Abstract
Objective
Determine the effects of body-weight-supported treadmill training (BWSTT) and tilt-table standing (TTS) on clinically assessed and self-reported spasticity, motor neuron excitability, and related constructs in individuals with chronic spinal cord injury (SCI).
Design
Random cross-over.
Methods
Seven individuals with chronic SCI and spasticity performed thrice-weekly BWSTT for 4 weeks and thrice-weekly TTS for 4 weeks, separated by a 4-week wash-out. Clinical (Modified Ashworth Scale, Spinal Cord Assessment Tool for Spinal reflexes) and self-report (Spinal Cord Injury Spasticity Evaluation Tool, Penn Spasm Frequency Scale) assessments of spasticity, quality of life (Quality of Life Index Spinal Cord Injury Version – III), functional mobility (FIM Motor Subscale), plus soleus H-reflex were measured at baseline, after the first training session and within 2 days of completing each training condition.
Results
In comparison with TTS, a single session of BWSTT had greater beneficial effects for muscle tone (effect size (ES) = 0.69), flexor spasms (ES = 0.57), and the H/M ratio (ES = 0.50). Similarly, flexor spasms (ES = 0.79), clonus (ES = 0.66), and self-reported mobility (ES = 1.27) tended to benefit more from 4 weeks of BWSTT than of TTS. Participation in BWSTT also appeared to be favorable for quality of life (ES = 0.50). In contrast, extensor spasms were reduced to a greater degree with TTS (ES = 0.68 for single session; ES = 1.32 after 4 weeks).
Conclusion
While both BWSTT and TTS may provide specific benefits with respect to spasticity characteristics, data from this pilot study suggest that BWSTT may result in a broader range of positive outcomes.
Keywords: Paraplegia, Tetraplegia, Spasticity, Quality of life, Activity-based therapy, Muscle stretching exercises, Treadmill training, Tilt-table training, Spinal cord injury spasticity evaluation tool, Penn spasm frequency scale
Introduction
Spinal cord injury (SCI) is most commonly sustained by individuals who are relatively young, making it important to focus on the long-term effective management of co-morbidities that can impact quality of life. It has been reported that a majority of individuals with chronic SCI have spasticity1 and that spasticity has been identified by individuals with SCI as an important research priority.2 Spasticity may be considered beneficial by individuals with SCI for some aspects of daily life, such as sitting, standing, transferring, and some activities of daily living.1,3 There are also, however, numerous potentially problematic effects of spasticity, including inhibition of effective walking and self-care, pain, fatigue, disturbed sleep, compromised safety, and negative self-image.1,3 Consequently, many individuals with SCI seek strategies to manage their spasticity.
There are various approaches to manage spasticity following SCI.3 Physical therapy/rehabilitation is considered an essential component both as a first line of defense and in a long-term regimen during and after the implementation of pharmacological or surgical strategies.3 There is evidence suggesting that single sessions of active or passive ‘activity-based therapies’, such as passive standing assisted by a tilt-table, can have immediate effects on spasticity in individuals with SCI.4,5 In many cases, however, it has been reported that the effects of single sessions of activity on spasticity are short lasting (i.e. <24 hours).5 It is also important to note that there have been reports of increased spasticity by some study participants immediately following a single session of activity-based therapy.6 Participation in multiple sessions of active or passive movement activities by individuals with SCI has also been shown to modify spasticity symptoms.4 There is evidence that motor neuron excitability plays a role in spasticity following SCI7 and it has been shown to be modified following single8 and multiple9 sessions of activity-based intervention.
Body-weight-supported treadmill training (BWSTT) allows for assisted ambulation by individuals with impaired mobility. During BWSTT, the lower-limb musculature of an individual with motor-complete or -incomplete SCI bears weight, undergoes stretch, and exhibits electromyographic activity.10 BWSTT has also been reported to reduce the amplitude of the soleus maximum H-reflex/maximum M-wave ratio (H/M ratio) in people with SCI, which is believed to be a reflection of reduced motor neuron excitability8. In comparison, tilt-table standing (TTS) places load on the lower limbs and imposes stretch on the skeletal muscles that cross the hip, knee, and ankle joints, but it is not associated with lower-limb movement or electromyographic activity.
To our knowledge, there are no studies examining the immediate or longer-term effects of BWSTT and TTS on multiple measures of spasticity in individuals with SCI. Therefore, the objective of this pilot study was to compare the effects of single and multiple sessions of BWSTT and TTS on clinically assessed and self-reported spasticity, motor neuron excitability, and related constructs in individuals with chronic SCI. It was hypothesized that BWSTT would demonstrate a greater benefit on spasticity-related outcomes compared with TTS.
Methods
Participants
Six males and one female were recruited from the Hamilton Health Sciences outpatient SCI rehabilitation program. Eligibility criteria were chronic (>1 year) complete or incomplete paraplegia or tetraplegia (ASIA A–C), self-reported presence of ‘stable’ spasticity, consistent medication and physical activity/physiotherapy routines during the previous 6 months, and reliance on a wheelchair as a primary mode of mobility. Participation in BWSTT during the previous 6 months and/or any medical contraindications to the performance of BWSTT or TTS were exclusion criteria. This study received ethics approval from the Hamilton Health Sciences Research Ethics Board and all participants provided informed consent.
Study design and overall protocol
A random cross-over design was used to compare intervention effects. Participants were scheduled to perform 12 sessions of BWSTT over 4 weeks and 12 sessions of TTS over 4 weeks, with a 4-week detraining period between the two conditions. Each participant served as his own control, with the order of conditions for each participant determined randomly. Each session was scheduled to last up to 45 minutes, while aiming to match time spent in the two conditions.
Testing was performed in the following order: (1) self-report questionnaires, (2) maximum H-reflex to maximum M-wave ratio (H/M ratio), (3) muscle tone, (4) flexor spasms, (5) extensor spasms, and (6) clonus. Post-testing occurred immediately following the first session (30–60 minutes post-activity) and 24–48 hours following the final session of each 4-week activity condition. All assessments were performed by the same examiner and at the same time of the day. Participants were asked to refrain from caffeine, nicotine, marijuana, and physical activity 20 hours before testing and to empty their bladders prior to testing.
Activity conditions
BWSTT was performed using a motor-driven treadmill (Woodway USA Inc., Foster, CT) with a harness and overhead pulley system capable of supporting a percentage of the participant's body weight. Body-weight support (BWS) and speed of the treadmill were chosen to enable appropriate gait with full knee extension during stance. When necessary, lower-limb movement was assisted by therapists. Participants were encouraged to place their entire body weight over a fully extended leg during the stance phase of the walking cycle. Modifications of the training parameters over the 4-week BWSTT period were made according to our training protocol.11
TTS was performed on a motorized tilt-table (Midland Manufacturing Co., Inc., Columbia, SC, USA) at the greatest tolerated tilt angle (maximum 80°). Velcro straps at the knees, hips, and torso (if necessary) were used to support the individual in an upright position. Participants were allowed to increase gradually to their maximum tilt angle and to reduce their tilt angle during a session if they felt light headed.
Outcome measures
The Modified Ashworth Scale (MAS) was used to assess passive resistance to movement in the left- and right-hip flexors, extensors, and adductors, knee flexors and extensors, and ankle plantar-flexors and dorsiflexors. The single scores from the muscle groups of both lower limbs were summed for each participant.12 The Spinal Cord Assessment Tool for Spastic reflexes was used to assess spasm and clonus severity.13
The soleus H/M ratio was assessed with participants seated in their chairs. A single rectangular biphasic pulse with a pulse width of 500 µs was applied to the tibial nerve in the popliteal fossa using a constant-voltage stimulator (Digitimer Devices, Hertfordshire, UK). The Ag-AgCl gel, self-adhesive recording electrodes (Kendall Meditrace 530, Chicopee, MA, USA) were placed in a bi-polar arrangement; one electrode was secured over the right soleus muscle and the other over the right Achilles tendon. The data were collected using customized LabVIEW software (National Instruments, Austin, TX, USA). Stimulus intensity was increased in steps of ∼5 mV until Hmax and Mmax were approached and then increased in steps of ∼1 mV until peak values were obtained. The three greatest measurements of Hmax and the three greatest measurements of Mmax were averaged. An average of the right and left soleus H/M ratios for each participant was used for all analyses.
Study participants were asked to answer four self-report questionnaires while considering the previous 7 days. The Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET) measured the impact of spasticity on daily life.14 The Penn Spasm Frequency Scale15 assessed spasm frequency. Perceived health and functioning were assessed with the Quality of Life Index16 SCI Version – III (QLI) and the Motor Subscale of the FIM.17
Data treatment and statistical analyses
A paired samples t-test was used to compare the amount of BWSTT and TTS training time within the single-session and multiple-session protocols. To check for possible carryover effects of activity between conditions, independent samples t-tests were performed for each outcome measure, with Bonferroni correction for multiple comparisons. Given the relatively small sample size, Cohen's d effect sizes (ESs) were calculated to compare baseline scores to post-activity scores within condition and to compare change scores between conditions for each outcome; relevant medium (≥0.50) or large (≥0.80) ESs are discussed.18 ES calculations provide an estimate of the strength of a relationship.
Results
Participants
Six men and one woman (age: 37.1 ± 7.7 years) with chronic (5.0 ± 4.4 years post-injury) complete or incomplete paraplegia or tetraplegia and self-reported spasticity participated in this study (Table 1). Current medication and physical activity/physiotherapy routines had been consistent for at least 6 months prior to the start of the study and remained consistent throughout the duration of the study.
Table 1.
Participant characteristics
| Participant ID | Sex | Age (years) | Severity | Level | Post-injury (years) | Cause of SCI | Daily spasticity medications |
|---|---|---|---|---|---|---|---|
| 1 | M | 40 | C | T2 | 2 | Fall | None |
| 2 | M | 37 | A | C6 | 14 | MVA | 40 mg Baclofen |
| 3 | M | 32 | B | T5 | 7 | MVA | 80 mg Baclofen Marijuana (2–3 mg/day) |
| 4 | F | 24 | C | C5 | 5 | MVA | None |
| 5 | M | 39 | A | T5 | 3 | MVA | None |
| 6 | M | 49 | C | T10 | 3 | Bleed | 60 mg Baclofen |
| 7 | M | 39 | C | T2 | 1 | MVA | 80 mg Baclofen 5 mg Tizanidine |
Activity conditions
All participants completed the activity protocol with attendance at 12 sessions of BWSTT and 12 sessions of TTS within the respective 4-week training blocks (Tables 2 and 3). Total training time of BWSTT and TTS did not differ, at 453.7 ± 72.3 and 471.6 ± 52.0 minutes, respectively (P = 0.23). BWSTT training parameters included a group mean treadmill speed of 0.23 ± 0.10 m/second and a group mean provision of 50.7 ± 24.7% BWS. TTS was performed at a group mean table angle of 68.6 ± 11.3°.
Table 2.
BWSTT and TTS durations and parameters for the single-session protocol
| ID | BWSTT |
TTS |
|||
|---|---|---|---|---|---|
| Duration (minutes) | Avg. speed (minutes/second) | Avg. BWS (%) | Duration (minutes) | Avg. tilt angle (°) | |
| 1 | 30 | 0.28 | 8.0 | 30 | 70.0 |
| 2 | 30 | 0.28 | 50.3 | 30 | 63.3 |
| 3 | 30 | 0.33 | 32.0 | 30 | 56.7 |
| 4 | 25 | 0.07 | 40.0 | 20 | 43.0 |
| 5 | 30 | 0.19 | 72.0 | 30 | 70.0 |
| 6 | 30 | 0.17 | 24.0 | 30 | 66.7 |
| 7 | 30 | 0.14 | 40.0 | 30 | 70.0 |
Table 3.
BWSTT and TTS durations and parameters for the multiple session protocol
| ID | BWSTT |
TTS |
|||
|---|---|---|---|---|---|
| Duration (minutes) | Avg. speed (minutes/second) | Avg. BWS (%) | Duration (minutes) | Avg. tilt angle (°) | |
| 1 | 487 | 0.33 | 4.8 | 487 | 78.8 |
| 2 | 510 | 0.33 | 71.7 | 515 | 71.6 |
| 3 | 495 | 0.33 | 68.6 | 510 | 66.9 |
| 4 | 320 | 0.10 | 55.0 | 418 | 46.8 |
| 5 | 460 | 0.19 | 72.9 | 456 | 62.8 |
| 6 | 513 | 0.17 | 34.3 | 525 | 78.7 |
| 7 | 391 | 0.19 | 47.8 | 390 | 74.9 |
Effects of BWSTT and TTS
The mean scores for all outcome measures are presented in Table 4. There was no evidence of carryover effects between activity conditions, nor any statistically significant differences between baseline scores for either condition.
Table 4.
Summary of mean ± SD scores for all outcome measures at baseline and post-activity (single session and multiple session) for the BWSTT and TTS conditions
| Outcome measures | BWSTT |
TTS |
||||
|---|---|---|---|---|---|---|
| Baseline | Post-activity (single session) | Post-activity (multiple session) | Baseline | Post-activity (single session) | Post-activity (multiple session) | |
| Ashworth | 29.6 ± 16.0 | 25.6 ± 13.0 | 29.9 ± 8.5 | 29.4 ± 14.4 | 29.1 ± 13.5 | 30.1 ± 14.5 |
| Flexor spasms | 2.9 ± 1.2 | 2.7 ± 1.1 | 2.4 ± 2.0 | 2.4 ± 1.6 | 3.0 ± 1.5 | 3.0 ± 1.4 |
| Extensor spasms | 3.7 ± 1.5 | 3.3 ± 1.6 | 4.0 ± 2.1 | 4.6 ± 1.1 | 3.7 ± 1.4 | 3.6 ± 1.0 |
| Clonus | 2.6 ± 2.5 | 2.4 ± 2.3 | 1.7 ± 1.8 | 1.9 ± 1.7 | 1.7 ± 1.5 | 2.0 ± 1.3 |
| SCI-SET | −0.77 ± 0.46 | N/A | −0.64 ± 0.46 | −0.72 ± 0.36 | N/A | −0.64 ± 0.41 |
| Penn | 2.6 ± 0.8 | N/A | 2.3 ± 0.8 | 2.3 ± 1.0 | N/A | 2.1 ± 0.4 |
| QLI | 62.0 ± 12.4 | N/A | 68.1 ± 12.6 | 61.7 ± 12.0 | N/A | 64.7 ± 12.9 |
| FIM motor | 73.6 ± 11.4 | N/A | 76.3 ± 11.7 | 77.4 ± 10.2 | N/A | 76.1 ± 12.5 |
| H/M ratio | 0.61 ± 0.25 | 0.60 ± 0.22 | 0.63 ± 0.26 | 0.64 ± 0.26 | 0.66 ± 0.24 | 0.65 ± 0.27 |
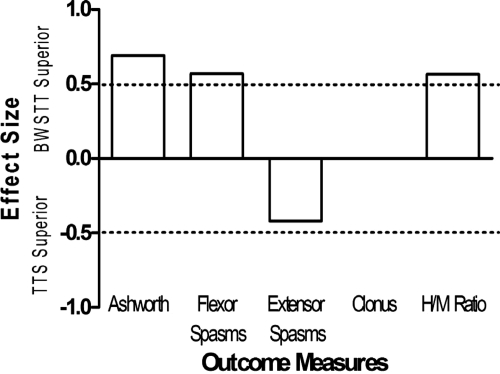
After the first session, there was a tendency for extensor spasms to decrease following TTS (ES = 0.68; Fig. 1), but not following BWSTT. A comparison of the change scores suggested that there was a greater reduction in passive resistance to movement (Ashworth; ES = 0.69; Fig. 2) and flexor spasms (ES = 0.57; Fig. 2) after BWSTT compared to TTS. It was also suggested that the H/M ratio decreased to a greater extent following a single session of BWSTT compared to TTS (ES = 0.50; Fig. 2).
Figure 1.
ESs of the differences between baseline and post-activity outcome measure scores after one session of BWSTT (open bars) and TTS (solid bars) Positive and negative ESs represent favorable and unfavorable changes, respectively, in the outcome measures. Absolute ESs greater than 0.50 (dashed line) are discussed in the text.
Figure 2.
ESs of the differences in change scores between outcome measures following a single session of BWSTT and a single session of TTS. Positive ESs represent a superiority of BWSTT, whereas negative ESs represent a superiority of TTS in terms of the induction of a favorable change. Absolute ESs greater than 0.50 (dashed line) are discussed in the text.
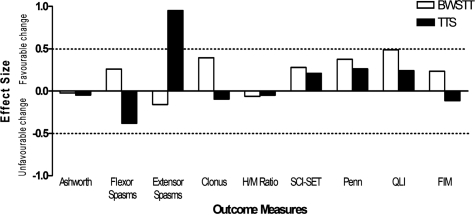
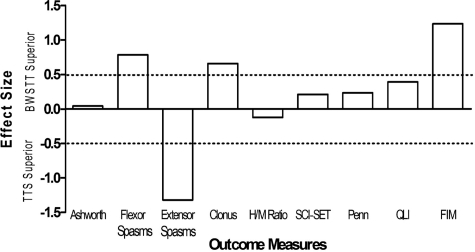
Four weeks of TTS appeared to reduce extensor spasms (ES = 0.95; Fig. 3). There were also indications that extensor spasms were affected differently from flexor spasms depending on the intervention. Extensor spasms tended to be reduced to a greater extent following TTS compared to BWSTT (ES = 1.32; Fig. 4), while flexor spasms tended to be reduced to a greater extent following BWSTT compared to TTS (ES = 0.79; Fig. 4). Clonus also appeared to be improve more following BWSTT (ES = 0.66; Fig. 4). There were no observed changes in the H/M ratio following participation in 12 sessions of either BWSTT or TTS, nor any observed differences in effects between activity conditions (Table 1; Figs 3 and 4).
Figure 3.
ESs of the differences between baseline and post-activity outcome measure scores for BWSTT (open bars) and TTS (solid bars) multiple session conditions. Positive and negative ESs represent favorable and unfavorable changes, respectively, in the outcome measures. Absolute ESs greater than 0.50 (dashed line) are discussed in the text.
Figure 4.
ESs of the differences in change scores between outcome measures following multiple sessions of BWSTT and of TTS. Positive ESs represent a superiority of BWSTT, whereas negative ESs represent a superiority of TTS in terms of the induction of a favorable change. Absolute ESs greater than 0.50 (dashed line) are discussed in the text.
Participation in 12 sessions of BWSTT or TTS did not result in group changes in scores on the SCI-SET or the Penn Spasm Frequency Scale. A medium ES supported the potential beneficial effect of 12 sessions of BWSTT on quality of life (ES = 0.50; Fig. 3). BWSTT also resulted in more positive changes in FIM Motor Subscale scores (ES = 1.24; Fig. 4).
Discussion
To our knowledge, this is the first study comparing the effects of single and multiple sessions of BWSTT and TTS on various measures of spasticity in individuals with chronic ASIA A to C SCI. The group mean activity durations and the amount of BWS provided were similar between conditions for both protocols. Only participant #4 had a notable difference in training duration between the BWSTT and TTS conditions (98 fewer minutes of BWSTT). This participant felt that the work required by BWSTT was greater than with TTS and, therefore, was consistently unable to match her BWSTT activity session durations with those of her previously completed TTS activity sessions.
There was no evidence of change in muscle tone (MAS) following 4 weeks of BWSTT or TTS. Others have found similar results following BWSTT or overground locomotor training for 12 weeks.19 This could reflect limitations of the MAS or a true inability of activity to reduce muscle tone. Results did suggest, however, that participation in single and multiple sessions of TTS-reduced extensor spasms, a finding that has been reported previously.5 Despite this, the study results indicated that a single session of BWSTT may be superior to TTS for several spasticity-related outcomes (reduction of muscle tone, flexor spasms, and motor neuron excitability). Participation in multiple sessions of BWSTT also appeared to be superior to TTS in terms of management of flexor spasms and clonus. We were unable to detect changes in the soleus H/M ratio following multiple sessions of activity. A previous study was also unable to demonstrate a change in the H/M ratio following 4 months of treadmill walking by an individual with incomplete SCI.20 Although this may cast doubt on the role of motor neuron excitability in spasticity, it is more likely that various mechanisms play a role depending on the symptom, the timeline, and the activity.
It is considered that the best judge of spasticity is the individual, as he/she is best able to assess the impact of spasticity on daily life.1 It has also been suggested that short-term benefits of a single session of activity may not be obvious to an examiner beyond a few hours.5 Of note, the majority of participants in the present study identified smaller problematic effects of spasticity on daily life following 4 weeks of either BWSTT or TTS. There was moderately strong evidence, however, that self-reported quality of life improved following participation in multiple sessions of BWSTT, but not TTS. This finding is encouraging given the previous finding of no change in quality of life following 1 year of intrathecal pharmacological treatment for spasticity.21 Therefore, the improvement in quality of life might have been related to the tendency of BWSTT to increase self-reported functional mobility compared to TTS.
Overall, our finding that various outcome measures did not respond similarly to activity is not surprising given numerous reports of poor correlations between different measures of spasticity.7,13,12 The variability of results within the present study also reflects the generally inconsistent findings in the literature for the effects of single and multiple sessions of activity-based therapy on lower-limb spasticity in humans with SCI.4 Although little information exists, it is likely that the heterogeneous nature of spasticity and of SCI contributes to a variable response to activity-based intervention in this population. Anecdotal reports by the participants from the present study revealed that some participants perceived short-term decreases in spasticity following a training session (up to ∼4 hours), whereas others experienced a decrease in spasticity frequency, but an increase in intensity when spasticity did occur. Because spasticity exerts different consequences in the lives of different individuals with SCI, it has been stated that a repertoire of interventions would be beneficial to target the various issues.2
While the present study identified several interesting findings related to the effects of BWSTT and TTS on spasticity-related measures, the small and heterogenous nature of our sample made it difficult to draw conclusions based on group changes. A limitation of the study may have resulted from an inability of the examiner to be blinded to the study intervention. It is also possible that our multiple-session intervention durations were not long enough for the benefits to be gained from BWSTT or TTS. The participants in the present study exerted efforts that were uncustomary for them and, in some cases, an increase in spasm severity during the intervention period was reported. Perhaps a longer intervention period would have allowed for the development of favorable physiological adaptations to activity, eventually making the activity sessions beneficial to spasticity management.
Conclusion
The results from this pilot study indicate that some individuals with SCI and spasticity may benefit from participation in weight-bearing activity and that BWSTT and TTS may have different effects for certain outcomes. Compared to more invasive management strategies (e.g. pharmacological, surgical), activity-based interventions offer the potential for further health-related benefits and fewer possible problematic side effects. Therefore, this area of research would benefit from investigations of longer activity periods which address individual characteristics that may affect responses to participation.
Acknowledgment
Ontario Neurotrauma Foundation (studentship, M. Adams) and Gatorade Sport Science Foundation (student grant, M. Adams)
References
- 1.Sköld C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil 1999;80(12):1548–57 [DOI] [PubMed] [Google Scholar]
- 2.Hammell KR. Spinal cord injury rehabilitation research: patient priorities, current deficiencies and potential directions. Disabil Rehabil 2010;32(14):1209–18 [DOI] [PubMed] [Google Scholar]
- 3.Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43(10):577–86 [DOI] [PubMed] [Google Scholar]
- 4.Hsieh JTC, Wolfe DL, Connolly S, Townson AF, Curt A, Blackmer J, et al. Spasticity following spinal cord injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Aubut J, et al. (eds.) Spinal cord injury rehabilitation evidence. Vancouver, BC; 2006. pp. 21.1–21.56 [Google Scholar]
- 5.Bohannon RW. Tilt table standing for reducing spasticity after spinal cord injury. Arch Phys Med Rehabil 1993;74(10):1121–22 [DOI] [PubMed] [Google Scholar]
- 6.Thoumie P, Le Claire G, Beillot J, Dassonville J, Chevalier T, Perrouin-Verbe B, et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: physiological evaluation. Paraplegia. 1995;33(11):654–9 [DOI] [PubMed] [Google Scholar]
- 7.Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54(8):1574–82 [DOI] [PubMed] [Google Scholar]
- 8.Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J Spinal Cord Med 2001;24(2):74–80 [DOI] [PubMed] [Google Scholar]
- 9.Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, et al. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med 2005;28(3):241–5 [DOI] [PubMed] [Google Scholar]
- 10.Wirz M, Colombo G, Dietz V. Long term effects of locomotor training in spinal humans. J Neurol Neurosurg Psychiatry 2001;71(1):93–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks AL, Adams MM, Martin GK, Giangregorio L, Latimer A, Phillips SM, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 2005;43(5):291–8 [DOI] [PubMed] [Google Scholar]
- 12.Lechner HE, Frotzler A, Eser P. Relationship between self- and clinically rated spasticity in spinal cord injury. Arch Phys Med Rehabil 2006;87(1):15–9 [DOI] [PubMed] [Google Scholar]
- 13.Benz EN, Hornby TG, Bode RK, Scheidt RA, Schmit BD. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil 2005;86(1):52–9 [DOI] [PubMed] [Google Scholar]
- 14.Adams MM, Martin Ginis KA, Hicks AL. Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET): development and evaluation. Arch Phys Med Rehabil 2007;88(9):1185–92 [DOI] [PubMed] [Google Scholar]
- 15.Penn RD. Intrathecal baclofen for severe spasticity. Ann N Y Acad Sci 1988;531:157–66 [DOI] [PubMed] [Google Scholar]
- 16.Ferrans C, Powers M.Quality of life index: Spinal Cord Injury Version III. 1998. [accessed 2011 Jul 25]. Available from: http://www.uic.edu/orgs/qli/index.htm .
- 17.Dittmar SS. Selection and administration of functional assessment and rehabilitation outcome measures. In: Dittmar SS, Gresham GE. (eds.) Functional assessment and outcome measures for the rehabilitation professional. Gaithersburg, MA: Aspen Publishers, Inc.; 1997. p. 11–6 [Google Scholar]
- 18.Cohen J. A power primer. Psychol Bull 1992;112(1):155–9 [DOI] [PubMed] [Google Scholar]
- 19.Dobkin B, Apple D, Barbeau H, Behrman A, Deforge D, Ditunno J, et al. Spinal Cord Injury Locomotor Trial (SCILT) Group Weight-supported treadmill vs. overground training for walking after acute incomplete SCI. Neurology. 2006;66(4):484–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trimble MH, Kukulka CG, Behrman AL. The effect of treadmill gait training on low-frequency depression of the soleus H-reflex: comparison of a spinal cord injured man to normal subjects. Neurosci Lett 1998;246(3):186–8 [DOI] [PubMed] [Google Scholar]
- 21.Gianino JM, York MM, Paice JA, Shott S. Quality of life: effect of reduced spasticity from intrathecal baclofen. J Neurosci Nurs 1998;30(1):47–54 [DOI] [PubMed] [Google Scholar]