Abstract
Background
Photosensitive epileptic (SZ) baboons demonstrate different cerebral blood flow (CBF) activation patterns from asymptomatic controls (CTL) during intermittent light stimulation (ILS). This study compares “resting” CBF between PS and CTL animals, and CBF correlations with ketamine dose and interictal epileptic discharges (IEDs) between PS and CTL animals.
Methods
Continuous intravenous ketamine was administered to eight PS and eight CTL baboons (matched for gender and weight), and maintained at subanesthetic doses (4.8–14.6 mg/kg/hr). Three resting H215O-PET studies were attempted in each animal (CTI/Siemens HR+ scanner). Images were acquired in 3D mode (63 contiguous slices, 2.4 mm thickness). PET images were co-registered with MRI images (3T Siemens Trio, T1-weighted 3D Turboflash sequence, TE/TR/TI = 3.04/2100/785 msec, flip angle=13 degrees). EEG was used to monitor depth of sedation and for quantification of IED rates. Regional CBF was compared between PS and CTL groups and correlations were analyzed for ketamine dose and IED rates.
Results
When subsets of animals of either group, receiving similar doses of ketamine were compared, PS animals demonstrated relative CBF increases in the occipital lobes and decreases in the frontal lobes. Correlation analyses with ketamine dose confirmed the frontal and occipital lobe changes in the PS animals. The negative correlations of CBF with ketamine dose and IED rate overlapped frontally. While frontal lobe CBF was also negatively correlated with IED rate, positive correlations were found in the parietal lobe.
Conclusions
“Resting” CBF differs between PS and CTL baboons. Correlation analyses of CBF and ketamine dose reveal that occipital lobe CBF increases and frontal lobe in PS animals are driven by ketamine. While frontal lobe CBF decreases may be related to ketamine’s propensity to activate IEDs, positive CBF correlations with IED rate suggest involvement of the parietal lobes in their generation.
Keywords: CBF, PET, Ketamine, Baboon, Idiopathic generalized epilepsy
The baboon represents a model of idiopathic generalized epilepsy with in humans (Killam, 1979; Naquet and Meldrum, 1972). The epileptic baboons exhibit myoclonic and generalized tonic-clonic seizures, predominantly in the morning. Their epilepsy tends to present in adolescence. The initial interest in this model was sparked by the recognition of photosensitivity, i.e. that interictal epileptic discharges (IEDs) or seizures could be provoked by intermittent light stimulation (ILS). The electroclinical features of the generalized epilepsy in the baboon closely resemble juvenile myoclonic epilepsy, a relatively common, inherited epilepsy syndrome in humans.
In a previous study characterizing the electroclinical phenotypes of photosensitive, epileptic baboons in a pedigreed colony at the Southwest National Primate Research Center (SNPRC) at the Southwest Foundation of Biomedical Research (SFBR) in San Antonio, Texas, ketamine was used as a sedative to immobilize the baboons during scalp electroencephalography (Szabó et al., 2005). Ketamine is a short-acting dissociative anesthetic that does not suppress respiratory drive or systemic blood pressure. It also does not suppress cortical responsiveness to ILS at subanesthetic doses. Furthermore, similar to humans (Ferrer-Allado et al., 1973; Celesia et al., 1975), ketamine can activate IEDs or even seizures in animals predisposed to epileptic seizures (Szabó et al., 2005). For these reasons, we used ketamine in a recent H215O-positron emission tomography (PET) study that characterized regional CBF changes associated with ILS in six photosensitive, epileptic baboons (PS) compared to four asymptomatic control animals (Szabó et al., 2007). But while regional CBF patterns were dramatically different between the PS and CTL animals during ILS, there were no regional differences at resting state. This may have been due to similar ketamine-induced CBF changes in both groups, or the large variance of ketamine doses used in each group. In healthy humans, ketamine demonstrated a dose-dependent increase in CBF, even at subanesthetic doses (Holcomb et al., 2001; Långsjö et al., 2003; Långsjö et al., 2005). While CBF was increased globally, regional differences were also observed. The greatest CBF increases were found in the frontal lobe convexity, anterior cingulate, putamen and insula, followed by the thalamus, caudate and parietal cortex, while occipital CBF was largely unchanged (Långsjö et al., 2003; Långsjö et al., 2005) or decreased (Holcomb et al., 2001).
Therefore, to better understand the regional CBF changes that occurred during ILS in photosensitive and asymptomatic baboons, it is essential to evaluate potential dose-dependent effects of ketamine in the resting state. To evaluate the dose-dependent effect of ketamine on regional CBF, subgroups receiving the same weight-adjusted doses were compared. Furthermore, as ketamine infusions were continuously titrated during the imaging studies to maintain sedation and immobilization in both groups, correlation analyses using the dose of ketamine as an external pattern vector were performed in each group to better understand the associated CBF changes. Finally, because of neurovascular coupling, regional CBF changes could reflect IEDs or seizure activity occurring at rest in the photosensitive, epileptic animals. To account for this effect, correlation analyses were also performed using IED rate as a separate external pattern vector.
Methods
Eight female CTL and eight female PS baboons (Papio hamadryas anubis and hybrids) were selected from a database of animals that had undergone scalp EEG studies to characterize their epilepsy phenotypes in baboons housed at the SNPRC (Szabó et al., 2005). PS animals included baboons with previously witnessed spontaneous seizures that demonstrated IEDs and photosensitivity, while CTL baboons did not have a history witnessed seizures or EEG abnormalities (Szabó et al., 2007).
Female baboons were chosen in order to maintain relative homogeneity of body size, weight, physiology, and brain size. The mean age was 18 (range 7–25) years old, and the mean weight was 18.4 (range 14.2–23.9) kilograms. There were no significant differences in age or weight between the PS and CTL groups (Table 1). PS and CTL groups were statistically compared for age and weight using two-tailed Student t-tests (α < 0.05).
Table 1.
Demographics of Seizure and Control Baboons
| Seizure Group | Control Group | |
|---|---|---|
| Gender | 8 females | 8 females |
| Age | 21 (+/− 5) years oldNS | 15 (+/− 6) years oldNS |
| Weight | 18.8 (+/− 3.6) kgNS | 18.0 (+/− 1.2) kgNS |
| Ketamine dose | 6.5 (+/− 1.4) mg/kg/hr* | 10.1 (+/− 2.5) mg/kg/hr* |
| Resting EEG Findings | ||
| EEG samples (total) | 20 | 20 |
| IEDs (animals/samples) | 7 animals/14 samples | 1 animal/3 samples |
| Mean IED Rate | 4 (range 0–12)/90 seconds | 3 (range 1–5)/90 seconds |
| Seizures (animals/samples) | 3 animals/5 samples | None |
IEDs (interictal epileptic discharges), NS statistically not significant,
statistically significant by two-tailed t-test (p<0.0001).
Baboons were transported in squeeze cages from the SFBR to the Research Imaging Center (RIC) at the University of Texas Health Science Center at San Antonio (UTHSCSA). The baboons were imaged in PET and MRI and returned to SFBR on the same day. The baboons were treated in strict accordance with the U.S. Public Health Service’s Guide for the Care and Use of Laboratory Animals (Grossblatt, 1996) and the Animal Welfare Act. This study was approved by the Institutional Animal Care and Use Committees of UTHSCSA and SFBR.
Ketamine Anesthesia
The baboons were sedated with an intramuscular injection of s-ketamine 5–6 mg/kg (KetaVed, Phoenix Scientific, St. Joseph, Missouri). They were removed from their squeeze cages and their scalp, shoulders and one leg were shaved. A catheter was placed in the saphenous vein. All animals received atropine 0.5 mg intramuscularly to reduce oropharyngeal secretions. EEG electrodes were affixed and the placement of soft restraints and facemask or Velcro straps fitted to limit head movement in the scanner. A continuous ketamine infusion (initially at 6 mg/kg/hr) was then started. The animals’ EEG, respiration and movement were monitored to assure adequate, but minimal, sedation. The ketamine dose was titrated to restrain movement while avoiding anesthesia (suppression of background activity). The doses ranged between 4.8 and 14.6 mg/kg/hr. The CTL group received statistically higher doses of ketamine than the PS group (two-tailed Student t-test, (p<0.05).
PET scans
Dynamic PET images were acquired on a CTI/Siemens HR+ scanner (Siemens, Munich, Germany; 63 contiguous slices, 2.4 mm thickness, 12 frames of 10 seconds each) in 3D mode. Transmission scans were performed for attenuation correction. Five CBF measurements were acquired in each animal. The first, third and fifth scans measured resting CBF, while ILS was delivered during the second and fourth scans. The baboons received a bolus dose of 20 mCi 15O-labelled water (H215O) every 8–10 minutes. The total acquisition time for each scan was 120 seconds
A total of twenty resting scans were acquired for each group. Three resting CBF measurements were available in all but two PS and three CTL animals. The remaining resting scans were excluded due to motion artifact.
MRI scans
At the end of each PET session, the baboons were intubated and connected to a ventilator (Datex-Ohmeda Aestiva 5, Madison, Wisconsin, U.S.A.). The ketamine drip was discontinued, and the baboons received isoflurane at 1–1.5% inspiratory MAC. The baboons were placed supine into a 3T Trio MRI scanner (Siemens, Munich, Germany) using soft restraints. Heart rate, pulse oximetry and respiratory rates were monitored. High-resolution (500 μm isotropic), high gray-matter/white-matter contrast (~25%) and T1-weighted (~25 signal-to-noise) images were acquired using a retrospective motion-corrected protocol. With this protocol, six full-resolution volumes were acquired using a T1-weighted, 3D TurboFlash sequence with an adiabatic inversion contrast pulse (TE/TR/TI = 3.04/2100/785 msec, flip angle=13 degrees).
Image Pre-Processing
PET images were reconstructed to a matrix size of 128 x 128 x 75, using a 5 mm Hahn filter resulting in images with a spatial resolution of about 7 mm full-width at half-maximum (FWHM). For each injection of H215O, the time of bolus arrival in the brain was confirmed to be in the third frame (20–30 seconds). Therefore, the first two frames (20 seconds) of the data were excluded from further analysis. Frames 3–12 were corrected for intra-scan motion using MCFLIRT tool in FSL software (FMRIB, Oxford, UK) and averaged. All resting H215O scans were also corrected for inter-scan motion using MCFLIRT. The PET images were value-normalized to a whole-brain mean count of 1000. Each animal’s PET scan was co-registered with its own MRI using the Convex Hull algorithm (Lancaster et al., 1999). MRI scans of all baboons were aligned to the first baboon MRI. Further analysis of PET and MRI data were performed once the images were processed to a common space.
Image Analysis
Images were analyzed using a combination of voxel-wise statistical parametric images (SPIs) and correlation analyses. SPIs were computed inter-group for the PS and CTL. Cross-group z-score image (SPI{z}) were obtained by comparing averaged resting scans for PS and CTL groups. Because of the significant differences in ketamine doses between the two groups, three initial scans were identified in each group, matched for weight-adjusted ketamine doses. Initial scans were chosen as CBF was not affected by prior to exposure to ILS. These scans belonged to three PS animals receiving ketamine doses of 6.2, 7.2 and 8.4 mg/kg/hr, and three CTL animals receiving 7.0, 7.8 and 7.9 mg/kg/hr, respectively. Averaged resting scans of the CTL group were subtracted from the PS group (Figure 1).
Figure 1. SZ–CTL Subtraction of Averaged “Resting” Scans.
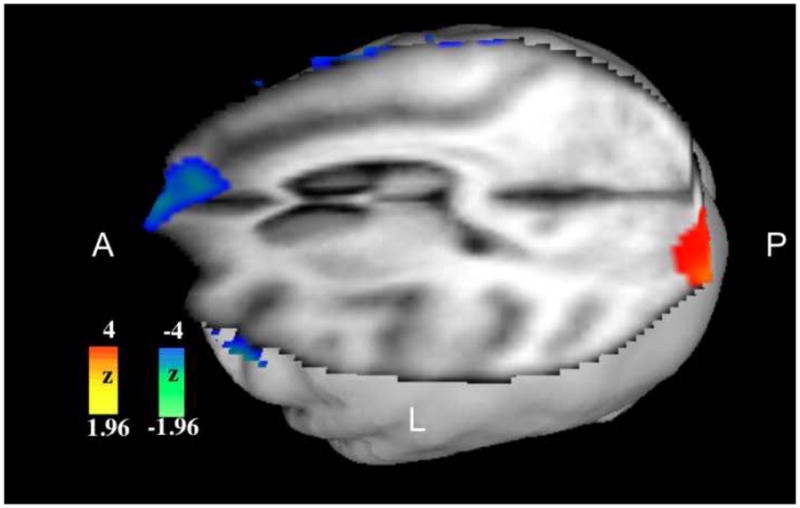
Activations are noted in the left occipital region, deactivations in the right orbitofrontal region.
Activation and deactivation clusters were localized using MRI templates described elsewhere (Greer et al., 2002). Because of small numbers of scans available for the parametric analyses, mean value normalized PET counts were obtained from volumes of interest (VOI) of 125 mm3 from bilateral orbitofrontal and occipital cortices in both groups. The mean counts from each group was computed and compared by two-tailed t-tests for differences in means.
Finally, Pearson correlations were used to evaluate the relationship between regional CBF and ketamine dose changes in PS and CTL groups.
EEG studies
EEG recordings were performed during the PET scans to monitor the level of sedation, interictal epileptic discharges (IEDs), seizure activity, as well as photoparoxysmal and photoconvulsive responses. The EEG techniques have been described elsewhere (Szabó et al., 2007). The resting state EEG was reviewed over the 120-second PET acquisition period for each animal, and the presence of IEDs and brief myoclonic seizures, predominantly consisting of eyelid myoclonus, were documented in the resting state (Table 1). All EEG samples were reviewed by one author (CÁS), who was blinded to identity and prior EEG diagnosis of the baboons.
IEDs were noted in seven of eight PS animals and in one of 8 CTL animals. While all of the animals had been grouped on the basis of a clinical history of seizures and photosensitivity on a prior EEG, both of these animals demonstrated ILS-induced CBF patterns typical for their respective groups (Szabó et al., 2007). IEDs were documented in a total of 17 EEG samples. Brief myoclonic seizures, with motor involvement restricted to the eyelids and face, were observed on 5 studies in three PS animals. In the eight baboons with IEDs, Pearson correlations were used to evaluate the relationship between IED rate and regional CBF changes. In addition, Pearson correlations were also used to evaluate the relationship of ketamine dose and IED rate, but no correlation was found (r2=0.02).
Results
CBF was increased in the occipital regions and decreased in the orbitofrontal regions, particularly on the right, in PS animals compared to CTL baboons (Figure 1). Resting CBF was significantly increased in the PS group in the left occipital region when compared with CTL animals (mean ± SEM – 1066 ± 19 vs 1000±20, p=0.01). There was a nonsignificant trend of increased CBF in right orbitofrontal region in the control group (1096 ± 36 vs 1026 ± 38, p=0.06). There was no significant difference in CBF between the two groups in left orbitofrontal and right occipital regions.
Regional CBF changes were correlated with increasing ketamine dose (Figure 2) and IED rate (Figure 3). Regional positive and negative CBF correlations with ketamine dose did not overlap between the PS and CTL groups. In the animals with IEDs, ketamine and IED-related correlations were concordant in the frontal lobes, but not in other brain regions.
Figure 2. Correlation Analyses of CBF with Ketamine Dose.

Top two images show correlations in CTL, the bottom two in PS animals, respectively. Note the positive correlations (yellow-red) in the PS animals in the occipital lobes, and the negative correlations (green-blue) in the frontal lobe.
Figure 3. Correlation Analyses of CBF with IED Rate.
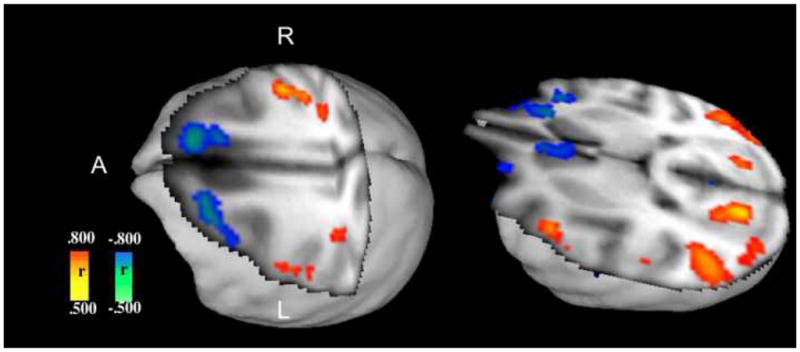
Positive correlations are evident in the primary sensorimotor cortices and parietal lobe association cortices, while negative correlations are observed frontally, in the right anterior cingulate gyrus, and in the medial orbitofrontal gyri bilaterally.
Regional CBF correlations with ketamine dose for the CTL group
Significant positive correlations with CBF were noted bilaterally in the frontal regions (lateral motor cortices, striatum and insulae), and in the left supplementary motor area and lingular gyrus. Negative correlations were noted bilaterally in the orbitofrontal, affecting the right side more expansively, and temporal lobes, affecting more the left side, and in the right medial parieto-occipital region.
Regional CBF correlation with ketamine dose for the PS group
Positive correlations were noted with ketamine dose only in the occipital cortices in the PS animals. Significant negative correlations were noted in the left anterior cingulate, lateral motor cortex and striatum, right orbitofrontal and insular cortices, and in the posterior cingulate region.
Regional CBF correlation with IED rate
Positive correlations were noted for the post-more than precentral gyri, cuneus, superior temporal, lingular and angular gyri bilaterally. Negative correlations were noted for caudate heads and orbitofrontal gyri bilaterally, and in the right anterior cingulate and left superior frontal gyrus.
Discussion
In a previous H215O-PET study, regional cerebral blood flow (CBF) differed dramatically between the photosensitive epileptic (PS) and asymptomatic control (CTL) animals during intermittent light stimulation (ILS), though no differences were noted during the resting scans (Szabó et al., 2007). In the current study, with larger numbers of PS and CTL baboons, there were still no CBF differences between the averaged resting scans for each group. The expected CBF changes in PS animals, based upon CBF studies in humans with generalized ictal or interictal epileptic discharges (IEDs), may have been masked by ketamine-induced CBF increases or the large variance in ketamine doses in each group. By comparing scans performed at similar weight-matched doses of ketamine, however, CBF differences became apparent. Occipital lobe CBF was increased, while frontal lobe CBF was decreased in the PS animals. While these findings need to be confirmed in larger numbers of baboons, correlation analyses of CBF with ketamine dose and IED rate proved helpful in their validation.
Occipital CBF changes may not be surprising as the PS animals were photosensitive. But rather than being decreased in the resting state, CBF was increased. As only the initial resting scans were compared, the relative increase of occipital CBF cannot be explained by the effect of ILS. Although the occipital lobe plays an essential role in human and baboon photosensitivity, the occipital lobe is not believed to be the generator of the photoparoxysmal or photoconvulsive responses in the baboon (Fischer-Williams et al., 1968; Naquet and Meldrum, 1972). In the absence of occipital lobe ictal or IEDs, it is unclear why the occipital CBF would be increased, but positive occipital CBF correlations with ketamine dose in the PS baboons suggest a particular susceptibility to ketamine. As NMDA-receptors are less concentrated in the visual cortices of the baboon, the CBF changes may be mitigated by neurotransmitters other than glutamate (Geddes et al., 1989; Ticku et al., 1992). If occipital GABA concentrations are decreased in the photosensitive baboon as they are in patients with juvenile myoclonic epilepsy, which is associated with a high prevalence of photosensitivity (Petroff et al., 2001), CBF increases may reflect decreased regional inhibition of a global excitatory effect of ketamine. Magnetic resonance spectroscopy of occipital lobe glutamate or GABA levels of the PS baboons may help to confirm this hypothesis.
The CBF decrease in the frontal regions of the PS baboons, when compared to the CTL baboons, was also not unexpected. The CBF decrease was predominantly in the right anterior cingulate and orbitofrontal regions in PS animals, the same areas demonstrating activation during ILS (Szabó et al, 2007). Although ketamine causes a dose-dependent increase in frontal CBF in healthy human subjects, particularly in the right orbitofrontal region (Långsjö et al., 2003; Långsjö et al., 2005), minimal positive frontal lobe CBF correlations were noted with increased ketamine doses in the CTL baboons, while the frontal lobe CBF was negatively correlated in the PS animals. In contrast to the occipital lobe findings, the negative frontal lobe correlations may have been related to IED rate or seizure activity in the PS group, and not solely to ketamine dose. Why ictal and interictal epileptic discharges, which are maximally expressed in the frontal regions on EEG, are associated with decreased CBF is unclear. CBF may be decreased in the frontal regions during the late phase of the ictal or IEDs as well as between discharges (Gotman et al., 2005). CBF studies in patients with absence seizures or sustained generalized IEDs using blood oxygen level dependent (BOLD) MRI also demonstrated predominantly frontal lobe deactivations (Archer et al., 2003; Salek-Haddadi et al., 2003; Aghakhani et al., 2004; Hamandi et al., 2006).
The positive correlation of CBF in the primary sensorimotor cortices with IED confirms electrophysiological and neuroimaging findings in baboons and rodents (Naquet and Meldrum, 1972; van Luijtelaar and Sitnikova, 2006). In the baboon, early electrophysiological studies identified the primary sensorimotor area as a potential generator of IEDs, occurring spontaneously or induced by ILS, and motor seizures (Naquet and Meldrum, 1972). The ictal discharges and IEDs propagated only secondarily to the medial frontal and subcortical structures. Parietal lobe activation, particularly of the primary somatosensory (barrel) cortex, was also demonstrated by electrophysiological and functional MRI studies in WAG/Rij rat during generalized IEDs and absence seizures, with secondary propagation to the somatomotor and visual cortices and the thalami (Nersesyan et al., 2004; van Luijtelaar and Sitnikova, 2006). The CBF increases in the baboon also involved the parietotemporal and parieto-occipital association cortices. Caudal parietal lobe involvement in the generation of ictal or IEDs could explain the initial and frequently restricted involvement of the eyelids and face in myoclonic or generalized motor seizures in the baboon. Unfortunately, the early electrophysiological studies may not have adequately sampled the parietal lobe association cortices to demonstrate its role as a generator of seizures or IEDs (Fischer-Williams et al., 1968).
In summary, the baboon provides an important neuroimaging model for the evaluation of CBF changes associated with IEDs activated by ILS or occurring under the influence of ketamine sedation. From the subtraction scans comparing the PS and CTL animals, it was evident that differences in CBF were most apparent occipitally and frontally. Correlation studies provided a sensitive tool for the detection of subtle CBF shifts in regions affected by ketamine or IED rates, confirming the frontal deactivations found on the subtraction scans. Positive CBF correlations with IED rate were noted in brain regions previously associated with the generation of ictal and IEDs in the baboon and rodent models. Functional MRI may better delineate the spatial and temporal resolution of CBF during IEDs, but, ultimately, intracerebral electrophysiological monitoring in the baboon will be necessary to determine if the regional CBF changes in the frontal, parietal and occipital lobes correspond to abnormal cortical excitability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aghakhani Y, Bagshaw AP, Bénar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- 2.Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulated during generalized spike and wave. NeuroImage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 3.Celesia GG, Chen R-C, Bamforth BJ. Effects of ketamine in epilepsy. Neurology. 25:169–172. doi: 10.1212/wnl.25.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer-Allado T, Brechner VL, Dymond A, Cozen H, Crandall P. Ketamine-induced electroconvulsive phenomena in the human limbic and thalamic regions. Anesthesiology. 1973;38:333–344. doi: 10.1097/00000542-197304000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fischer-Williams M, Poncet M, Riche D, Naquet R. Light-induced epilepsy in the baboon, Papio Papio: Cortical and depth recordings. Electroenceph Clin Neurophys. 1968;25:557–569. doi: 10.1016/0013-4694(68)90235-6. [DOI] [PubMed] [Google Scholar]
- 6.Geddes JW, Cooper SM, Cotman CW, Patel S, Meldrum BS. N-methy-D-aspartate receptors on in the cortex and hippocampus of baboon (Papio anubis and Papio papio) Neurosci. 1989;32:32–47. doi: 10.1016/0306-4522(89)90106-1. [DOI] [PubMed] [Google Scholar]
- 7.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalzied epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greer PJ, Villemagne VL, Ruszkiewicz J, Graves AK, Meltzer CC, Mathis CA, Price JC. MR atlas of the baboon brain for functional neuroimaging. Brain Res Bull. 2002;58:429–438. doi: 10.1016/s0361-9230(02)00810-9. [DOI] [PubMed] [Google Scholar]
- 9.Grossblatt N, editor. Guide for the Care and Use of Laboratory Animals. Washington D.C: National Academy Press; 1997. [Google Scholar]
- 10.Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, Duncan JS, Lemieux L. EEG-fMRI of idiopathic and secondarily generalized epilepsies. NeuroImage. 2006;31:1700–1710. doi: 10.1016/j.neuroimage.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA. Sequential regional cerebral blood flow brain scans using PET with H215O demonstrate ketmaine actions in CNS dynamically. Neuropsychopharmacology. 2001;25:165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 12.Killam EK. Photomyoclonic seizures in the baboon, Papio papio. Federation Proc. 1979;38:2429–2433. [PubMed] [Google Scholar]
- 13.Lancaster JL, Fox PT, Downs H, Nickerson DS, Hander TA, El Mallah M, Kochunov PV, Zamarripa F. Global spatial normalization of human brain using convex hulls. J Nucl Med. 1999;40:942–955. [PubMed] [Google Scholar]
- 14.Långsjö JK, Kaisti KK, Aalto S, Hinkka S, Aantaa R, Oikonen V, Sipilä H, Kurki T, Silvanto M, Scheinin H. Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:614–623. doi: 10.1097/00000542-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Långsjö JK, Mkasimow A, Salmi E, Kaisti K, Aalto S, Oikonen V, Hinkka S, Aantaa R, Sipilä H, Viljanen T, Parkkola R, Scheinin H. S-Ketamine anesthesia increases cerebral blood flow in excess of the metabolic needs in humans. Anesthesiology. 2005;103:258–268. doi: 10.1097/00000542-200508000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Naquet R, Meldrum BS. Photogenic seizures in baboon. In: Purpura DP, Penry JK, Tower DK, Woodbury DM, Walter RD, editors. Experimental Models of Epilepsy. New York: Ravens Press Publishers; 1972. pp. 374–406. [Google Scholar]
- 17.Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow & Metab. 2004;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- 18.Petroff OA, Hyder F, Rothman DL, Mattson RH. Homocarnosine and seizure control in juvenile myoclonic epilepsy and complex partial seizures. Neurology. 2001;56:709–715. doi: 10.1212/wnl.56.6.709. [DOI] [PubMed] [Google Scholar]
- 19.Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–667. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- 20.Szabó CÁ, Leland MM, Knape K, Elliott JJ, Haines VL, Williams JT. Clinical and EEG phenotypes of epilepsy in the baboon (Papio hamadryas spp) Epilepsy Res. 2005;65:71–80. doi: 10.1016/j.eplepsyres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Szabó CÁ, Narayana S, Kochunov PV, Franklin C, Knape KD, Davis MD, Fox PT, Leland MM, Williams JT. PET Imaging in the photosensitive baboon: A case- controlled study. Epilepsia. 2007;48:245–253. doi: 10.1111/j.1528-1167.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- 22.Ticku MK, Lee JC, Murk S, Mhatre MC, Story JL, Kagan-Hallet K, Luther JS, MacCluer JW, Leland MM, Eidelberg E. Inhibitory and excitatory amino acid receptors, c-fos expression, and calcium-binding proteins in the brain of baboons (Papio hamadryas) that exhibit 'spontaneous' grand mal epilepsy. Epilepsy Res Suppl. 1992;9:141–149. [PubMed] [Google Scholar]
- 23.van Luijtelaar G, Sitnikova E. Global and focal aspects of absence epilepsy: The contribution of genetic models. Neuroscience and Biobehavioral Reviews. 2006;30:983–1003. doi: 10.1016/j.neubiorev.2006.03.002. [DOI] [PubMed] [Google Scholar]


