Abstract
Background/objective
Vitamin D deficiency is prevalent in chronic spinal cord injury (SCI). A 3-month course of oral vitamin D3 to ‘normalize’ serum vitamin D levels was investigated.
Design
Prospective drug-intervention study.
Setting
VA Medical Center; private rehabilitation facility.
Methods
Seven individuals with chronic SCI and vitamin D deficiency completed 3 months of oral vitamin D3 (i.e. cholecalciferol) supplementation. At screening, baseline, and months 1 and 3, blood was collected for serum calcium, 25 hydroxyvitamin D [25(OH)D], intact parathyroid hormone (iPTH), and N-telopeptide (NTx); 24-hour urine for calcium, creatinine, and NTx was performed. Oral vitamin D3 (2000 IU daily) and elemental calcium (1.3 g daily) were prescribed for 90 days. The results are expressed as mean ± standard deviation (SD). Analysis of variance with a Fisher's post-hoc analysis was performed to test for differences between study visits. Subjects were classified as deficient (<20 ng/ml), relatively deficient (20–30 ng/ml), or not deficient (>30 ng/ml) in 25(OH)D.
Results
Serum 25(OH)D levels were greater at months 1 and 3 than at baseline (26 ± 6 and 48 ± 17 vs. 14 ± 2 ng/ml; P = 0.005). Six of seven subjects were no longer deficient [25(OH)D >30 ng/ml] by month 3. Serum iPTH levels were significantly decreased at month 1 and month 3; serum NTx levels were significantly lower at month 3 than at baseline. Serum and urinary calcium levels remained within the normal range.
Conclusion
A daily prescription of 2000 IU of oral vitamin D3 for 3 months safely raised serum 25(OH)D levels into the normal range in persons with chronic SCI on calcium supplementation.
Keywords: Vitamin D deficiency, Nutritional supplementation, Calcium, Vitamin D, Osteoporosis, Spinal cord injuries, Bone loss, Cholecalciferol, Paraplegia, Tetraplegia, Veterans
Introduction
Our earlier work suggested that approximately 32% of veterans with spinal cord injury (SCI) were absolutely deficient in vitamin D (25 hydroxyvitamin D [25(OH)D]), whereas only 16% of able-bodied individuals were found to be deficient.1 In our initial report, the primary consideration was the effect of vitamin D deficiency on calcium metabolism and its potential to further accelerate sublesional bone loss.1 Other investigators have confirmed and extended our original findings of a higher prevalence of vitamin D deficiency in persons with chronic SCI.2–5
This study was performed to determine whether oral supplementation with 2000 IU vitamin D3 and supplemental calcium for 3 months in persons with SCI who were vitamin D deficient is safe and a sufficient dosage to raise 25(OH)D levels to >30 ng/ml to improve calcium metabolism by suppressing parathyroid hormone and bone turnover. Hence, such an approach would be anticipated to reduce bone loss over the long term. However, the importance of this work may not relate solely to the effect of adequate levels of vitamin D on calcium absorption and progression of osteoporosis, but also on the overall health benefits in persons with SCI who are predisposed to numerous other health issues that may be ameliorated by sufficient vitamin D supplementation.
Methods
Subjects
Seven subjects with chronic SCI were enrolled for study participation. The mean age of the subjects was 35 ± 7 years (range: 25–46 years). Six subjects were male and one female. There were four subjects with paraplegia and three with tetraplegia; four subjects had motor complete lesions [American Spinal Injury Association (ASIA) Impairment Scale AIS A or B], and three motor incomplete lesions (AIS C). The mean duration of injury was 15 ± 7 years; mean height was 175 ± 7 cm and weight was 84 ± 17 kg. Individuals were excluded from participation in the study if they were pregnant, or had a history of bone disorders, moderate or severe constipation, or chronic kidney stones. Participants signed informed consent prior to being screened for vitamin D deficiency and again prior to beginning the vitamin D replacement study, in accordance with the policies of the Institutional Review Boards of the James J. Peters VA Medical Center, Bronx, NY and Kessler Foundation Research Center, West Orange, NJ. For purposes of this investigation, subjects who had 25(OH)D levels <20 ng/ml (50 nmol/l) were categorized as vitamin D deficient and were invited to participate in the study (to convert ng/ml to nmol/l, multiply by 2.5).
Laboratory assessments
Subjects had blood and urine collected (e.g. 24-hour) at screening, baseline, month 1, and month 3. Serum calcium, phosphate, albumin, alkaline phosphatase, and creatinine were measured in the general chemistry laboratory of the Veterans Affairs Medical Center by sequential multiple analyzer (SMAC, Technicon Instruments, Tarrytown, NY, USA). The ionized calcium was analyzed by Laboratory Corporation of America (LabCorp, Raritan, NJ, USA). Blood samples for measurement of serum intact parathyroid hormone (iPTH), [25(OH)D], and 1, 25 dihydroxyvitamin D3 [1,25(OH)2D] were collected, promptly separated by centrifugation at 4°C, and kept at −70°C until analyzed. The serum for iPTH (radioimmunoassay kit, Diagnostic Systems Laboratories, Inc., Webster, TX, USA), 25(OH)D (competitive protein binding assay), and 1,25(OH)2D (radioreceptor assay, Quest Diagnostics, Teterboro, NJ, USA) were assayed. Urine and serum N-telopeptide (NTx) were measured by radioimmunoassay (Osteomark, Ostex International, Inc., Seattle, WA, USA). The sensitivity of the PTH assay was 10 pg/ml; the within-assay coefficients of variation were 3.1, 5.2, and 2.6% at 13, 23, and 472 pg/ml, respectively. The sensitivity of the 25(OH)D assay was 4 ng/nl; the within-assay coefficients of variation are not available from Quest Diagnostics because of facility-specific company disclosure rules (Teterboro, NJ, USA). The sensitivity of the 1,25(OH)2D assay was 8 pg/ml; the within-assay coefficient of variation was 7% for samples distributed throughout the calibration range (i.e. 30–40, 70–80, and 140–160 pg/ml, respectively). The sensitivity of the serum NTx assay was 5 nm bone collagen equivalent (BCE); the within-assay coefficient of variation was 4.6% for samples distributed throughout the assay range (i.e. 5.4–24.2 nM BCE). The sensitivity of the urine NTx assay is 30 nm BCE; the within-assay coefficients of variation for urine were 10 and 7% at 439 and 1537 nm BCE. The 24-hour urinary determinations were collected into a container containing boric acid and measured for urinary calcium, creatinine, and NTx by methods described above. The baseline serum 25(OH)D value was computed as the average of the screening and day zero blood collections. The baseline values for all other determinations were from a single blood collection on day zero.
Vitamin D and calcium supplementation
Subjects were orally administered 2000 IU vitamin D3 (i.e. cholecalciferol) daily and 3.25 g calcium carbonate daily, which provided 1.3 g of elemental calcium. Subjects were prescribed vitamin D and calcium supplements for a total of 3 months (90 days) after the baseline visit.
Statistical analysis
The results are expressed as mean plus or minus standard deviation (SD). Analysis of variance (ANOVA) with a Fisher's post-hoc analysis was performed to test for differences between study visits. At baseline, month 1, and month 3, subjects were classified as being vitamin D deficient (<20 ng/ml), relative deficient (20–30 ng/ml), or not deficient (>30 ng/ml).
Results
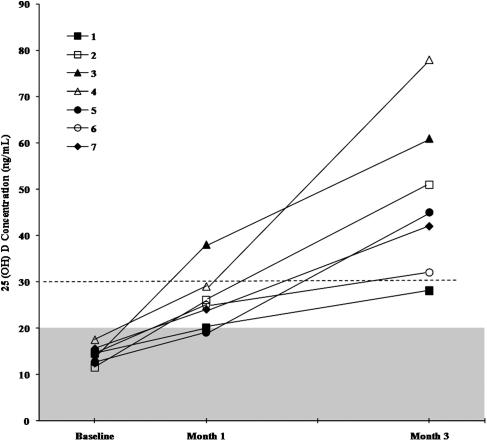
At baseline, by study inclusion criteria, all subjects were vitamin D deficient. Mean serum levels of 25(OH)D were significantly greater at months 1 and 3 compared to values at baseline, and were significantly greater at month 3 than at month 1 (Table 1 and Fig. 1). The mean serum levels of 25(OH)D values for the total group were 14 ng/ml at baseline, 26 ng/ml at month 1, and 48 ng/ml at month 3 (Table 1). At month 1, one subject remained vitamin D deficient, five subjects were relatively deficient, and one subject was no longer deficient (>30 ng/ml); at month 3, six of seven subjects were no longer deficient. The only subject who failed to normalize the 25(OH)D level at month 3 had a baseline 25(OH)D value of 17 ng/dl that rose to 20 ng/dl at month 1, and further increased to 28 ng/ml at month 3 (Fig. 1). Mean serum iPTH levels were significantly decreased at months 1 and 3 compared to the value at baseline. The mean serum NTx levels were significantly lower at month 3 than at baseline. Average values for serum albumin, creatinine, alkaline phosphatase, calcium (total and ionized), phosphate, and 1,25(OH)2D, and for urinary calcium and NTx, were not significantly different at baseline from values at month 1 or month 3.
Table 1.
Serum and urine values of vitamin D and bone markers
| Baseline | Month 1 | Month 3 | |
|---|---|---|---|
| Serum | |||
| 25(OH)D (ng/ml)† | 14 ± 2 | 26 ± 6 | 48 ± 17 |
| 1,25(OH)2D (pg/ml) | 38 ± 7 | 38 ± 17 | 45 ± 12 |
| Total calcium (mg/dl) | 8.6 ± 1.4 | 9.3 ± 0.4 | 9.2 ± 0.5 |
| Ionized calcium (mg/dl) | 5.5 ± 0.3 | 5.5 ± 0.3 | 5.5 ± 0.2 |
| NTx (nm BCE)§ | 11.4 ± 4.1 | 8.7 ± 4.3 | 8.5 ± 3.6 |
| Creatinine (mg/dl) | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.7 ± 0.3 |
| Phosphate (mg/dl) | 3.6 ± 0.5 | 3.8 ± 0.4 | 3.5 ± 0.4 |
| Alkaline phosphatase (U/l) | 70.7 ± 14.1 | 61.7 ± 33.0 | 60.6 ± 22.5 |
| Albumin (g/dl) | 4.3 ± 0.4 | 4.4 ± 0.4 | 4.3 ± 0.4 |
| iPTH (pg/ml)¥ | 79.3 ± 12.6 | 44.9 ± 13.9 | 39.8 ± 13.1 |
| Urine | |||
| Calcium (mg/24 hour) | 202.1 ± 233.3 | 189.3 ± 126.2 | 155.8 ± 38.0 |
| Creatinine (mg/24 hour) | 1301.8 ± 713.1 | 1519.3 ± 1313.3 | 1222.3 ± 255.5 |
| NTx (nm BCE) | 86.8 ± 85.2 | 67.9 ± 86.9 | 59.7 ± 77.2 |
†P < 0.05 between baseline, month 1, and month 3 study visits; ¥P < 0.05 between baseline and month 1 and baseline and month 3; §P < 0.05 between baseline and month 3.
NTx = N-telopeptide; iPTH = intact parathyroid hormone; BCE = bone collagen equivalent.
Figure 1.
Serum vitamin D [25(OH)D] levels after supplementation with 2000 IU/day. Values are displayed for seven subjects after month 1 and month 3 of replacement vitamin administration. Note that six of seven subjects had 25(OH)D levels >30 ng/ml by month 3; the only subject who did not normalize the 25(OH)D level by month 3 approached the lower limit of normal (i.e. 28 ng/ml) from a baseline level of 17 ng/ml.
Discussion
The Institute of Medicine's (IOM) current recommended daily allowance for vitamin D is 600 IU daily for non-pregnant/lactating adults aged ≤70 years and 800 IU daily for those > 70 years,6 levels of supplementation that are probably not sufficient for people with chronic SCI.7 For reasons of safety, the upper limit of daily intake was not to exceed 4000 IU, as recommended by the IOM. Vitamin D deficiency was originally defined from studies in patients with osteomalacia; thus, as historically defined, not surprisingly the prior accepted values for vitamin D deficiency were extremely low by current standards. The concept of a relative deficiency of vitamin D arose initially from the work of Heaney et al. and Dawson-Hughes et al. in studies of calcium metabolism; these investigators have suggested that gut absorption of calcium reaches a plateau at 25(OH)D levels >32 ng/ml.8,9 Thus, serum 25(OH)D levels between 30 and 40 ng/dl, the range proposed because of variation in assay reproducibility and standardization, were suggested by these investigators as a preferable range to achieve for optimum bone metabolism.8,9 In 2007, the Thirteenth Workshop Consensus for Vitamin D Nutritional Guidelines, and in 2011 the IOM both conservatively recommended an acceptable serum 25(OH)D value to be ≥20 ng/ml.6,10 This value was chosen for the general population because of the realization that there was lack of sufficient evidence by prospective, controlled clinical trials to support the benefits of a 25(OH)D level of >30 ng/ml.6 There was also concern since a subpopulation of individuals would be at increased risk of renal lithiasis if serum 25(OH)D levels were raised >30 ng/ml, as well as findings from meta-analysis suggested increased cardiovascular mortality with supplemental calcium and vitamin D intake.6,11,12
Because of multiple etiologies, vitamin D deficiency has been reported to be extremely prevalent in the SCI population, as well as in those with other disabilities.1–5 Persons with SCI often have a lower calcium intake than the general population, including vitamin D-fortified milk, which is the major dietary source of vitamin D, excluding supplements.13 Those with disability tend to have a much reduced sunlight exposure, and conversion of vitamin D precursors to the active form requires ultraviolet exposure. Several of the drugs prescribed in persons with SCI, especially anti-convulsants and psychotropic agents, may accelerate hydroxylation of vitamin D and increase its renal clearance.14 African Americans may have decreased benefit of sunlight exposure due to increased skin pigmentation, as well as being more frequently lactose intolerant, leading to a self-imposed reduction in the intake of dairy products, including milk.15
Bauman et al. demonstrated that approximately one-third of a veteran SCI population had an absolute deficiency of vitamin D.1 Oleson and Wuermser recently reported that in subjects with chronic SCI, 81% had 25(OH)D levels <32 ng/ml in the summer, and the percentage deficient in the winter increased to 96%; 54% of these subjects had 25(OH)D levels <13 ng/dl during the winter months.2 In a retrospective inpatient study of 100 SCI patients who were consecutively admitted to an acute inpatient rehabilitation facility, the prevalence of 25(OH)D relative or absolute deficiency was 93%; the mean 25(OH)D value was 16.3 ± 7.7 ng/ml, and 21% of the admissions were reported as severely deficient.3 In a retrospective outpatient study of 136 patients, 67% were reported as partially or absolutely deficient in 25(OH)D.4 Smith et al. studied persons with disability (i.e. SCI, traumatic brain injury, or lower-limb amputation) and noted that 63% of their study cohort had vitamin D deficiency and 15% had vitamin D insufficiency (e.g. relative deficiency) by their definition.5
Persons with SCI have marked sublesional loss of bone mass and disruption of the normal trabecular architecture with associated reduction in the bone strength to withstand stress, predisposing them to fractures.16–18 Although the initial loss of bone during the acute and subacute phases of SCI is dramatic and undoubtedly responsible for the osteoporosis evident in those with chronic injury, there is evidence of continued bone loss progressing decades after injury that would be expected to increase the risk of fracture.19 As a result of this observation, those caring for persons with SCI should make an effort to reduce all modifiable secondary causes of bone loss.20 Vitamin D deficiency and less than optimal calcium intake and gastrointestinal absorption will lead to secondary hyperparathyroidism that would be anticipated to be associated with increased bone turnover and progressive bone loss. As such, safely maximizing calcium absorption with the goal of reducing bone turnover should be a therapeutic goal. Indeed, serum levels of NTx were significantly reduced by month 3 of supplementation. Bone densitometry measurements were not performed in our study because we would not have been expected to detect any improvement after such a relatively short course of vitamin D replacement therapy.
From the previous discussion and considerations, it is apparent that individuals with SCI should have vitamin D levels monitored, and if found to be deficient, supplemented appropriately. However, the question remains as to the optimum threshold level of vitamin D and the appropriate replacement dose to attain it in a consistent, predictable manner in those with SCI. The IOM recommended daily intake of 600/IU day for adults ≤70 years is likely inadequate for the SCI population, judging from our previous work in which healthy persons with chronic SCI with vitamin D deficiency had remained absolutely (about one-third) or relatively (about two-thirds) deficient while being supplemented with vitamin D 800 IU daily for 12 months.7
The classic concept of vitamin D in the regulation of calcium absorption has been expanded to include its function as a compound that facilitates gene expression through an autocrine mechanism of action.21 Thus, it has been suggested that vitamin D plays an important role in several organ systems, in addition to that related to the PTH–vitamin D axis, including those of the endocrine, cardiovascular, pulmonary, and immune systems, and 25(OH)D levels >30 ng/dl has been demonstrated in various observational studies to reduce the risk of a wide and ever-increasing list of chronic ailments.22–37 Many adverse conditions and diseases have been shown to be more prevalent in persons with SCI, including disorders of carbohydrate metabolism (insulin resistance, impaired glucose tolerance, and diabetes mellitus), cardiovascular disease, and pulmonary disease (impaired pulmonary function, asthma, and infection).38–42 Although the current consensus of opinion by advisory panels in the able-bodied population is that 25(OH)D levels >20 ng/ml are probably consistent with general health, strong observational evidence suggests that levels >30 ng/ml may have medical benefit, and all-cause mortality has been observed to be decreased with levels >30 ng/ml,25,26 albeit markedly higher levels may be associated with increased mortality.12
A 10-year observational population study has shown a significantly reduced risk of ischemic heart disease events in persons with known cardiovascular disease if 25(OH)D levels were >30 ng/ml compared to the subgroups with levels of <15 and/or 15 to <30 ng/ml.31 Thus, it may be speculated that higher levels of vitamin D may also reduce vascular morbidity and mortality in higher-risk populations. A recent observational study in a large able-bodied population found a significant association between vitamin D deficiency and decreased pulmonary function, increased respiratory tract infection, and asthma.34 Because persons with higher cord lesions have both a restrictive and obstructive airway disease,41,42 it is not inconceivable that a somewhat higher serum level of vitamin D may serve to improve pulmonary function and reduce infection in these individuals. Thus, select populations, possibly those with SCI because they are predisposed to specific health issues, may be hypothesized to benefit from vitamin D levels that are somewhat higher than those recently recommended for the general population.
To our knowledge, there has not been a successful oral regimen reported for vitamin D replacement therapy in persons with disability. In our study, vitamin D3 was administered at a dose of 2000 IU daily for 3 months in conjunction with daily oral calcium supplementation (1.3 g elemental calcium/day) in an effort to restore serum vitamin D levels to ‘normal’ that would serve to optimize gastrointestinal absorption of calcium. After 3 months of vitamin D supplementation in subjects who were defined as vitamin D deficient, the mean level of vitamin D for the total group was well above the lower limit of the normal range [25(OH)D >30 ng/ml], and six of seven subjects had values that were raised above this threshold value. Serum and urine calcium values remained within the normal range despite subjects having received supplemental daily oral calcium and vitamin D supplementation. Thus, it would appear that the daily administration of vitamin D3 2000 IU is a practical, safe, and effective manner of replacement therapy in those with SCI who are deficient. The improved levels of vitamin D in our subjects would be anticipated to benefit the sublesional skeleton by reducing turnover, as evidenced by suppressed levels of serum iPTH and NTx at month 3, and, hence, associated bone loss over the years, as well as potentially having favorable impact on other health issues.
Although large and repetitive doses of vitamin D2 (e.g. 50,000 to 100,000 IU) have been prescribed to treat vitamin D deficiency, and this approach has been fairly widely employed in the general population, it may not be judicious to treat persons with SCI in this manner. In persons with incomplete motor SCI who are ambulatory, one consideration is that high-dose vitamin D therapy in the general population has been reported to be associated with an increased incidence of falls and fractures.43 Once elevated, vitamin D levels may take months to return to acceptable levels. The prevalence of renal insufficiency may be increased in those with chronic SCI due to various insults to the kidney over time due to urinary tract infection with or without nephrocalcinosis, urinary tract stones, vesicular-ureteral reflux, and high-pressure hydronephrosis. With intermittent high-dose vitamin D therapy, the risk of unsuspected and intermittent hypercalcemia and hypercalciuria may be heightened, with the possibility of increased renal insufficiency. Although the possibility of hypercalciuria also exists with oral doses of vitamin D supplementation, urinary calcium excretion can be closely monitored, with the intake of vitamin D and/or calcium appropriately reduced, if indicated. Oral administration also avoids the unfavorable pharmacokinetics of parenteral administration – that is, the associated inevitable peaks and valleys in circulating concentrations of vitamin D after large, single-dose therapy.
Conclusions
Persons with SCI have been reported to have a higher prevalence of vitamin D deficiency than the general population. In people with SCI who have sublesional osteoporosis, remediable causes of increased bone loss should be prevented, such as vitamin D deficiency. The need for the clinician caring for those with chronic SCI to have an effective and safe vitamin D replacement protocol is apparent. This study found that oral therapy with vitamin D3 2000 IU daily generally raises 25(OH)D levels from absolute or relative deficiency into the acceptable range in subjects being supplemented with calcium, without any adverse effects noted. There is strong observational evidence to suggest that ‘sufficient’ levels of vitamin D are obligate for good health. Although the authors have advanced an argument for raising 25(OH)D levels to >30 ng/ml in the SCI population, randomized clinical trials in those with SCI are certainly needed to confirm the overall health benefit of supplementation to this level, and, as such, our recommendation is somewhat tentative in the absence of more compelling evidence supporting this proposed threshold value. Caution should be advised with vitamin D and calcium supplementation, with careful monitoring of the urinary calcium excretion and adjustment of the vitamin D and calcium intake, if indicated.
Acknowledgement
Veteran Affairs Rehabilitation Research and Development Service (#B4162-C), the James J. Peters VA Medical Center and Kessler Institute for Rehabilitation. The authors thank Quest Diagnostics for their support in providing the vitamin D assays.
References
- 1.Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism 1995;44(12):1612–6 [DOI] [PubMed] [Google Scholar]
- 2.Oleson CV, Patel PH, Wuermser LA. Influence of season, ethnicity, and chronicity on vitamin D deficiency in traumatic spinal cord injury. J Spinal Cord Med 2010;33(3):202–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemunaitis GA, Mejia M, Nagy JA, Johnson T, Chae J, Roach MJ. A descriptive study on vitamin D levels in individuals with spinal cord injury in an acute inpatient rehabilitation setting. PM R 2010;2(3):202–8 [DOI] [PubMed] [Google Scholar]
- 4.Pellicane AJ, Wysocki NM, Schnitzer TJ. Prevalence of 25-hydroxyvitamin D deficiency in the outpatient rehabilitation population. Am J Phys Med Rehabil 2010;89(11):899–904 [DOI] [PubMed] [Google Scholar]
- 5.Smith EM, Comiskey CM, Carroll AM. A study of bone mineral density in adults with disability. Arch Phys Med Rehabil 2009;90(7):1127–35 [DOI] [PubMed] [Google Scholar]
- 6.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. editors. Institute of Medicine of the National Academies. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011 [PubMed] [Google Scholar]
- 7.Bauman WA, Morrison NG, Spungen AM. Vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord Med 2005;28(3):203–7 [DOI] [PubMed] [Google Scholar]
- 8.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005;16(7):713–6 [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. editor. Vitamin D. Biosynthesis, metabolism, and mode of action. 2nd ed WB Saunders Company; Philadelphia, PA; 1989 [Google Scholar]
- 10.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3–5):204–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worcester EM, Coe FL. Nephrolithiasis. Prim Care 2008;35(2):369–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson C. Nutrition: Calcium supplements and osteoporosis: the heart of the matter. Nature Rev Endocrinol 2011;7(7):373. [DOI] [PubMed] [Google Scholar]
- 13.Walters JL, Buchholz AC, Martin Ginis KA. SHAPE-SCI Research Group. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord 2009;47(4):318–22 [DOI] [PubMed] [Google Scholar]
- 14.Hahn TJ, Hendin BA, Scharp CR, Haddad JG., Jr. Effect of chronic anticonvulsant therapy on serum 25-hydroxycalciferol levels in adults. N Engl J Med 1972;287(18):900–4 [DOI] [PubMed] [Google Scholar]
- 15.Simoons FJ. Progress report. New light on ethnic differences in adult lactose intolerance. Am J Dig Dis 1973;18(7):595–611 [DOI] [PubMed] [Google Scholar]
- 16.Comarr AE, Hutchinson RH, Bors E. Extremity fractures of patients with spinal cord injuries. Am J Surg 1962;103:732–9 [DOI] [PubMed] [Google Scholar]
- 17.Zehnder Y, Lüthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004;15(3):180–9 [DOI] [PubMed] [Google Scholar]
- 18.Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil 2005;86(3):498–504 [DOI] [PubMed] [Google Scholar]
- 19.Bauman WA, Spungen AM, Schwartz E, Wang J, Pierson RN., Jr. Continuous loss of bone in chronic immobilization: a monozygotic twin study. Osteoporos Int 1999;10(2):123–7 [DOI] [PubMed] [Google Scholar]
- 20.Bauman WA, Zhang RL, Morrison N, Spungen AM. Acute suppression of bone turnover with calcium infusion in persons with spinal cord injury. J Spinal Cord Med 2009;32(4):398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol 2008;3(5):1535–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85(6):1586–91 [DOI] [PubMed] [Google Scholar]
- 23.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 2005;97(1–2):179–94 [DOI] [PubMed] [Google Scholar]
- 24.Rheem DS, Baylink DJ, Olafsson S, Jackson CS, Walter MH. Prevention of colorectal cancer with vitamin D. Scand J Gastroenterol 2010;45(7–8):775–84 [DOI] [PubMed] [Google Scholar]
- 25.Jia X, Aucott LS, McNeill G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br J Nutr 2007;98(3):593–9 [DOI] [PubMed] [Google Scholar]
- 26.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168(15):1629–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59(1):242–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010;33(6):1379–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010;2010:351385 Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci 2009;338(1):40–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasson LT, Shimbo D, Rubin MR, Shaffer JA, Schwartz JE, Davidson KW. Is vitamin D deficiency a risk factor for ischemic heart disease in patients with established cardiovascular disease? 10-year follow-up of the Nova Scotia Health Survey. Int J Cardiol 2011;148(3):387–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol 2009;158(1):20–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginde AA, Mansbach JM, Camargo CA., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 2009;169(4):384–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res 2011;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 2004;806 Suppl:1717S–20S [DOI] [PubMed] [Google Scholar]
- 36.Ishihara J, Inoue M, Iwasaki M, Sasazuki S, Tsugane S. Dietary calcium, vitamin D, and the risk of colorectal cancer. Am J Clin Nutr 2008;88(6):1576–83 [DOI] [PubMed] [Google Scholar]
- 37.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat 2010;121(2):469–77 [DOI] [PubMed] [Google Scholar]
- 38.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–56 [DOI] [PubMed] [Google Scholar]
- 39.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008;46(7):466–76 [DOI] [PubMed] [Google Scholar]
- 40.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005;43(7):408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linn WS, Spungen AM, Gong H, Jr, Adkins RH, Bauman WA, Waters RL. Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord 2001;39(5):263–8 [DOI] [PubMed] [Google Scholar]
- 42.Dicpinigaitis PV, Almenoff PL, Spungen AM, Bauman WA. Bronchial hyperresponsiveness after cervical spinal cord injury. Chest 1994;105(4):1073–6 [DOI] [PubMed] [Google Scholar]
- 43.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303(18):1815–22 [DOI] [PubMed] [Google Scholar]