Abstract
Objective
To examine the association of microbial contamination of the meal preparer’s hands with microbial status of food and kitchen/utensil surfaces during home preparation of a “Chicken and Salad” meal.
Design and Setting
Observational home food safety assessment. Before starting meal preparation, participants’ hands were tested to estimate total bacterial and coliform counts and the presence of Campylobacter, Salmonella, Listeria, and Staphylococcus aureus (S. aureus). Microbiological testing was conducted on samples from kitchen/utensil surfaces, and on food ingredients obtained before and during meal preparation.
Participants
Sixty Puerto Rican women residing in inner-city Hartford, CT.
Main Outcome Measures
Total bacterial and coliform counts, and presence of S. aureus in target samples.
Analysis
Bivariate tests and multiple logistic regression.
Results
Participants considering food safety as “very important” were less likely to test positive for S. aureus on hands (P < .05). S. aureus on post-handling chicken, counter/cutting board, and salad was positively associated with S. aureus on participants’ hands (P < .05). Coliform count on the counter/cutting board and sink was significantly higher at baseline when participants’ hands tested positive for coliform before starting meal preparation.
Conclusions and Implications
Meal preparer’s hands can be a vehicle of pathogen transmission during meal preparation.
Keywords: food safety attitude, hands coliform count, S. aureus, household, Latinas
INTRODUCTION
Occurrence of foodborne illness is a public and clinical health problem causing economic losses related to lower work productivity, hospitalization, and other health care expenses. In the US, known pathogens account for an estimated 38.6 million food-borne illnesses each year and 13% (or 5.2 million) of those illnesses are caused by bacteria genera including Listeria, Salmonella, and Campylobacter.1,2 In the year 2000, an estimated 1.3 million cases of salmonellosis led to $2.4 billion in medical costs and lost productivity.3 There is a consensus that sporadic foodborne illnesses, typically characteristic of home-based food infections, are seriously underreported.1,2,4
Consumer food handling and sanitation practices play a major role in foodborne illnesses. Food safety surveillance data show that after restaurants, the household or private residence is the second most common venue for foodborne illnesses.4 Studies have consistently shown that consumers have insufficient knowledge to prevent foodborne illnesses in their homes.5–8 Redmond and Griffith concluded in their review that consumers do not consider food safety at home as a major issue, leading to an “optimistic bias” and downplay of potential food safety threats in their own homes.7 According to the 2006 Food and Drug Administration (FDA)/Food Safety and Inspection Service survey results, 56% of consumers believe that foodborne illnesses are much more likely to occur at a restaurant than at home.9 This belief might reduce consumer motivation to follow safe food-handling behaviors at home.
Kohl and colleagues conducted a case-control study in Louisiana to understand the mechanisms of transmission for salmonellosis at the household level.10 These investigators found that cases were significantly more likely than controls to report “never” or “rarely” washing their hands in between preparation of meat and nonmeat items (odds ratio [OR]: 8.3, 95% confidence interval [CI]: 1.1–61.8, P < .05). In the US, Mead et al identified poor hand and surface hygiene as a significant causative factor for Escherichia coli (E. coli) O157:H7 infections from hamburgers prepared by consumers at home.11 The importance of preventing cross-contamination by washing hands and kitchen surfaces during meal preparation has been demonstrated in simulation studies conducted in homes and laboratory settings.12–14 A household study in Ireland demonstrated transfer of pathogenic genera such as Staphylococcus aureus (S. aureus) and Campylobacter from chicken to the participants’ hands and kitchen surfaces during meal preparation.13 The results of a study by Aycicek et al indicated that although S. aureus was found most commonly (70%), E. coli was found on 7% of hospital food handlers’ hands.15
Home kitchens are particularly vulnerable to unsafe food-handling practices, as they do not have the benefit of tight food safety monitoring systems similar to commercial food facilities. Consumer food safety education is key for ensuring food safety in home kitchens that often represent the final line of defense.16 A meta-analysis of food safety studies in the US demonstrated that food safety practices vary as a function of a meal preparer’s gender, age, income, and region of residence.17 For instance, men were more likely than women to report greater consumption of raw or undercooked food, poorer hygiene, practices leading to cross-contamination, and unsafe defrosting practices. The results of the meta-analysis also indicated that there is less knowledge of hygiene and greater cross-contamination in high-income individuals. Thus, to design effective and targeted consumer food safety education, behavioral differences between and within subpopulations must be better understood.
In the US, the Latino population is growing at a very fast rate, increasing from 22.4 million in 1990 to 44.5 million in 2006 and representing 15% of the total US population.18 The rapid growth of this population is likely to have major public health implications, because Latinos experience a high degree of poverty, low literacy, and a disproportionate incidence of chronic diseases.19 Hartford, CT, is the second-poorest midsized city in the US, with Latinos and especially Puerto Ricans accounting for the majority of the population. This community follows risky food safety practices at home, including thawing meat on the counter, not washing fresh produce, and inadequate hand washing.20,21 A household observation study in this community showed that only one fourth of the participants washed their hands with soap and water before and during meal preparation.21
The first objective of this study was to examine the association of microbial contamination of the meal preparer’s hands with microbial status of food and of kitchen and utensil surfaces during home preparation of a “Chicken and Salad” meal. The second objective was to find out if the level of microbial contamination on the hands of meal preparers varies by sociodemographics, food safety attitude, and acculturation (measured by proxy indicators such as language spoken at home and place of birth). This study is part of a research project conducted to identify critical control points during a “Chicken and Salad” meal preparation at the household level.20
METHODS
Sixty Puerto Rican women were recruited by distributing flyers in local schools; grocery stores; the Supplemental Program for Women, Infants, and Children (WIC) offices; and on inner-city streets of Hartford, CT. The sample size for the study was designed to detect a significant correlation in total bacterial counts at different stages of meal preparation with 80% statistical power and a tolerable α error of .05 (n = 60).
To be eligible, study participants were required to: (a) be Puerto Rican females; (b) be the primary meal preparers of the household; and (c) reside in inner-city Hartford. Upon meeting these criteria and obtaining signed consent, in consultation with the study participants, a trained bilingual (Spanish-English) outreach worker scheduled household visits for food delivery, microbial testing, and interviewing. Each participating household was visited 3 times: first visit: delivery of the food ingredients to the participant’s home for the preparation of a “Chicken and Salad” meal; second visit: household observation and food and kitchen/utensil surface sample collection during “Chicken and Salad” meal preparation; third visit: food safety interview survey using a closed-ended questionnaire. The University of Connecticut, in collaboration with the Hispanic Health Council, Inc., conducted the study. The institutional review boards of both institutions approved the study.
Pilot Study
To streamline and standardize microbiological testing, a pilot study (n = 10) was conducted prior to collecting the data for the main study (n = 60). In addition, the feasibility of collecting samples without disrupting the kitchen settings and the participants’ routine behaviors was tested during the pilot phase. During this phase, 10 simulations were conducted to rule out unintended research-driven secondary microbial contamination during the delivery of food ingredients and transportation of samples from the households to the microbiology laboratory.22
Main Study
First visit
For each participant to be able to prepare the “Chicken and Salad” meal, the research staff purchased a package of uncooked chicken breasts (chicken) with skin and bones, lettuce and tomatoes, oil, salad dressing, and common Puerto Rican spices and condiments from the local grocery store. After purchase, food ingredients were taken to the microbiology laboratory. At the laboratory, chicken and lettuce/tomato samples (approximately 25 g) were removed and tested to determine the presence of target pathogenic genus and to establish baseline total bacterial and coliform counts. After removing the sample, the chicken and lettuce/tomatoes were repacked and transferred to coolers until delivery to the participant’s home. From purchase to household delivery, a 3-hour period, the chicken and lettuce/tomatoes samples were maintained at ≤ 4°C in ice coolers, and the oil and spices were kept at room temperature. At the time of home delivery, participants were asked to freeze the chicken and defrost it using their usual method so that the research staff could observe them preparing the “Chicken and Salad” meal during the second visit. At the time of the delivery, participants were also asked to refrigerate the vegetables.
Second visit
Microbial samples were obtained and household observations were conducted during the meal preparation, scheduled 1 day after the first visit, when the food ingredients were delivered. During this visit, food, kitchen surfaces, and meal preparation utensils were sampled before and after the participant had handled the food.
Before starting any meal preparation activity including hand washing (if any), participants’ hands were sampled for microbial testing. Participants were asked to dip their hands, one at a time, for 30 to 50 seconds in 250 mL of 0.1% peptone buffer (Difco/Becton Dickinson, Franklin Lakes, NJ) in sterile stomacher bags (Difco/Becton Dickinson). Immediately upon arrival, participants’ hands were tested to estimate general personal hygiene level and predict cross-contamination risk. In addition, the kitchen counter or cutting board, refrigerator/freezer handles, and knife and sink surfaces were sampled before meal preparation. A sample of the kitchen counter was used if the participant did not have a cutting board and she planned to use the counter as a cutting surface. Similarly, the cutting surface of the knife was swabbed just before being used. Because of their small surface, the entire surfaces of the knife and refrigerator/freezer handles were swabbed for microbial estimation. Two 30 cm2 (template area 6.5 × 4.5 cm) cutting/counter and sink samples were swabbed using sterile templates and prepackaged sterile swabs (Difco/Becton Dickinson) dipped in Tryptic soy broth (TSB; Difco/Becton Dickinson) + 0.6% yeast extract (YE).
All participants handled the chicken first and then the lettuce/tomatoes. A defrosted chicken sample (approximately 25 g) was collected after the participant began handling the chicken but before cooking (ie, after cutting or removing skin and bones, and washing, if applicable). The knife and cutting surfaces (counter or cutting board) were swabbed using pre-packaged sterile swabs immediately after being used to cut or clean the chicken. As with the baseline samples, 30 cm2 counter or cutting board samples and the entire knife were swabbed for microbial testing. Lettuce/tomatoes (approximately 25 g) samples were collected after washing (if applicable), cutting, and mixing, or once the salad was ready to serve. Food samples (chicken and lettuce/tomatoes) were transferred into stomacher bags using sterilized tongs. All the household samples collected for microbial testing were transported to the laboratory at ≤ 4°C.
Microbiological testing
Participants’ hands, food, and surface area samples (counter, cutting board, sink) were tested for total bacterial and coliform counts and the presence of Campylobacter, Salmonella, Listeria, and S. aureus. The refrigerator/freezer handle and knife surface samples were tested only for the presence of pathogens, since the surface size was not obtained using a predefined surface area template. The collection and testing of food and surface samples for total bacterial and coliform counts has been previously reported in detail.20 Likewise, the procedures to test for the presence of pathogens including incubation temperature/environment, selective agar, and standardized confirmatory tests have been previously reported.20
To estimate the total bacterial and coliform counts on participant’s hands, the hand wash sample collected in 250 mL of 0.1% peptone buffer was serially diluted to prepare 101 and 102 dilutions. A volume of 100 μL of the original sample and serial dilutions were spread plated on Tryptic Soy Agar (Difco/Becton Dickinson) and Violet Red Bile Agar (Difco/Becton Dickinson) for total bacterial and coliform counts, respectively. The plates were incubated at 37°C for 24 hours. For enriching, a volume of 5 mL of the original hand wash sample was transferred to 50 mL of TSB + 0.6 YE (Salmonella, Listeria, S. aureus) and Brucella broth + 0.5% sheep’s blood (for Campylobacter testing).
Third visit
The day after the second visit, a meal preparation food safety survey was conducted with the participant. The questionnaire was available in English and Spanish, and a bilingual outreach worker conducted the interview in the participant’s preferred language. During the interview, sociodemographic and acculturation proxy information such as language spoken at home and place of birth was collected. In addition, attitude toward food safety was assessed through a question, “How important is food safety for you?” The response options were: (a) very important; (b) important; (c) somewhat important; and (d) not at all important.
STATISTICAL ANALYSES
SPSS version 14.0 (SPSS, Inc., Chicago, IL, 2006) was used to analyze the microbiological and survey data. Analysis of variance or t test and multiple logistic regression were used to estimate the differences in risk of microbial estimation on the participants’ hands by sociodemographic, acculturation proxy indicators and attitudinal indicators. Bivariate parametric tests were conducted to examine the relationship between microbial contamination on participants’ hands and microbial counts in food, kitchen surfaces, and utensil samples before and after food preparation began. The paired t test was used to compare pre- and post-handling changes in coliform count in food samples among individuals who tested positive or negative for coliform on their hands, sampled at the beginning of the second household visit. Statistical significance was set at a probability value of P ≤ .05.
RESULTS
According to study inclusion criteria, all the participants were Puerto Rican and the main meal preparer of the household, living in Hartford. The average age of the participants was 40 years. Almost half of the participants (n = 33) had less than a high school education. Similarly, almost half of the participants (n = 36) reported speaking only Spanish at home, and 56% (n = 34) reported an income less than or equal to $1,000 per month (Table 1). The majority of the participants were unemployed (n = 51). Some of the participants (30%) reported receiving benefits from the Housing Assistance Program.
Table 1.
Relationship Between Total Bacterial and Coliform Count on Meal Preparers’ Hands and Sociodemographic Variables, Language Spoken, and Attitude (n = 60)
| n | Total Count | Pa | Coliform Count | Pa | |
|---|---|---|---|---|---|
| log CFU Mean ± SD | log CFU Mean ± SD | ||||
| Age | |||||
| 18 to 30 y | 21 | 4.68 ± 1.68 | .691 | .80 ± 1.71 | .047b |
| 31 to 40 y | 18 | 5.12 ± 1.49 | 1.67 ± 2.27 | ||
| ≥41 y | 21 | 5.07 ± 1.87 | 2.42 ± 2.23 | ||
| Monthly income | |||||
| $1000 or less | 34 | 4.70 ± 1.85 | .205 | 1.73 ± 2.12 | .670 |
| $1001 or more | 26 | 5.29 ± 1.37 | 1.48 ± 2.20 | ||
| Education | |||||
| Less than high school | 33 | 4.75 ± 1.96 | .347 | 2.05 ± 2.21 | .094 |
| High school or more | 27 | 5.18 ± 1.25 | 1.11 ± 1.96 | ||
| Attitude | |||||
| Important or somewhat important | 14 | 5.50 ± .65 | .152 | 2.63 ± 2.44 | .046b |
| Very important | 46 | 4.76 ± 1.87 | 1.32 ± 1.97 | ||
| Language spoken at home | |||||
| English only or English and Spanish | 24 | 5.41 ± .68 | .086 | 1.93 ± 2.38 | .370 |
| Spanish only | 36 | 4.62 ± 2.07 | 1.42 ± 1.97 | ||
| Place of birth | |||||
| Puerto Rico | 51 | 4.88 ± 1.80 | .485 | 1.73 ± 2.15 | .371 |
| US | 9 | 5.33 ± .51 | 1.03 ± 2.06 | ||
log CFU indicates logarithmic colony forming unit; SD, standard deviation; y, years.
Analysis of variance or t test;
Significant.
Microbial analyses of the participants’ hands demonstrated that 91% tested positive for any type of bacteria, and 38% were positive for coliforms. The mean total bacterial and coliform counts for the participants’ hands were 5.0 ± 1.68 logarithmic colony forming unit (log CFU) and 1.63 ± 2.14 log CFU, respectively. None of the participants’ hands were found to be positive for Campylobacter, Listeria, or Salmonella genera. However, S. aureus was found on the hands of 42% of participants.
Coliform counts were significantly higher among the older (> 40 years) than the younger (< 40 years) individuals. With regard to food safety attitudes, no participant reported that food safety was “not at all important.” For the other 3 categories, 77% reported food safety as “very important,” 20% reported food safety as “important,” and the remaining 3% considered food safety “somewhat important.” The coliform counts of the hands of participants who reported that food safety was “important” or “somewhat important” were significantly higher (P < .05) than among participants who considered food safety to be “very important” (Table 1). Coliform counts on hands did not differ by language preference or birthplace of the participants.
Multiple logistic regressions showed that the odds of having S. aureus on the hands was 4 times higher among the income group earning $1,000 or less per month when compared to the income group earning $1,001 or more (P < .05). Those who considered food safety as “very important” were less likely to test positive for S. aureus on their hands than those who considered food safety as “important/somewhat important” (P < .05) (Table 2).
Table 2.
Predictors for the Presence of S. aureus on the Hands of the Puerto Rican Meal Preparers at the Household Level (n = 60)a
| Predictors | n | OR | 95% CI | Pb |
|---|---|---|---|---|
| Age | ||||
| 18 to 30 y | 21 | .43 | 0.091 – 2.05 | .290 |
| 31 to 40 y | 18 | .60 | 0.146 – 2.50 | .486 |
| 41 y and above | 21 | 1.00 | ||
| Income | ||||
| $1,000 or less | 34 | 4.15 | 1.03 – 16.875 | .045c |
| $1,001 or more | 26 | 1.00 | ||
| Education | ||||
| Less than high school | 33 | 0.33 | 0.082 – 1.401 | .117 |
| High school or more | 27 | 1.00 | ||
| Attitude | ||||
| Important or somewhat important | 14 | 4.95 | 1.10 – 22.30 | .037c |
| Very important | 46 | 1.00 | ||
| Language spoken at home | ||||
| English only or English and Spanish | 24 | 1.54 | 0.435 – 5.503 | .501 |
| Spanish only | 36 | 1.00 | ||
CI indicates confidence interval; OR, odds ratio; y, years.
S. aureus on hands (dependent variable): Absence (0), n = 35; Presence (1), n = 25;
Multivariate logistic regression;
Significant.
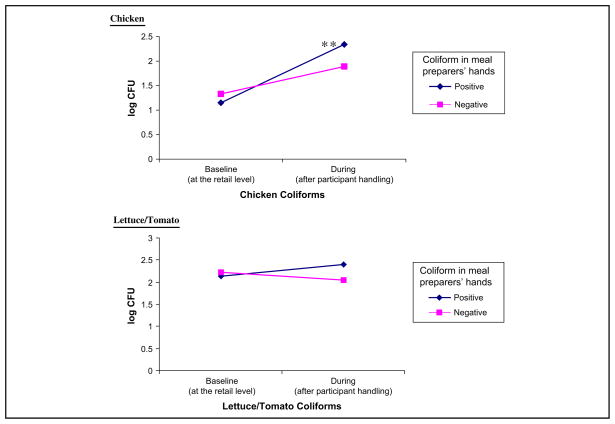
The presence of S. aureus on the participants’ hands was correlated with the presence of this pathogen on the food and kitchen surface and utensil samples collected during the “Chicken and Salad” meal preparation, that is, once the participant had handled the food (Table 3). The presence of S. aureus on chicken and lettuce/tomato samples was significantly higher when the participants’ hands also tested positive for this pathogen. Likewise, the coliform count on the sink and cutting surface before food preparation started was significantly higher when the participant’s hands tested positive for coliforms (Table 3). As shown in Figure, change in coliform counts in chicken differed by presence or absence of coliform on participants’ hands. Compared to the retail level, chicken coliforms during meal preparation increased significantly (P < .05) only among participants whose hands tested positive for coliform (Figure).
Table 3.
Association Between Microbial Contamination (S. aureus and Coliform) on the Meal Preparers’ Hands and Food and Kitchen Surfaces During the Preparation of “Chicken and Salad” Meal at the Household Level
| Presence of S. aureus on Handsa | Absence of S. aureus on Handsa | Pb | Presence of Coliform on Hands log CFU | Absence of Coliform on Hands log CFU | Pc | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | Mean ± SD | Mean ± SD | |||
| Before starting meal preparation | ||||||
| Refrigerator/freezer handles (n = 60) | 9 (36) | 11 (31) | .711 | na | na | |
| Knife (before used to cut chicken) (n = 60) | 8 (33) | 7 (21) | .305 | na | na | |
| Counter or cutting boardsd (n = 60) (cutting board: 45; counter: 15) | 8 (40) | 7 (26) | .241 | 1.22 ± 1.27 | .54 ± 1.07 | .053 |
| Sink (n = 60) | 13 (52) | 10 (29) | .066 | 1.77 ± 1.30 | .85 ± 1.19 | .007h |
| During/after meal preparation | ||||||
| Counter/cutting board (n = 42)e | 11 (55) | 0 | .007h | 1.40 ± 1.06 | .68 ± .96 | .021h |
| Chicken (n = 60)f | 15 (60) | 7 (20) | .002 | 2.34 ± 1.55 | 1.89 ± 1.46 | .255 |
| Lettuce/tomato (n = 60)g | 10 (40) | 4 (11) | .010 | 2.82 ± 1.23 | 2.25 ± 1.35 | .109 |
log CFU indicates logarithmic colony forming unit; na, refrigerator/freezer handles and knives were not sampled for coliform count; SD, standard deviation.
Column represents surface or food sample positive for S. aureus by S. aureus status of participants’ hands. For instance, among participants whose hands tested positive for S. aureus, 36% refrigerator/freezer handles tested positive for S. aureus. The corresponding value among those whose hands tested negative for S. aureus was 31%. Overall, 25 (42%) of the study participants tested positive for S. aureus on their hands;
Chi-square;
t test;
Counter or cutting board sample before cutting chicken; the sample collected was analyzed if the participant used the counter/cutting board for cutting the chicken;
Counter or cutting board sample after cutting chicken on it; the sample collected was analyzed only if the same surface was used to cut lettuce/tomatoes;
Chicken sample collected after: freezing, thawing, washing (if done), removing skin and bones (if done), and cutting;
Lettuce/tomato sample collected after refrigeration, washing (if done), and cutting, or serving as a final dish;
Significant.
Figure.
Changes in coliform counts in chicken and lettuce/tomato by baseline coliform status on participants’ hands (n = 60). Log CFU indicates logarithmic colony forming unit; positive or negative, meal preparers’ hands tested positive/negative for coliform before starting “Chicken and Salad” meal preparation.
Paired t-test, **P < .05.
DISCUSSION
To the authors’ knowledge, this is the first household food safety study in the US designed to examine factors associated with microbial contamination on the hands of low-income Latina meal preparers. In this study, the authors did not detect pathogenic genera such as Listeria, Salmonella, and Campylobacter on the participants’ hands. However, S. aureus was commonly isolated from the hands of meal preparers. Consistent with these findings, Gorman et al in Ireland found that of the 4 pathogens that they tested (Campylobacter, Salmonella, E. coli, and S. aureus), the highest cross-contamination was observed for S. aureus (19.7%).13 S. aureus is highly susceptible to heat and sanitizing agents, thus its presence on kitchen, hands, or other surfaces is generally an indication of poor sanitation.23 Some S. aureus strains are capable of producing a highly heat-stable protein toxin that causes illness in humans. The authors tested for S. aureus genus and not specifically for the toxin-producing strain. The authors hypothesize, however, that the high prevalence of S. aureus on participants’ hands indicates poor personal hygiene and a relatively higher food safety risk in this community.
Compared to specific fecal coliform tests, the generic coliform count is relatively easy and inexpensive to measure; thus, it is widely used in field or natural environment microbiological studies as an indicator of fecal contamination.13,24,25 Coliform counts as high as 5.33 logCFU were recovered from the participants’ hand samples. In developing countries, poor personal hygiene, especially unwashed hands before cooking and feeding children, has been identified as an important risk factor for diarrhea in children.24,25 In a study in India by Sheth et al,24 mean coliform count on meal preparers’ hands was 102, a log higher than the average coliform count found on the participants’ hands in the present study. This difference is expected, given the major differences in poverty levels and access to sanitation in both countries. Similar to these study findings for coliform, a study conducted in Turkey among hospital food handlers found that the E. coli count ranged from 101 to 105, indicating poor or improper hand hygiene.15
The US FDA has recommended hand washing as one of the major foodborne illness prevention strategies. This recommendation is well justified, as several studies have determined the efficacy of hand washing in preventing cross-contamination and transmission of pathogens from hands to food and other objects during meal preparation.12–14 Previously published household observation results of this project showed that only 25% of the participants washed their hands with soap and water before starting the preparation of the “Chicken and Salad” meal.21 The presence of coliforms and S. aureus on the participants’ hands along with limited hand washing before starting meal preparation perhaps explain in part the high likelihood of cross-contamination identified by this group in this community. Indeed, through this study the authors have demonstrated that the presence of S. aureus was significantly more likely on post-handling samples of chicken, kitchen surfaces, and the salad when the participants’ hands were also contaminated with the pathogen. In agreement with these findings, Scott and Bloomfield found that S. aureus could cause cross-contamination for up to 24 hours via the fingertips.26
Among this group of low-income participants, those living in households earning less than $1,000 per month were more likely than their counterparts earning ≥ $1,000 to test positive for S. aureus on their hands. The inability to afford sanitation materials owing to very low household income may partly explain the disparity by income level. In line with the authors’ income differential findings, Roseman et al found in a telephone survey that the respondents with an annual household income of $15,000 or less were less likely to use a meat thermometer compared to participants living in higher-income households.27 The present findings call for delivering kitchen surface and hand-washing education to the target community and to facilitate access to the tools they need. It is essential that meal preparers follow the appropriate kitchen sanitation and hand-washing techniques including thorough rinsing. In a study by Cogan et al in the United Kingdom, domestic kitchens were examined for pathogen transfer following the preparation of a chicken dish using different hygiene regimes. The researchers found that bowl washing paired with thorough rinsing in running water was more effective than just bowl washing at reducing Salmonella and Campylobacter loads.12 Consistent with this finding, through a simulation study, Barker et al demonstrated the spread of Salmonella from food to other surfaces, such as telephone and tap handles, via the hands. When the hands were thoroughly washed for 2 minutes with soap and water followed by rinsing, the recovery of bacteria on hands decreased by 90%, indicating the importance of time and mechanical action for effective hand washing.28
Results of this study revealed that attitude toward food safety plays a significant role in predicting microbial presence and levels on the hands of Latina meal preparers. After controlling for age, education, and language spoken at home, participants who considered food safety as “very important” were less likely to test positive for S. aureus on their hands. It has been proposed that attitudes toward food safety affect behavior, which in turn is influenced by beliefs and risk associated with it.7 A consumer survey in Kentucky by Roseman et al revealed a significant positive relationship between self-reported food safety perception and behaviors.27 For instance, those who believed that food-borne illness was not common were significantly less likely to wash their hands with soap and water after handling meat. In a national telephone survey, respondents who correctly identified a food vehicle for Salmonella spp. were more likely than their counterparts to report washing their hands and cutting boards after handling raw meat.5 In the 2002 Home Food Safety … in Your Hands survey, respondents did not generally think that it is extremely common or very common to become sick because of the way food is prepared in their homes (70%), and 60% did not associate symptoms such as fever, chills, and nausea to food prepared at home.29 Furthermore, most (85%) consumers gave themselves an “A” or “B” food safety behavior rating (on an A-to-F scale). This finding implies that consumers believe that their current home food safety behaviors are sufficient to protect them from foodborne illnesses. Trepka et al conducted focus groups in Miami, Florida with WIC participants to understand the barriers and beliefs affecting safe food-handling practices. In that study, WIC participants did not perceive foodborne illness as a major concern and were largely unaware of the importance of safe food-handling practices in the home.8
Previous studies have shown that acculturation influences dietary intake and other lifestyle behaviors.30 This finding may be explained at least in part by differences in lifestyles between the country or territory of origin and the US mainland. The lack of association between acculturation proxies and food safety outcomes in the present study suggests that food safety behaviors may not be different in Puerto Rico and the US.
LIMITATIONS
The present study used a single question to assess food safety attitude that could not be tested for its reliability, although its association with hard microbiological outcomes suggests that the measure is of value. Considering that food safety attitude is a complex construct, future research is needed to better understand how to measure it in diverse populations. As a start, it is important to test the validity and psychometric behavior of previously developed scales in Latino populations,31 and if necessary to continue developing and testing the validity and reliability of food safety attitude scales that are culturally appropriate.
During the interview, participants were not asked about their understanding of the term “food safety.” Hence, difference in this understanding was not controlled for in the food safety attitude analysis. The fact that this project involved direct household observation and the collection of samples for microbial analysis during meal preparation may have led participants to practice better food safety behaviors than usual. However, the authors believe that this potential bias was greatly attenuated in this study given the extensive exploratory work that preceded it and the highly trained, culturally skilled staff that supported the research. Regarding the external validity of the study, it is important to acknowledge that Latinas represent a very diverse group and that the results from 1 subgroup (Puerto Ricans) do not necessarily apply to others such as Mexicans and Central and South American Latino groups.
IMPLICATIONS FOR RESEARCH AND PRACTICE
Inadequate cooking and storage of food is considered to be the main cause of foodborne infection, but in addition, poor hand hygiene and cross-contamination from it seem to be very common at the household level. Transfer of microorganisms from hands to surfaces such as refrigerator/freezer handles poses a continuous risk of food contamination or spreading of microorganisms from one meal to the next. Results of this study underscore the importance of hand hygiene and kitchen sanitation education targeting low-income Latina household meal preparers.
Considering the low food safety risk perception among consumers, efforts are needed to draw consumer attention to the frequency and severity of foodborne illnesses associated with food handling and preparation at home. Moreover, considering the extent to which attitudes toward food safety affect food-handling behaviors, there is a need to educate consumers on the risks associated with improper food handling. In this study, language spoken at home and place of birth were used as proxy indicators for acculturation. Future studies should use valid, multidimensional acculturation scales to better understand a possible relationship between acculturation and food safety risks at home. Culturally appropriate, goal-oriented, and behavior-change–based food safety curriculums providing information on pathogens, the vehicles of transmission, and food safety practices to prevent transmission (including hand washing) could be very effective at addressing food safety risk behaviors among Latinos and other low-income communities in the US.
Supplementary Material
Acknowledgments
This study was funded through a grant awarded to Dr. Pérez-Escamilla by the US Department of Agriculture Integrated Research, Education, and Extension Competitive-National Integrated Food Safety Initiative Program.
Dr. Rafael Pérez-Escamilla received support for the development of this manuscript from the NIH National Center on Minority Health and Health Disparities (grant # P20MD001765). This study was a collaboration between the Department of Nutritional Sciences, Animal Science at the University of Connecticut and the Hispanic Health Council, Inc., in Hartford, CT. This study was funded through a grant awarded to Dr. Rafael Pérez-Escamilla by the USDA-Integrated Research, Education, and Extension Competitive-National Integrated Food Safety Initiative Program. The authors are grateful to the community outreach workers involved with this study. The authors are also thankful to Sofia Segura-Pérez from the Hispanic health Council, Inc., for assisting in outreach worker training and recruitment for the study.
References
- 1.Mead PS, Slustsker L, Dietz V, et al. Food related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruhn CM, Schlutz HG. Consumer food safety knowledge and practices. J Food Safety. 1999;1:73–87. [Google Scholar]
- 3.Buzby JC, Roberts T, Lin CT, MacDonald JM. Bacterial foodborne disease: medical costs and productivity losses. Economic Research Service, US Department of Agriculture; [Accessed September 14, 2009.]. Agriculture Economic Report No. 741. Available at: http://www.ers.usda.gov/publications/aer741/AER741fm.PDF. Published August 1996. [Google Scholar]
- 4.Lynch M, Painter J, Woodruff R, Braden C. Surveillance for foodborne disease outbreaks—United States, 1998–2002. MMWR. 2006;55:1–34. [PubMed] [Google Scholar]
- 5.Aletkruse SF, Street DA, Fein SB, Levy S. Consumer knowledge of food-borne microbial hazards and food-handling practices. J Food Prot. 1996;59:287–294. doi: 10.4315/0362-028x-59.3.287. [DOI] [PubMed] [Google Scholar]
- 6.Bermúdez-Millán A, Pérez-Escamilla R, Damio G, González A, Segura-Pérez S. Food safety knowledge, attitudes, and behaviors among Puerto Rican caretakers living in Hartford. Connecticut. J Food Prot. 2004;67:512–516. doi: 10.4315/0362-028x-67.3.512. [DOI] [PubMed] [Google Scholar]
- 7.Redmond EC, Griffith CJ. Consumer food handling in the home: a review of food safety studies. J Food Prot. 2005;66:130–161. doi: 10.4315/0362-028x-66.1.130. [DOI] [PubMed] [Google Scholar]
- 8.Trepka MJ, Violet M, Syreeta C, Fatma GH, Zisca D. Food safety beliefs and barriers to safe food handling among WIC program clients, Miami, Florida. J Nutr Edu Behav. 2006;38:371–377. doi: 10.1016/j.jneb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Lando A, Verill L Centerfor Food Safety and Applied Nutrition. FDA/FSIS Food Safety Survey. Washington DC: 2006. [Accessed September 14, 2009.]. Available at: http://www.fda.gov/Food/ScienceResearch/ResearchAreas/ConsumerResearch/ucm080374.htm. [Google Scholar]
- 10.Kohl KS, Rietberg K, Wison S, Farley TA. Relationship between home food-handling practices and sporadic salmonellosis in adults in Louisiana, United States. Epidemiol Infect. 2002;129:267–276. doi: 10.1017/s0950268802007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead PA, Finelli L, Lambert-Flair MA, et al. Risk factors for sporadic infection with Escherichia coli 0157: H7. Arch Intern Med. 1997;157:204–208. [PubMed] [Google Scholar]
- 12.Cogan TA, Slader J, Bloomfield SF, Humphrey TJ. Achieving hygiene in the domestic kitchen: the effectiveness of commonly used cleaning procedures. J Appl Microbiol. 2002;92:885–892. doi: 10.1046/j.1365-2672.2002.01598.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorman R, Bloomfield S, Adley CC. A study of cross-contamination of food-borne pathogens in the domestic kitchen in the Republic of Ireland. Int J Food Microbiol. 2005;76:143–150. doi: 10.1016/s0168-1605(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhao P, Zhao T, Doyle MP, Rubino JR, Meng J. Development of a model for evaluation of microbial cross-contamination in the kitchen. J Food Prot. 1998;61:960–963. doi: 10.4315/0362-028x-61.8.960. [DOI] [PubMed] [Google Scholar]
- 15.Aycicek H, Aydogan H, Kucukkaraaslan A, Baysallar M, Celal Basustaoglu A. Assessment of the bacterial contamination on hands of hospital food handlers. Food Control. 2004;15:253–259. [Google Scholar]
- 16.Griffith C, Worsfold D, Mitchell R. Food preparation, risk communication and the consumer. Food Control. 1998;9:225–232. [Google Scholar]
- 17.Patil SM, Cates S, Morales R. Consumer food safety knowledge, practices, and demographic differences: findings from a meta-analysis. J Food Prot. 2005;68:1884–1894. doi: 10.4315/0362-028x-68.9.1884. [DOI] [PubMed] [Google Scholar]
- 18.Facts for Features: Hispanic Heritage Month. US Census Bureau; 2007. [Accessed September 14, 2009.]. Available at: http://www.census.gov/Press-Release/www/2007/cb07ff-14.pdf. [Google Scholar]
- 19.Aguirre-Molina M, Molina CW, Zambrana RE. Health Issues in the Latino Community. San Francisco, CA: Jossey-Bass; 2001. [Google Scholar]
- 20.Dharod JM, Pérez-Escamilla R, Paciello S, Venkitanarayanan K, Bermúdez-Millán A, Damio G. Critical control points for home prepared ‘Chicken and Salad’ in Puerto Rican households. Food Protection Trends. 2007;27:544–552. [Google Scholar]
- 21.Dharod JM, Pérez-Escamilla R, Paciello S, Bermúdez-Millán A, Venkitanarayanan K, Damio G. Comparison between self-reported and observed food handling behaviors among Latinas. J Food Prot. 2007;70:1927–1932. doi: 10.4315/0362-028x-70.8.1927. [DOI] [PubMed] [Google Scholar]
- 22.Paciello S. Masters Thesis. University of Connecticut; 2003. Validation of a “Chicken and Salad” household HACCP-like analysis protocol in a Puerto Rican community. [Google Scholar]
- 23.Bacteria associated with food borne diseases. Report. [Accessed September 14, 2009.];Institute of Food Technologists: Scientific Status Summary. 2004 August; Available at: http://members.ift.org/NR/rdonlyres/3DEA7A91-DF48-42CE-B195-06B01C14E273/0/bacteria.pdf. Published August 2004.
- 24.Sheth M, Patel J, Sharma S, Seshadri S. Hazard analysis and critical control points of weaning foods. Indian J Pediatr. 2000;67:405–410. doi: 10.1007/BF02859455. [DOI] [PubMed] [Google Scholar]
- 25.Bryan FL, Teufel P, Roohi S, Qadar F, Riaz S, Malik ZR. Hazards and critical control points of food preparation and storage in homes in a village and a town in Pakistan. J Food Prot. 1992;55:714–721. doi: 10.4315/0362-028X-55.9.714. [DOI] [PubMed] [Google Scholar]
- 26.Scott E, Bloomfield SF. The survival and transfer of microbial contamination via cloths, hands, and utensils. J Appl Bacteriol. 1990;68:271–278. doi: 10.1111/j.1365-2672.1990.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 27.Roseman M, Kurzynske J. Food safety perceptions and behaviors of Kentucky consumers. J Food Prot. 2006;69:1412–1421. doi: 10.4315/0362-028x-69.6.1412. [DOI] [PubMed] [Google Scholar]
- 28.Barker J, Naeeni M, Bloomfield SF. The effects of cleaning and disinfection in reducing Salmonella contamination in a laboratory model kitchen. J Appl Microbiol. 2003;95:1351–1360. doi: 10.1046/j.1365-2672.2003.02127.x. [DOI] [PubMed] [Google Scholar]
- 29.Cody MM, Hogue MA. Result of the Home Food Safety—It’s in Your Hands 2002 survey: comparison to the 1999 benchmark survey and Healthy People 2010 food safety behaviors objective. J Am Diet Assoc. 2003;103:1115–1125. doi: 10.1016/s0002-8223(03)01064-2. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Escamilla R, Putnik P. The role of acculturation in nutrition, lifestyle, and incidence of type 2 diabetes among Latinos. J Nur. 2007;137:860–870. doi: 10.1093/jn/137.4.860. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros LC, Hillers VN, Chen G, Bergmann V, Kendall P, Schroeder M. Design and development of food safety knowledge and attitude scales for consumer food safety education. J Am Diet Assoc. 2004;104:1671–1677. doi: 10.1016/j.jada.2004.08.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.