.g., in affectively charged contexts), which will need to be defined in future work. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2. Stimulus-driven vs. goal-driven attention/behavior
Attention is the initial process that orients brain resources towards an object/event/situation, and primes specific cognitive control processes (e.g., inhibiting, set-shifting, planning) for being recruited to respond to the object/event/situation (see Fig. 1). Cognitive control represents the ability to orchestrate a collection of cognitive processes in the pursuit of an internal goal. Attention modulates, i.e., facilitates or hampers, the coming on-line of these processes.
2.1. Definition
Attention can be captured by external stimuli (i.e., exogeneous capture of attention), or follow internal goals (voluntary steering of attention). For example, while talking with a friend, you suddenly hear your favorite song on the radio. You can interrupt your conversation to turn your attention towards your favorite song (stimulus-driven attention). Or, you can continue talking with your friend, and ignore the song playing in the background (goal-driven attention). These two attention modes operate constantly in tandem according to a balance that is tailored to external and internal demands. In the absence of endogenous attention, behavior would be controlled solely by conspicuous (salient) stimuli, and goal-directed behavior would be impossible to achieve. Conversely, would exogenous attention be absent, behavior could not adapt to sudden environmental changes, exposing the organism to danger or preventing the organism to take advantage of opportunities. These attention modes are interactive. For example, if a goal (goal-driven) is to look for snakes in a forest, anything moving on the ground (stimulus-driven) will attract attention.
Unique characteristics distinguish stimulus-driven vs. goal-driven attention. Saccadic eye movements differentiate these two types of behavior quite well. A prosaccade is a rapid eye movement that is directed towards a suddenly appearing stimulus. This ocular movement places in the fovea visual stimuli, which become the target of “attention”. This action is stimulus-driven, based on exogeneous information. In contrast, an antisaccade is an eye movement that is directed away from a suddenly appearing stimulus (Hallett, 1978). This ocular movement requires the inhibition of the prepotent response towards the stimulus, and the generation of a saccade away from this stimulus. In other words, this action is goal-driven, based on endogenous information (e.g., internalized instruction to look at the opposite side of an appearing stimulus).
Simple stimulus-driven responses are easy, fast, often prepotent or reflexive. They depend on the integrity of a perceptual attentional network. This network has been described as the ventral attentional network (Corbetta and Shulman, 2002, Kincade et al., 2005), which detects behaviorally relevant sensory events. It encompasses temporoparietal and ventral frontal regions (Corbetta and Shulman, 2002).
Goal-driven responses are complex, slow, and reflective. They depend on the integrity of the dorsal attentional network including dorsal posterior parietal and frontal cortex, which directs the selection of sensory-information and responses based on internal goals (Corbetta and Shulman, 2002, Kincade et al., 2005). Both stimulus-driven and goal-driven attention/responses also involve the specific components of the oculomotor circuitry for saccadic eye movement.
Both reflexive and reflective networks can be influenced by emotion and motivation states, through bottom-up modulation from amygdala and striatum-related circuits (see Fig. 2). It is not clear whether these two functional networks (reflexive, reflective) are similarly modulated by bottom-up circuits (Engelmann et al., 2009), and whether age affects the efficiency of this modulation. This question will be important to address, particularly in view of the purported hyper-responsivity of the reward system in adolescents.
Our central question is whether there is evidence of a unique biologically-driven balance between stimulus-driven and goal-driven attention in adolescents, and to what extent this unique pattern could contribute to the propensity for risk-taking exhibited by adolescents.
2.2. Empirical evidence
Behavioral evidence in support of a weighted balance towards stimulus-driven over goal-driven attention, would entail better performance on tasks with prepotent action, and worse performance on tasks with reflective action, in adolescents relative to adults. We will use eye-movement developmental studies to examine this point.
In saccadic eye movement tasks, age has a differential effect on pro- and antisaccade. Adolescent performance, relative to adult performance, is consistently found to be worse on antisaccades (enhanced latency, reduced accuracy) (Abel et al., 1983, Fischer et al., 1997, Fukushima et al., 2000, Jazbec et al., 2006, Klein and Foerster, 2001, Klein et al., 2003, Luna et al., 2001, Munoz et al., 1998, Velanova et al., 2009), but shows facilitation on prosaccade performance (Fioravanti et al., 1995, Funk and Anderson, 1977, Geier et al., 2010, Irving et al., 2006, Jazbec et al., 2006), although not consistently (Luna et al., 2001, Luna et al., 2004, Munoz et al., 1998).
Prosaccade accuracy often approaches a perfect score in both adolescents and adults (Velanova et al., 2009). Such high performance level suggests the possibility of a ceiling effect in both age groups, which makes it difficult to capture modulation of this performance variable. However, prosaccade velocity and peak velocity have been found to be higher in adolescents than in adults, suggesting the facilitation of prosaccade execution in youths (Fioravanti et al., 1995, Funk and Anderson, 1977, Irving et al., 2006, Jazbec et al., 2006). Most notably, one study reported that saccade velocity increased throughout childhood, and peaked at ages 10–15 years, before decreasing again in adulthood (Irving et al., 2006). However, other studies failed to detect such age effects (Luna et al., 2001, Luna et al., 2004, Munoz et al., 1998). Another support for the facilitation of prosaccades in adolescents comes from the analysis of prosaccade errors, i.e., prosaccades mistakenly executed instead of antisaccades. Geier et al. (2010) reported that prosaccade errors were facilitated (faster latency) by incentives in adolescents but not in adults. This finding suggests a greater reward sensitivity of prosaccades (stimulus-driven attention), when they override the behavioral rule (antisaccade), in adolescents than in adults.
Saccadic eye-movement studies consistently report performance improvement on antisaccade accuracy and latency from childhood to adolescence to adulthood (Abel et al., 1983, Fischer et al., 1997, Fukushima et al., 2000, Jazbec et al., 2006, Klein and Foerster, 2001, Klein et al., 2003, Luna et al., 2001, Munoz et al., 1998, Velanova et al., 2009). This age-related improvement on antisaccade has been attributed to maturing inhibitory control, while the notion of a more efficient goal-driven attention has not been entertained. Both mechanisms of improved inhibition and attention can manifest together, and may even synergize with each other through maturation.
To test this hypothesis and dissociate attentional from inhibitory processes, it will be important to consider the problem of “task impurity”. Task impurity refers to the fact that a number of cognitive processes may operate simultaneously in a task, and complicate the analysis of any single process. Strategies to remedy this problem can involve the computation of latent variables from performance scores on several cognitive tasks that have in common the cognitive process of interest while differing on the other processes. These latent variables are considered to represent purer measures of the single process under study (Miyake et al., 2000). Another approach is to design paradigms that comprise different conditions that vary only along the process under study. The comparison of the performance between the different conditions can then be attributed to the process under consideration (Kincade et al., 2005).
Overall, this brief consideration of saccade studies suggests that adolescents, relative to adults, might perform better on prosaccades, and worse on antisaccades. This pattern of behavioral responses is consistent with facilitated stimulus-driven attention and hampered goal-driven attention in adolescents.
Neural evidence in support of the hypothesis of a weighted balance towards stimulus-driven over goal-driven attention in adolescents would entail an age-related differential engagement of the circuits associated with stimulus-driven attention, i.e., dorsal parietal frontal network, from those associated with goal-driven attention, i.e., ventral parietal frontal network (Corbetta and Shulman, 2002). However, the direction of age-related differences is difficult to predict, because both higher and lower neural activation can be interpreted as evidence of facilitation of behavior. Lower activation can reflect greater efficiency, whereas higher activation can reflect facilitation of engagement of the neural circuitry (Luna et al., 2010). Alternate interpretations are also commonly proposed, such as enhanced effort to complete a given task for regional hyperactivation, or different cognitive strategy for qualitatively distinct patterns of activation.
Despite these difficulties, the neurocircuitry of saccades may help inform predictions about directionality. Prosaccades recruit the frontal eye fields, supplementary eye fields and intraparietal cortex, but also more ventral regions, including the middle temporal gyrus, temporo-parietal junction and inferior parietal lobule (Sestieri et al., 2007). These ventral regions have also been described as part of the ventral attentional network (Corbetta et al., 2000, Kincade et al., 2005). Antisaccades engage the frontal eye fields, supplementary eye fields in the parietal cortex, and posterior parietal cortex (including precuneus). Thalamus, basal ganglia and superior colliculus also belong to this oculomotor circuit (Luna et al., 2008). The degree of reliance on these structures varies as a function of the saccade type. Antisaccades recruit the oculomotor network, but, in addition, engage the dorsolateral prefrontal cortex (DLPFC) (Velanova et al., 2008).
The DLPFC has been shown to be the main region that distinguishes preparation for antisaccades from preparation for prosaccades (Brown et al., 2007, Everling et al., 1999, Everling and Munoz, 2000). Here, we suggest that the role in biasing the oculomotor system for generating antisaccades can be ascribed to activation of the goal-driven attention network, to which the DLPFC belongs. In this function, DLPFC activation may prime (i.e., enhance response-readiness of) executive function processes, and, thus, facilitate the engagement of inhibitory processes. In a recent event-related fMRI study of saccadic eye movements comparing adolescents and adults, Velanova et al. (2009) showed greater DLPFC activation in adolescents compared to adults for correct antisaccades vs. prosaccades errors (i.e., mistaken execution of prosaccades instead of antisaccades). This finding could reflect that greater “effort” is required to engage the goal-driven network in adolescents compared to adults. These findings are also supported by an ERP work on antisaccades, which shows that adolescents exhibit greater frontal activity for antisaccades relative to adults (Klein and Feige, 2005).
Taken together, these neuroimaging findings suggest two conclusions. (1) In addition to relying on the oculomotor circuit, prosaccades recruit more ventral brain regions, which are also associated with the stimulus-driven attention network; and antisaccades recruit the DLPFC, which is associated with the goal-driven attention network. (2) In comparing antisaccades to prosaccades, adolescents activate the DLPFC more strongly compared to adults, perhaps reflecting more effort at engaging the goal-driven attention network. Because of the paucity of developmental studies focusing on prosaccades, no definite conclusion can be drawn regarding the facilitation of the stimulus-driven network in adolescents compared to adults. However, the data in adolescents are consistent with a deficit in goal-directed attention (still to be separated from weaker inhibitory processes), and a potential facilitated stimulus-driven attention compared to adults. In addition, the review of the saccade work across adolescence into adulthood does not present any evidence against the proposed model.
3. Conditioning across adolescence
3.1. Definition
Affective conditioning is a form of associative conditioning, by which neutral stimuli are imbued with an emotional/motivational salience, through the repeated pairing of these neutral stimuli with other appetitive (reward) or aversive (punishment) stimuli (Martin-Soelch et al., 2007). Aversive conditioning is learning to avoid threat or injury in the form of generally negative stimuli, such as aggressive conspecifics or predators, bitter tastants, electric shock, or bright light. Appetitive conditioning is learning to satisfy needs with generally positive stimuli, such as food, water, beneficial social interactions, or money.
Classical conditioning paradigms (i.e., Pavlovian conditioning) take advantage of these innate relationships between aversive or appetitive stimuli and avoidance or approach responses, respectively. Repeatedly pairing a neutral stimulus (conditioned stimulus; CS) with an inherently meaningful stimulus (unconditioned stimulus; US) eventually causes the conditioned stimulus to elicit an avoidance or approach response (conditioned response; CR) that is often indistinguishable from the unconditioned response (UR). Many studies include differential conditioning in which two CSs are tested: one is paired with the US as just described (CS+) whereas the other is presented repeatedly but never paired with the US (CS−), thus serving as an experimental control, or ‘safe’ stimulus. In the instance of fear (aversive) conditioning, these paradigms consist of (1) the repeated pairing of a previously neutral stimulus (e.g., unemotional face, CS+) with a threat (e.g., fearful face and recorded scream, US) until the neutral stimulus provokes an aversive response, and (2) a different neutral stimulus never paired with threat (CS−). Establishing the association between US and CS+ (but not CS−) can be defined as the acquisition stage of conditioning.
Regardless of the conditioning model, extinction of the conditioned response is realized by repeated exposure to the conditioned stimulus in the absence of the unconditioned stimulus (unpairing CS–US), with extinction essentially requiring new learning about the lack of association between CS and US. Subsequent reinstatement of the conditioned response by re-exposure to the CS–US pair, to environmental cues that were present during acquisition, or to various stressors, can also be tested as measures of suppressed retention of the conditioned association.
Whether conditioning can be obtained in the absence of the awareness of the CS–US association is a matter of debate (Ohman et al., 2007). However, conditioning happens best if sufficient attention is paid to the stimuli. In this regard, attention capacity is bound to influence conditioning processes (see Fig. 1).
To our knowledge, human developmental work focusing on adolescence has not been conducted in the context of appetitive conditioning and very little in the context of aversive conditioning. One study by Oades et al. (1996) compared conditioned blocking (CB) across 4 age groups and found that CB was present throughout ages 10 to 22 years, but was weaker in younger children, up to 12 years of age. This lack of human studies does not reflect absence of interest (Casey et al., 2009, Pattwell et al., 2011, Soliman et al., 2010). In fact, clinical research on conditioning has been conducted in younger groups (up to 8 yo), particularly as a potential predictive measure of psychopathy (Gao et al., 2010a, Gao et al., 2010b, Gao et al., 2010c, Raine and Mednick, 1989), but none in adolescents in the context of normative development. In contrast, basic animal research evidences a growing interest in appetitive conditioning in the context of adolescent models of drug addiction, and adolescent aversive conditioning has been addressed with a fear conditioning model. Therefore, we will consider briefly conditioning in humans, and then in adolescent animal models, examining evidence for distinct properties of conditioning in adolescence and adulthood.
3.2. Empirical evidence
Human research in adolescent conditioning is scarce. Existing reports mainly explore aversive conditioning, including acquisition and extinction stages, in an effort to understand risk for and mechanisms of anxiety disorders (Lissek et al., 2005). Pediatric work is especially difficult because of ethical constraints related to acceptable aversive stimuli that can be used in youths (Neumann et al., 2008). Most studies have compared aspects of aversive conditioning in healthy and anxious adolescents using verbal and physiological indices of arousal (i.e., skin conductance; fear potentiated startle reflex) (Craske et al., 2008, Lau et al., 2008, Liberman et al., 2006, Waters et al., 2009). Unfortunately, these adolescent studies cannot be compared to adult work, because of the use of different types of aversive stimuli. For example, it is not clear whether a lack of discrimination between CS+ and CS− in children (Liberman et al., 2006), compared to good discrimination in adults (Lissek et al., 2005), is due to age differences or to the weaker aversive stimulus used in children (e.g., aversive tone) compared to that used in adults (e.g., electric shock).
To our knowledge, only one study directly compared adolescents (average 13 yo) and adults (average 28 yo) on aversive conditioning (Lau et al., 2011). This study employed a differential threat conditioning paradigm, and included both behavioral and fMRI experiments. The CS stimuli were two neutral faces, one face was presented always alone (CS−), and one face was presented with a US repeatedly (CS+). The US was the same actor of the CS+ face, but presented with a fearful expression and accompanied with a piercing scream. In the behavioral experiment conducted in the clinic, both adults and adolescents discriminated to a similar degree CS+ from CS− on skin conductance and self-report measures. This first experiment demonstrated the validity of the conditioning paradigm. The second experiment used this paradigm in an fMRI environment. During scanning, adolescents exhibited reduced discrimination of self-rated threat vs. safe cues relative to adults, although both adults and adolescents showed greater self-reported fear to the CS+ than CS−. Thus, conditioning in the clinic revealed no age-group difference in threat cue discrimination, while conditioning during scanning revealed reduced fear-cue discrimination in adolescents relative to adults. Although the behavioral and imaging experiments used independent samples, a possible explanation for the different age effects on fear-safe discrimination was the testing environment, which was more stressful during scanning than in the clinic. However, the neural findings indicated that adolescents showed more threat–safe discrimination than adults in the amygdala and hippocampus, while adults seemed to rely more on the DLPFC. From the perspective of the dual attentional theory, the poorer threat–safe cue discrimination in adolescents could be linked to the reduced recruitment of the goal-driven attention network, the network that facilitates other processes to take place in order to respond adaptively to the cues. As a result, in a scary context like the fMRI environment, modulation of subcortical responses (i.e., amygdala, hippocampus) to emotional cues may be hampered in adolescents, preventing the refined discrimination of the cues.
In summary, human developmental studies of affective conditioning in adolescents have a long way to go. It is surprising to realize that, to our knowledge, only one developmental study has assessed differences between healthy adolescents and healthy adults on aversive conditioning, and none on appetitive conditioning. We now turn to the animal literature.
3.3. Animal research
We can advance our understanding of human adolescence through laboratory research with animal models. Although maturation among non-human primate animals clearly mirrors human development more accurately than maturation in other species, relevant research with adolescent or ‘juvenile’ non-human primates is sparse. We focus here on rodent models and male subjects, given that numerous models of conditioning are well-validated, tractable, and more feasible in these subjects. Defined as a transitional period sometime between 28 and 60 days of age (Smith, 2003, Spear, 2000b, Spear and Brake, 1983), adolescence in rats and mice shares numerous characteristics with primate adolescence, such as sexual maturation, increases in peer-directed social interactions, elevations in novelty-seeking and/or risk-taking, and transition out of the early post-natal home environment (Crews et al., 2007, Laviola et al., 1999, Smith, 2003, Spear, 2000b).
Aversive conditioning paradigms for animals, as for humans, use aversive stimuli (e.g., footshock) or aversive states (e.g., withdrawal from drugs of abuse) to investigate the aversive control of behavior, generally avoidance behaviors. Different types of aversive conditioning can be studied. For example, conditioned place aversion (CPA) pairs footshock or drug withdrawal with a neutral environmental chamber; conditioned taste aversion (CTA) pairs malaise-producing drugs with a novel tastant; eyeblink conditioning pairs an air-puff to the eye with neutral visual and/or auditory cues; and fear conditioning pairs footshock with neutral discrete cues or an environmental context. More complex instrumental conditioning (i.e., operant conditioning) pairs a behavioral response with environmental consequences. If the consequence is aversive, then the behavior is usually punished. If the behavior removes an aversive state, then the behavior is usually reinforced (via negative reinforcement). Despite extensive research over many decades with these rodent models, surprisingly few studies include adolescent subjects. Those that do, however, suggest that adolescence may be a developmental stage of relative insensitivity to aversive stimuli and/or expression of aversive conditioning. With regard to fear conditioning, numerous studies address the ontogeny of conditioning from the early postnatal period up to juvenile or early adolescent phases of development (to about postnatal day 30; (Barnet and Hunt, 2005, Barnet and Hunt, 2006)), but few extend through adolescence with appropriate younger and older comparison groups (Hefner and Holmes, 2007, Quirk et al., 2010). One such study we would like to highlight, though, used a paradigm that permitted differentiation between context-conditioning and cue-conditioning, as well as acquisition vs. retrieval of the conditioned behavior (Pattwell et al., 2011). Male mice of various ages were exposed in a novel context (chamber) to three repeats of explicit pairings of a tone (CS) with footshock (US) that naturally produces a freezing response. Mice were tested the next day for retention of an association between the novel context and footshock (context-conditioned fear, or contextual fear), and the day after that for retention of the explicit association between the discrete tone (CS) and footshock (US; cue-conditioned fear). Mice of all ages demonstrated the cue-conditioned fear. However, the mice conditioned in early adolescence (29 days of age) failed to show the context-conditioned fear, as measured by freezing after placement back into the conditioning chamber, whereas younger (23 days of age) and older mice (39, 49, or 79 days of age) did exhibit the expected freezing response in the shock-paired context. Surprisingly, if tested for context-conditioned fear 14 days after conditioning, all age groups exhibited the expected freezing response. Moreover, if tested daily for 14 days after initial conditioning at 29 days of age, the freezing response emerged only 13 days later. Thus, acquisition of the association between shock and context was intact in early adolescence, but retrieval of the association was blocked during adolescence and only emerged later during the transition into early adulthood. As demonstrated here and in other studies (Richardson et al., 2000), cue-conditioned fear is different from contextual fear; it matures earlier, and may even be resistant to extinction during adolescence (Hefner and Holmes, 2007, McCallum et al., 2010). Differences in cue- and context-conditioned fear may relate to age-dependent reliance on stimulus-driven (cue) vs. goal-driven (contextual) attention, as described above, such that adolescent behavior might be guided more easily by discrete external stimuli than by more complex cognitive constructs, such as contexts or internal goals. Temporary suppression of contextual fear responses during adolescence has been interpreted as an evolutionary adaptation that might encourage exploration of potentially threatening new environments in a developmental stage that in many species requires establishment of new territories (Pattwell et al., 2011).
Animal models using drugs of abuse also provide some cases in which lower levels of aversive conditioning are observed among adolescent compared with adult rodents. For example, adolescent male rats exhibit less robust place aversions conditioned by high doses of nicotine (Torres et al., 2008), along with less robust taste aversions conditioned by nicotine (Shram et al., 2006, Wilmouth and Spear, 2004), cocaine (Schramm-Sapyta et al., 2006), amphetamine (Infurna and Spear, 1979), or ethanol (Anderson et al., 2010). Less robust taste aversion conditioned by lithium chloride (Schramm-Sapyta et al., 2006), although not place aversion (O’Dell et al., 2006), suggests that the adolescent resistance to aversive conditioning extends beyond drugs of abuse in some cases. In terms of simple sensitivity to aversive stimuli (as separate from conditioning), adolescent rats also show fewer affective and somatic signs of withdrawal from nicotine (O’Dell et al., 2006, O’Dell et al., 2007, Shram et al., 2008) or heroin (Doherty et al., 2010), and are less sensitive to numerous adverse effects of ethanol intake, such as hangover-related anxiety (Varlinskaya and Spear, 2006). Each of these studies uses slightly different age ranges within adolescence, and only a few provide specificity with regard to age differences in early, mid, or late adolescent development (Philpot et al., 2003, Varlinskaya and Spear, 2006). For example, whereas early and mid-adolescent male rats (PND 25 and 35, respectively) demonstrated a conditioned place aversion to 1 g/kg ethanol, late adolescents showed the opposite, a conditioned place preference, to the same dose (Philpot et al., 2003). Taken together, these experiments suggest that temporary suppression of aversive conditioning, coupled with relative insensitivity to aversive stimuli and/or fewer aversive side effects of drugs may contribute to functional imbalance between aversive and appetitive conditioning that minimizes the influence of aversive conditioning over behavior during adolescence. They also remind us that adolescence is a labile period, within which conditioning effects may change.
Appetitive conditioning is hypothesized to be particularly effective during adolescence, thereby adding to an imbalance between aversive and appetitive conditioning that could underlie risk-taking and sensation-seeking. A classical conditioning paradigm that contributes to the assessment of the approach or rewarding value of stimuli is conditioned place preference (CPP). This is similar to conditioned place aversion (CPA) described above except that repeated pairings between a stimulus and a conditioning chamber produce an increased preference for spending time in the paired chamber vs. a control (unpaired) chamber, interpreted to mean that the stimulus was in some way positive, attractive, or rewarding. An extension of this paradigm tests for extinction and reinstatement of the place preference, with extinction recorded as gradual diminution of the preference over repeated test days in the absence of the reward, and reinstatement recorded as renewal of the preference after acute re-exposure to the reward, some discrete reward-paired cues, and/or a stressor.
A prime example of heightened sensitivity to reward among adolescents is the demonstration of greater preference for a reward-paired context among adolescent male rats or mice compared to adults, which has indeed been reported in many CPP studies. For example, when the opportunity for social interaction with an age- and sex-matched peer was the reward (US), adolescent male rats demonstrated a stronger preference for a reward-paired environment than adults (Douglas et al., 2004). Similarly, when novel objects were the reward, adolescent male rats were more likely to demonstrate a preference than were their adult counterparts (Douglas et al., 2003). Several studies reported greater preference among adolescents than adults for environments paired with drugs such as nicotine or cocaine, or the opportunity to consume ethanol (Brenhouse and Andersen, 2008, Vastola et al., 2002). Notably, not all studies reported greater CPP or novel object preference among adolescents (Bolanos et al., 1996, Campbell et al., 2000, Pattwell et al., 2011), and some have specified narrower age ranges within adolescence over which CPP varies (Badanich et al., 2006, Philpot et al., 2003). Heightened sensitivity among adolescents to cocaine reward in the CPP paradigm extended to slower extinction of the preference and higher levels of reinstatement than adults on re-exposure to cocaine (Brenhouse and Andersen, 2008). Although often interpreted to reflect greater strength of the conditioned associations between environment and reward, slower extinction could instead reveal deficits in learning about the absence of the reward, as discussed towards the end of this section. Generally these studies of classical conditioning with rodents suggest that adolescents may be more likely than adults to demonstrate reward-related conditioning, and/or may be more sensitive to the rewarding stimuli themselves.
Operant conditioning has not been utilized extensively to test adolescent subjects. Most of the existing studies are conducted in the context of drug addiction with the intravenous (i.v.) drug self-administration model, in which lever-pressing or nose-poking in a conditioning chamber is reinforced with immediate presentation of an i.v. drug infusion. Consistent with heightened responses to rewards, adolescents acquired behaviors reinforced by cocaine, amphetamine, or components of cigarette smoke (e.g. nicotine and acetaldehyde) faster than adults (Belluzzi et al., 2005, Schramm-Sapyta et al., 2011, Shahbazi et al., 2008), or self-administered more cocaine, amphetamine, nicotine and acetaldehyde, or heroin than adults in some studies (Anker and Carroll, 2010, Belluzzi et al., 2005, Doherty and Frantz, 2009, Shahbazi et al., 2008), but not all (Belluzzi et al., 2005, Frantz et al., 2007, Kerstetter and Kantak, 2007, Li and Frantz, 2009). As above with CPP, heightened sensitivity to reinforcement by cocaine extended to slower extinction of cocaine-seeking among adolescent male rats compared with adults, as well as heightened drug-induced or stress-induced reinstatement of cocaine-seeking when tested towards the end of adolescence(Anker and Carroll, 2010). Also as above, slower extinction of conditioned behaviors is often interpreted to reflect greater strength of the conditioned associations, although it could instead reflect a deficit in learning about the absence of the reinforcer, an idea to which we return later. With regard to food as a reinforcer, adolescent rats acquired self-administration of sucrose pellets at the same rate as adults, but earned and consumed fewer pellets, perhaps related to their lower body mass (Li and Frantz, 2010). Adolescents also extinguished and reinstated their food-seeking in patterns similar to adults (Li and Frantz, 2010), suggesting that age differences in extinction and reinstatement do not generalize to all reinforcing stimuli. Together these operant conditioning studies demonstrate that adolescent rodents readily acquire behavior reinforced by positive stimuli, e.g. drugs or food, and in some cases they do so more quickly than adults or are more likely to retain the previously reinforced behavior in the absence of the reinforcer, i.e., in extinction and reinstatement conditions.
Distinctions between contextual conditioning and discrete cue-conditioning appear especially important during adolescence, both in the context of fear conditioning noted above and in operant conditioning. For example, reinstatement of drug-seeking after a period of abstinence can be triggered by re-exposure to the drug-paired environment (operant conditioning chamber), and/or discrete drug-paired cues. Two separate research groups have now demonstrated that reinstatement of cocaine-seeking triggered by re-exposure to discrete cocaine-paired cues is different in adolescents compared to adults; surprisingly cue-induced reinstatement is less robust among male rats that self-administered cocaine as adolescents, compared with those that self-administered as adults (Anker and Carroll, 2010, Li and Frantz, 2009). Preliminary data on cocaine- or heroin-seeking indicate, however, that when drug-seeking is tested in the absence of extinction sessions, as triggered by simultaneous re-exposure to both the operant conditioning chamber and the discrete drug-paired cues, it is instead similar across age groups (Doherty and Frantz, 2011, Li and Frantz, unpublished results). Therefore in this model, attenuations in cue-induced reinstatement among adolescents are restored to adult levels when the contextual construct is added. Moreover, the delayed extinction of a cocaine conditioned place preference among adolescent male rats mentioned above (Brenhouse and Andersen, 2008) is no longer observed if adolescents are restricted to the drug-paired context during several extinction sessions (Brenhouse et al., 2010). In this model, the delayed extinction in adolescents is accelerated to adult levels when un-pairing of the context from the drug experience is forced by the experimental procedure. Across the self-administration and CPP models, the adolescent subjects require salient contextual cues to demonstrate adult-like associations. These results seem at odds with the earlier maturation of cue-conditioned fear before contextual fear mentioned above, and thus may reflect additional differences between aversive and appetitive conditioning. Regardless, all the studies underscore the idea that explicit consideration of context, cue, and stimulus valence are important factors in tracking normative development of conditioning.
We can also use conditioned place preference and drug self-administration to consider potential interplay between aversive and appetitive conditioning, with focus on how less avoidance and greater approach might influence adolescent reward-related and risk-taking behavior. For example, one classic theoretical contributor to drug self-administration is the process of negative reinforcement, i.e., alleviation of aversive withdrawal states (a negative stimulus) reinforces continued drug-seeking or drug-taking behaviors. In fact, in adult rats, discrete cues paired with drug withdrawal can raise rates of reinstatement of heroin intake, as rats may associate the drug with alleviation of withdrawal symptoms through this process of negative reinforcement (Kenny et al., 2006). If adolescent subjects experience less aversive drug withdrawal than adults as outlined above, then their motivation to seek drugs after abstinence may be lowered in some cases (e.g. cue-induced reinstatement of morphine-, heroin- or cocaine-seeking (Anker and Carroll, 2010, Doherty and Frantz, 2011, Doherty et al., 2009, Li and Frantz, 2009), until increased drive to reinstate drug-seeking is provided, e.g. by adding contextual cues or stress (Anker and Carroll, 2010, Doherty and Frantz, 2011, Li and Frantz, unpublished results). With regard to classical conditioning models, pairing a context with aversive drug withdrawal results in a place aversion (CPA), rather than a preference (CPP) model. During extinction of a drug CPP, animals may be experiencing drug withdrawal effects that essentially create a new, negative, association between the context and the drug withdrawal state. This new association would be an aversion, and could speed extinction of the previous preference. Again if less aversive drug withdrawal is experienced by adolescents compared with adults, then it would be less likely to accelerate extinction of a place preference. Thus, delayed extinction of a CPP among adolescents could be misinterpreted as heighted sensitivity to reward instead of attenuated sensitivity to aversive drug withdrawal. Unfortunately, the role of aversive withdrawal in self-administration and CPP has not been mapped precisely enough to draw strong conclusions on this concept, but the possibility can be tested in future experiments. A final alternative interpretation of adolescent behavior that integrates aversive and appetitive conditioning also addresses extinction of conditioned behavior. Given that extinction processes require new learning about new relationships between stimuli (Bouton and Bolles, 1979), e.g., an environmental chamber is no longer associated with the reward after a conditioned place preference, slower extinction among younger animals could reflect an attenuated influence of these new relationships on behavior. Further, if the absence of the reward during extinction is aversive to the animal, then this interpretation provides additional support for the contention above that younger animals are less influenced by aversive stimuli than older animals.
Temporary suppression of the ability of aversive stimuli to exert control in conditioning and thus motivated behavior could be tested in adolescents using models of compulsive food- or drug-seeking in which lever-pressing previously reinforced by reward (food or drug) presentation is later also paired with aversive stimuli such as footshock. Continued responding in the presence of footshock is interpreted as compulsive behavior (Johnson and Kenny, 2010), and would be predicted for adolescents if they experience the same or higher sensitivity to rewards coupled with suppressed responsivity to aversive stimuli. Although not conceptualized as ‘compulsive’, adolescent-typical behaviors do continue in spite of potential harm or risk (compulsive), and also occur without appropriate planning or foresight (impulsive; see above). We have provided an example of how reduced aversive conditioning and amplified reward conditioning might lead to these characteristics.
In summary, we have highlighted some ways in which aversive and appetitive conditioning have been investigated during adolescence in rodent models. In support of our hypothesis, numerous studies point to a temporary suppression of aversive conditioning and/or reduced sensitivity to aversive stimuli, while other studies point to a transient amplification of appetitive conditioning with positive rewarding or reinforcing stimuli. Notably, many of the conditioning studies in adolescent rodents are conducted in the context of drug addiction. It will be important to determine to what extent findings with drugs of abuse reflect a general pattern of conditioning in adolescents or are specific to the drug action. Particularly, it will be important to test appetitive and aversive cue conditioning in different contexts. As reviewed here, most animal data on aversive cue conditioning were obtained in the context of reward-related drug administration. Such positive context may be associated with a relative reduction of the salience of negative stimuli in adolescents compared to adults, facilitating risk-taking and drug-taking. However, a negative context, such as in the presence of threat, may potentiate the salience of negative stimuli in adolescents more than in adults, contributing to the onset of psychopathology such as anxiety disorders. Continued research with a broader range of aversive and appetitive stimuli and in contexts of different valence, will contribute significantly to our understanding of the conditioning process across adolescence.
4. Conclusion
In conclusion, limited, but emerging evidence from the literature suggest the following two hypotheses:
-
1.
Adolescents, compared to adults, show relatively stronger stimulus-driven attention that favor stimulus-driven action, and conversely, present weaker goal-driven attention. Taken together, the functional profile of the dual-attention system in adolescents predisposes youths to respond relatively more prominently to external than internal stimuli, resulting in higher levels of impulsivity, and, together with a purported hypersensitive reward system, higher risk-taking behavior.
-
2.
Adolescents, compared to adults, may show stronger appetitive cue conditioning, that could be partly related to the putative dominance of stimulus-driven attention and hyperreactive reward system. No human studies today have tested this hypothesis. However, animal studies suggest that adolescents conditioned more easily to addictive drugs and show weaker extinction than adults. Regarding aversive conditioning, the one human study suggests that discrimination of aversive stimuli is weaker in adolescents than in adults in a stressful environment, suggesting perhaps a greater aversive conditioning to context than to cues in a stressful environment, in line with the proposed greater potentiation of cue aversiveness in a negative context for adolescents than adults. Animal studies identify a blocking of the retrieval of the cue aversive conditioning selectively during the adolescent period, suggesting a reduced efficacy of aversive cue conditioning in this period.
The functional profile of the dual attention system together with that of appetitive/aversive conditioning, may shape motivational patterns and be a critical determinant of risk-taking behavior in adolescents. Specifically, the predominance of stimulus-driven attention facilitates cue-conditioning. However, aversive conditioning in a stressful environment facilitates context conditioning and hampers differential conditioning, which may make it difficult for adolescents to take adaptive decisions. We hope that this review will stimulate research that could test these hypotheses.
Acknowledgements
This work was supported in part by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health.
References
- Abel L.A., Troost B.T., Dell’Osso L.F. The effects of age on normal saccadic characteristics and their variability. Vision Research. 1983;23:33–37. doi: 10.1016/0042-6989(83)90038-x. [DOI] [PubMed] [Google Scholar]
- Anderson R.I., Varlinskaya E.I., Spear L.P. Ethanol-induced conditioned taste aversion in male sprague–dawley rats: impact of age and stress. Alcoholism, Clinical and Experimental Research. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker J.J., Carroll M.E. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berlin) 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Badanich K.A., Adler K.J., Kirstein C.L. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. European Journal of Pharmacology. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Barnet R.C., Hunt P.S. Trace and long-delay fear conditioning in the developing rat. Learning and Behavior. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- Barnet R.C., Hunt P.S. The expression of fear-potentiated startle during development: integration of learning and response systems. Behavioral Neuroscience. 2006;120:861–872. doi: 10.1037/0735-7044.120.4.861. [DOI] [PubMed] [Google Scholar]
- Belluzzi J.D., Wang R., Leslie F.M. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bolanos C.A., Garmsen G.M., Clair M.A., McDougall S.A. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. European Journal of Pharmacology. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., Bolles R.C. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Brenhouse H.C., Andersen S.L. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behavioral Neuroscience. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Dumais K., Andersen S.L. Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience. 2010;169:628–636. doi: 10.1016/j.neuroscience.2010.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent D. Pergamon Press; London: 1958. Perception and Communication. [Google Scholar]
- Brown M.R., Vilis T., Everling S. Frontoparietal activation with preparation for antisaccades. Journal of Neurophysiology. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Campbell J.O., Wood R.D., Spear L.P. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiology and Behavior. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Duhoux S., Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Glatt C.E., Tottenham N., Soliman F., Bath K., Amso D., Altemus M., Pattwell S., Jones R., Levita L., McEwen B., Magarinos A.M., Gunnar M., Thomas K.M., Mezey J., Clark A.G., Hempstead B.L., Lee F.S. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164:108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Kincade J.M., Ollinger J.M., McAvoy M.P., Shulman G.L. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Waters A.M., Lindsey Bergman R., Naliboff B., Lipp O.V., Negoro H., Ornitz E.M. Is aversive learning a marker of risk for anxiety disorders in children? Behaviour Research and Therapy. 2008;46:954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F., He J., Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, Biochemistry, and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. Phasic vs. sustained fear in rats and humans: role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J. A conceptual and theoretical analysis of evaluative conditioning. Spanish Journal of Psychology. 2007;10:230–241. doi: 10.1017/s1138741600006491. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Changeux J.P., Naccache L., Sackur J., Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends in Cognitive Sciences. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Doherty, J., Dunigan, P., Lee, A., Williams, B., Frantz, K., 2010. Attenuated effects of experimenter-administered heroin in periadolescent vs. adult male rats: locomotor sensitization and somatic signs of withdrawal. Poster presentation at the Annual Meeting of the Society for Neuroscience.
- Doherty, J., Frantz, K., 2009. Self-administration of heroin and incubation of heroin-seeking in adolescent vs. adult rats. Poster presentation at the Annual Meeting of the Society for Neuroscience.
- Doherty, J.M., Frantz, K.J., 2011. Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology (Berlin). [DOI] [PubMed]
- Doherty J., Ogbomnwan Y., Williams B., Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacology, Biochemistry, and Behavior. 2009;92:164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L.A., Varlinskaya E.I., Spear L.P. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology and Behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas L.A., Varlinskaya E.I., Spear L.P. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Engelmann J.B., Damaraju E., Padmala S., Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Fudge J.L. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Romeo R.D., Andersen S.L. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacology, Biochemistry, and Behavior. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ernst M., Spear L. Reward systems. In: de Haan M., Gunnar M., editors. Handbook of Developmental Social Neuroscience. Guilford Press; New York: 2009. pp. 324–341. [Google Scholar]
- Everling S., Dorris M.C., Klein R.M., Munoz D.P. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. Journal of Neuroscience. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S., Munoz D.P. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.P. I don’t like it because it eats sprouts: conditioning preferences in children. Behaviour Research and Therapy. 2006;44:439–455. doi: 10.1016/j.brat.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Fioravanti F., Inchingolo P., Pensiero S., Spanio M. Saccadic eye movement conjugation in children. Vision Research. 1995;35:3217–3228. doi: 10.1016/0042-6989(95)00152-5. [DOI] [PubMed] [Google Scholar]
- Fischer B., Biscaldi M., Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Frantz K., O’Dell L.E., Parsons L.H. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Fukushima J., Hatta T., Fukushima K. Development of voluntary control of saccadic eye movements. I. Age-related changes in normal children. Brain and Development. 2000;22:173–180. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Funk C.J., Anderson M.E. Saccadic eye movements and eye-head coordination in children. Perceptual and Motor Skills. 1977;44:599–610. doi: 10.2466/pms.1977.44.2.599. [DOI] [PubMed] [Google Scholar]
- Gao Y., Raine A., Venables P.H., Dawson M.E., Mednick S.A. Association of poor childhood fear conditioning and adult crime. American Journal of Psychiatry. 2010;167:56–60. doi: 10.1176/appi.ajp.2009.09040499. [DOI] [PubMed] [Google Scholar]
- Gao Y., Raine A., Venables P.H., Dawson M.E., Mednick S.A. The development of skin conductance fear conditioning in children from ages 3 to 8 years. Developmental Science. 2010;13:201–212. doi: 10.1111/j.1467-7687.2009.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Raine A., Venables P.H., Dawson M.E., Mednick S.A. Reduced electrodermal fear conditioning from ages 3 to 8 years is associated with aggressive behavior at age 8 years. Journal of Child Psychology and Psychiatry. 2010;51:550–558. doi: 10.1111/j.1469-7610.2009.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A.M., Castellino S.M. Delineating the age ranges used to define adolescents and young adults. Journal of Clinical Oncology. 2011;29:e492–e493. doi: 10.1200/JCO.2011.35.5602. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berlin) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P.E. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hefner K., Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behavioural Brain Research. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W., De Houwer J., Perugini M., Baeyens F., Crombez G. Evaluative conditioning in humans: a meta-analysis. Psychological Bulletin. 2010;136:390–421. doi: 10.1037/a0018916. [DOI] [PubMed] [Google Scholar]
- Infurna R.N., Spear L.P. Developmental changes in amphetamine-induced taste aversions. Pharmacology, Biochemistry, and Behavior. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Irving E.L., Steinbach M.J., Lillakas L., Babu R.J., Hutchings N. Horizontal saccade dynamics across the human life span. Investigative Ophthalmology and Visual Science. 2006;47:2478–2484. doi: 10.1167/iovs.05-1311. [DOI] [PubMed] [Google Scholar]
- Jazbec S., Hardin M.G., Schroth E., McClure E., Pine D.S., Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.M., Kenny P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Prentice-Hall; Englewood Cliffs, NJ: 1973. Attention and Effort. [Google Scholar]
- Kenny P.J., Chen S.A., Kitamura O., Markou A., Koob G.F. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. Journal of Neuroscience. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter K.A., Kantak K.M. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berlin) 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Demler O., Frank R.G., Olfson M., Pincus H.A., Walters E.E., Wang P., Wells K.B., Zaslavsky A.M. Prevalence and treatment of mental disorders, 1990–2003. New England Journal of Medicine. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J., Caspi A., Moffitt T.E., Harrington H., Milne B.J., Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Kincade J.M., Abrams R.A., Astafiev S.V., Shulman G.L., Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. Journal of Neuroscience. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Feige B. An independent components analysis (ICA) approach to the study of developmental differences in the saccadic contingent negative variation. Biological Psychology. 2005;70:105–114. doi: 10.1016/j.biopsycho.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Klein C., Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6–26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- Klein C., Raschke A., Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40:17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- Kruschke J.K. Toward a unified model of attention in associative learning. Journal of Mathematical Psychology. 2001;45:812–863. [Google Scholar]
- Lau J.Y., Britton J.C., Nelson E.E., Angold A., Ernst M., Goldwin M., Grillon C., Leibenluft E., Lissek S., Norcross M., Shiffrin N., Pine D.S. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.Y., Lissek S., Nelson E.E., Lee Y., Roberson-Nay R., Poeth K., Jenness J., Ernst M., Grillon C., Pine D.S. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G., Adriani W., Terranova M.L., Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience and Biobehavioral Reviews. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Li C., Frantz K.J. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berlin) 2009 doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Li C., Frantz K.J. Time-dependent increases in cue-induced reinstatement of sucrose seeking after sucrose self-administration in adolescence. Behavioural Brain Research. 2010;213:109–112. doi: 10.1016/j.bbr.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Li, C., Frantz, K.J., unpublished results. No age differences in context- plus cue-induced reinstatement of cocaine-seeking in male rats.
- Liberman L.C., Lipp O.V., Spence S.H., March S. Evidence for retarded extinction of aversive learning in anxious children. Behaviour Research and Therapy. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S., Powers A.S., McClure E.B., Phelps E.A., Woldehawariat G., Grillon C., Pine D.S. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J., Keshavan M.S., Genovese C.R., Eddy W.F., Sweeney J.A. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B., Velanova K., Geier C.F. Development of eye-movement control. Brain and Cognition. 2008;68:293–308. doi: 10.1016/j.bandc.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C., Linthicum J., Ernst M. Appetitive conditioning: neural bases and implications for psychopathology. Neuroscience and Biobehavioral Reviews. 2007;31:426–440. doi: 10.1016/j.neubiorev.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurski E.J., Bond N.W., Siddle D.A., Lovibond P.F. Conditioning with facial expressions of emotion: effects of CS sex and age. Psychophysiology. 1996;33:416–425. doi: 10.1111/j.1469-8986.1996.tb01067.x. [DOI] [PubMed] [Google Scholar]
- McCallum J., Kim J.H., Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Broughton J.R., Goldring J.E., Armstrong I.T. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neumann D.L., Waters A.M., Westbury H.R. The use of an unpleasant sound as the unconditional stimulus in aversive Pavlovian conditioning experiments that involve children and adolescent participants. Behavior Research Methods. 2008;40:622–625. doi: 10.3758/brm.40.2.622. [DOI] [PubMed] [Google Scholar]
- O’Dell L.E., Bruijnzeel A.W., Smith R.T., Parsons L.H., Merves M.L., Goldberger B.A., Richardson H.N., Koob G.F., Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berlin) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell L.E., Chen S.A., Smith R.T., Specio S.E., Balster R.L., Paterson N.E., Markou A., Zorrilla E.P., Koob G.F. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Oades R.D., Roepcke B., Schepker R. A test of conditioned blocking and its development in childhood and adolescence: relationship to personality and monoamine metabolism. Developmental Neuropsychology. 1996;12:207–230. [Google Scholar]
- Ohman A., Carlsson K., Lundqvist D., Ingvar M. On the unconscious subcortical origin of human fear. Physiology and Behavior. 2007;92:180–185. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Pattwell S.S., Bath K.G., Casey B.J., Ninan I., Lee F.S. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot R.M., Badanich K.A., Kirstein C.L. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcoholism, Clinical and Experimental Research. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Cohen P., Gurley D., Brook J., Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Dehaene S. Attentional networks. Trends in Neurosciences. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Petersen S.E. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Pare D., Richardson R., Herry C., Monfils M.H., Schiller D., Vicentic A. Erasing fear memories with extinction training. Journal of Neuroscience. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A., Mednick S.A. Biosocial longitudinal research into antisocial behavior. Revue D’épidémiologie et de Santé Publique. 1989;37:515–524. [PubMed] [Google Scholar]
- Richardson R., Paxinos G., Lee J. The ontogeny of conditioned odor potentiation of startle. Behavioral Neuroscience. 2000;114:1167–1173. [PubMed] [Google Scholar]
- Robbins T.W., Everitt B.J. Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Rutter M., Kim-Cohen J., Maughan B. Continuities and discontinuities in psychopathology between childhood and adult life. Journal of Child Psychology and Psychiatry. 2006;47:276–295. doi: 10.1111/j.1469-7610.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta N.L., Cauley M.C., Stangl D.K., Glowacz S., Stepp K.A., Levin E.D., Kuhn C.M. Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology (Berlin) 2011;215:493–504. doi: 10.1007/s00213-011-2216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta N.L., Morris R.W., Kuhn C.M. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacology, Biochemistry, and Behavior. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C., Schoning S., Zwitserlood P., Pfleiderer B., Kircher T., Arolt V., Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. Public Library of Science. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C., Pizzella V., Cianflone F., Luca Romani G., Corbetta M. Sequential activation of human oculomotor centers during planning of visually-guided eye movements: a combined fMRI-MEG study. Frontiers in Human Neuroscience. 2007;1:1. doi: 10.3389/neuro.09.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M., Moffett A.M., Williams B.F., Frantz K.J. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berlin) 2008 doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Shallice T. Cambridge University Press; New York: 1988. From Neuropsychology to Mental Structure. [Google Scholar]
- Shram M.J., Funk D., Li Z., Le A.D. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berlin) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Shram M.J., Siu E.C., Li Z., Tyndale R.F., Le A.D. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berlin) 2008;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Smith R.F. Animal models of periadolescent substance abuse. Neurotoxicology and Teratology. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Soliman F., Glatt C.E., Bath K.G., Levita L., Jones R.M., Pattwell S.S., Jing D., Tottenham N., Amso D., Somerville L.H., Voss H.U., Glover G., Ballon D.J., Liston C., Teslovich T., Van Kempen T., Lee F.S., Casey B.J. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Research and Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Spear L., Brake S.C. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Torres O.V., Tejeda H.A., Natividad L.A., O’Dell L.E. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology, Biochemistry, and Behavior. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A.M. Contextual cues in selective listening. The Quarterly Journal of Experimental Psychology. 1960;12:242–248. [Google Scholar]
- Treisman A.M., Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G., Mishkin M. Two cortical visual systems. In: Ingle D.J., Goodale M.A., Mansfield R.J.W., editors. Analysis of Visual Behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- Varlinskaya E.I., Spear L.P. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Developmental Psychobiology. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Vastola B.J., Douglas L.A., Varlinskaya E.I., Spear L.P. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology and Behavior. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.M., Henry J., Neumann D.L. Aversive Pavlovian conditioning in childhood anxiety disorders: impaired response inhibition and resistance to extinction. Journal of Abnormal Psychology. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- Wilmouth C.E., Spear L.P. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Annals of the New York Academy of Sciences. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Wise R.A. The dopamine synapse and the notion of pleasure centers in the brain. Trends in Neurosciences. 1980;3:91–95. [Google Scholar]