Abstract
Objective
To evaluate artificial reflex arcs for micturition using urodynamics and electrophysiological recordings.
Design
Sixteen beagles were equally and randomly divided into two groups.
Methods
In group A, anastomosis of the proximal end of the left L7 ventral root (VR) and distal end of the left S2 VR was performed, as well as anastomosis of the L7 dorsal root (DR) and S2 DR to reconstruct the sensory and the motor function of the bladder. In group B the proximal end of the left L7 VR and the distal end of the left S2 VR were anastomosed, while the left L7 DR was kept intact to reconstruct the motor function of the bladder. Outcome measures included electrophysiological testing and the urodynamic measures. In addition, we also monitored urinary infection rates.
Results
Stimulation to the left S2 DR in groups A and B both elevated the bladder pressure before and after the spinal lower motor neuron lesion. Single stimulation of the two groups both elicited evoked action potentials. Urinary infections occurred in group A (three occurrences) and in group B (eight occurrences) during the 3 months after the spinal lower motor neuron lesion.
Conclusion
Data showed that both reconstructive methods could induce bladder micturition and evoked action potentials. However, in group A the micturition response was better and the urinary infection rates were lower after the spinal lower motor neuron lesion. Thus, the artificial physiological reflex arc reconstruction method used in group A, with sensory input above the lesion, might provide a better alternative in clinical practice.
Keywords: Urodynamics, Neurogenic bladder, Urination, Micturition, Neural pathway, Reconstruction, Detrusor–sphincter dyssynergia, Spinal cord injuries
Introduction
Spinal cord injury (SCI) often leads to the dysfunction of bladder, one of the most profound effects. For lower urinary tract problem (1) high urethral resistance appears to be the major factor. With SCI, the action of coordinating the relaxation of the sphincter muscle and contracting the detrusor muscle may be adversely affected, resulting in detrusor–sphincter dyssynergia. (2) Overactive bladder (incontinence). Injuries above the level of T12 that damage the spinal cord upper motor neurons, but leave the sacral segment intact will result in an overactive bladder. (3) The underactive bladder (atonic bladder) occurs when the injury is below the conus medullaris causing the damage of the micturition reflex arc, and nerve impulses from the stretch receptors in the bladder wall do not result in reflex voiding, leading to bladder overdistension. Therefore, restoring bladder function after SCI remains a concern for clinicians. Intermittent catheterization is the method of choice for functional recovery of the atonic bladder after SCI; micturition reflex arc reconstruction is being developed as an alternative.
Currently, there are two important micturition reflex arc reconstruction methods: (1) Lin et al.1 anastomosed T11 ventral root (VR) above the injury level and S2 VR, which primarily mediates bladder function below the injury level, keeping the T11 dorsal root (DR) intact; micturition was induced via the reconstructed artificial micturition reflex pathway. Because the T11 DR was kept intact, in this micturition reflex pathway the sensory input was above the lesions. (2) Zheng et al. separately anastomosed L5 VR and DRs above the SCI level and S2 VR and DRs below the SCI level in rats. They reconstructed the sensory and motor pathways of the atonic bladder. Bladder micturition function was recovered via the rebuilding of this artificial physiological bladder reflex arc.2–4 The S2 DR and L5 DR were anastomosed to restore the sensory function of bladder and in this micturition reflex arc, the sensory input was from below the lesions. Although it has been reported that both of these reconstruction methods can induce bladder micturition, little is known about the comparative effect of sensory input from above and below the lesions. In this study we reconstructed the two most important micturition reflex arcs in beagles with the acute SCI beagle model for comparison.
When bladder pressure reaches 5–15 cmH2O, bladder sensory nerves produce impulses and trigger the new micturition reflex arc, resulting in physiologically similar micturition. Urodynamic studies (UDS) and neural electrophysiological testing were generally used in previous studies to evaluate the bladder function.1,5 We attempted to identify the optimal procedure by measurement of neural electrophysiology, urodynamics, and urinary infection rates.
Methods
We studied 16, 1-year-old male beagles, weighing 7–12 kg (8.4 ± 1.5 kg) provided by the animal experiment center of Nanjing Drum Tower Hospital. The animals were treated in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ published by the Chinese National Institute of Health. The protocols were approved by Animal Ethics Committee of Nanjing Drum Tower Hospital. Medlab-U biological signal collection processing system was provided by Nanjing Meiyi Company, Nanjing, China. After a cystometry cannula was inserted into the bladder, we connected it with the pressure transducers of Medlab-U biological signal processing system. All signals were entered into the computer by Medlab-U biological signal processing system and analyzed by Medlab-U biological signal processing system software.
Animal preparation and surgical procedure
Sixteen beagles were randomly divided into the following groups: (1) Group A (N = 8). Dog's L7 VR and DR and S2 VR and DR were anastomosed, respectively, and in this micturition reflex arc the sensory input was below the spinal lower motor neuron lesions. (2) Group B (N = 8). Dog's L7 VR and S2 VR were anastomosed and L7 DR was kept intact so in this micturition reflex pathway the sensory input was from above the spinal lower motor neuron lesions. Ketamine 15 mg/kg, droperidol 5 mg, atropine 0.5 mg were used for the induction of anesthesia; the propofol was used to maintain anesthesia and the dosage of propofol was 0.2 ml/kg/minutes.
After tracheal intubation, the bladder and urethra were exposed via lower abdominal incision with the dogs lying supine position, and the cystometry cannula was inserted through the wall of urinary bladder, and the incision was sutured under sterile conditions. Then the cystometry cannula was fixed with 4–0 purse-string suture, and connected with the pressure transducer of Medlab-U biological signal processing system and the computer. The spinal canal of L5–S3 and the nerve root of L7–S2 were exposed via median incision in the lumbosacral region with the dog lying prone.1 The left side was the experimental side, while the right side served as a control. The bladder was injected with 300 ml of normal saline at 30 ml/minute. By stimulating S1–S4 nerve root on the experimental side with 300 mV at 20 Hz for 5 seconds, we determined by bladder pressure changes that the main nerve root controlled the bladder. In this experiment, S2 stimulation induced the highest bladder pressure change (S1–S4: 11.53 ± 2.35, 20.20 ± 1.12, 10.61 ± 1.33, 1.02 ± 1.22 cmH2O).
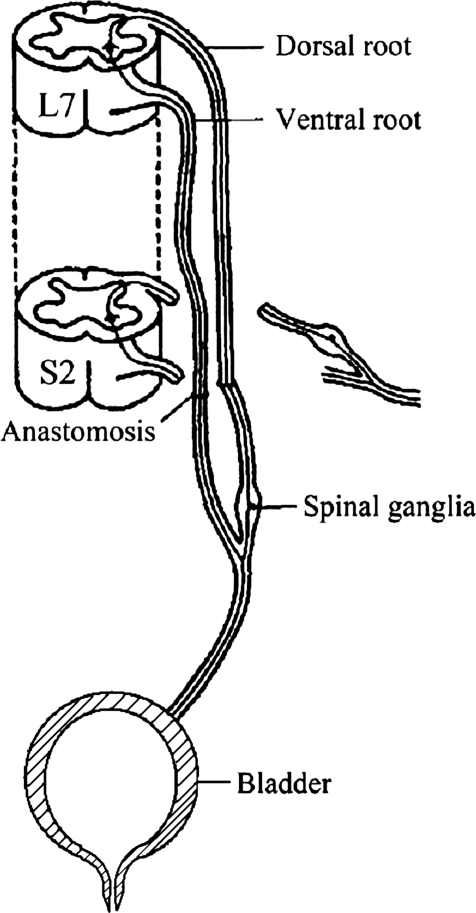
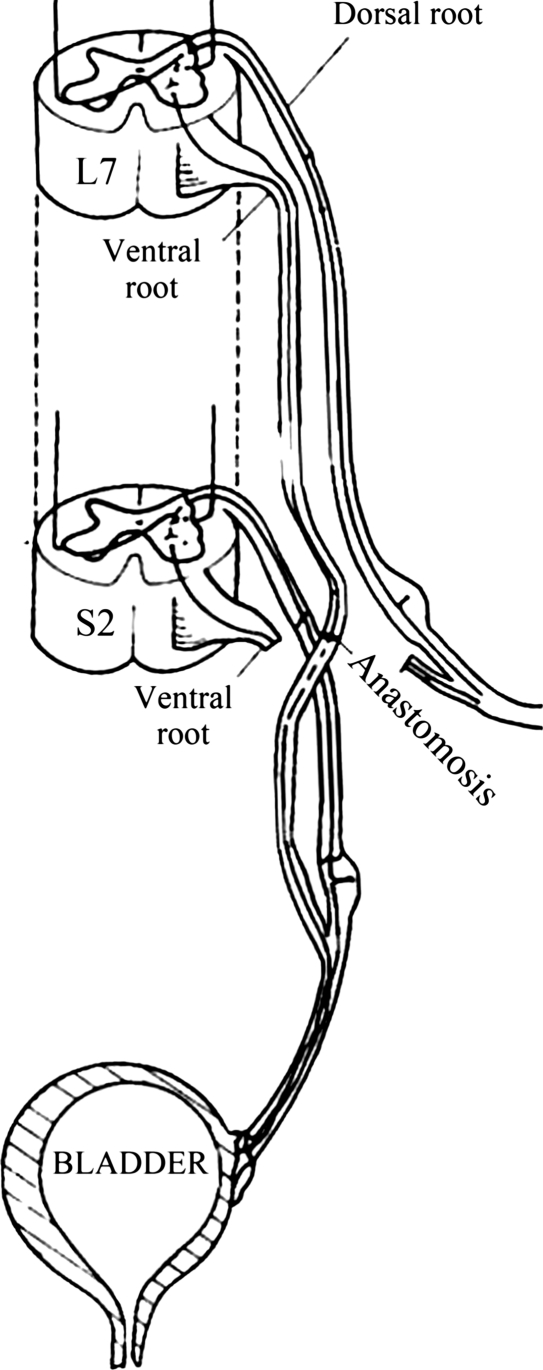
The dura was opened by a paramedian incision. In group A the left L7 nerve root was cut distally before it emerged from the dura mater, and the left S2 nerve root was cut close to the spinal cord. The VR and DR of L7 and S2 were carefully separated under a microscope. Coaptation between the L7 nerve roots and S2 nerve roots was achieved to reconstruct the artificial physiological bladder reflex arc. Intradural microanastomosis of the left L7 VR to S2 VR and L7 DR to S2 DR was performed to reconstruct the sensory and the motor functions of atonic bladder (Fig. 1). In group B the left L7 VR was cut distally before it emerged from the dura mater, and the left S2 VR was cut close to the spinal cord. L7 DR and S2 DR were kept intact. The left L7 VR and S2 VR were intradurally microanastomosed to reconstruct an artificial micturition reflex arc (Fig. 2).
Figure 1.
Physiological micturition reflex arc reconstruction in beagle.
Figure 2.
Artificial micturition reflex arc reconstruction in beagle.
The right sides in these two groups were kept intact and served as internal controls. Anastomotic level was marked with a ligation wire via zygapophysis. Medical biofilms (Di Kang Science Biopharmaceuticals Co., Ltd Chengdu, China) were used on the endorhachis to prevent conglutination. The basis of the Medical biofilms was poly-dl-lactide. After the operation, 0.8 million U/day of penicillin was administered via intramuscular injection for 3 days. When the wound was satisfactorily healing, the dogs were put in a feeding farm for 12 months.
Neural electrophysiological testing
After 12 months of feeding and nerve regeneration, the neural electrophysiological testing was performed to detect whether the regenerative nerve axons could conduct the nerve impulse. The survival dogs were anesthetized, and the VR and DR of the L7 and those of the S2 nerves were exposed via the original lumbosacral incision with the dog lying prone. The stimulation electrodes of Medlab-U biological signal processing system were fixed to the left and right S2 DR in group A with 37°C paraffin oil. Action potentials were recorded via the recording electrodes fixed with the left and right S2 VR. In group B the stimulation electrodes were placed on the left L7 DR and the right S2 DR with 37°C paraffin oil. Action potentials were recorded via the recording electrodes fixed with the left and right S2 VR. In the two groups, the action potentials of the bladder were recorded at a stimulation intensity of 300 mV for a period of 0.3 millisecond. Immediately, after the spinal lower motor neuron lesion, the action potentials of the left side were recorded again in both groups.
Urodynamic study
After the neural electrophysiological testing, UDS was performed in all dogs before the spinal lower motor neuron lesion to observe whether the bladder could micturition via the reconstructed micturition reflex. First, the bladder pressure was measured. The cystometry cannula was inserted into the bladder with the above method along hypogastrium incision through the bladder wall with the dog lying supine. The cystometry cannula was connected to the pressure transducer of Medlab-U biological signal processing system and a computer. Then 300 ml of 37°C normal saline was injected into the bladder through the cystometry cannula. The intensity of stimulation was 300 mV at 20 Hz for 5 seconds, and the pulse width was 0.3 milliseconds. Changes in bladder pressure were recorded by the Medlab-U biological signal processing system. Second, 300 ml of 37°C normal saline was injected to the bladder through the cystometry cannula again, and following the train stimulation the maximal urinary flow rate was recorded using WD-NL-100 (Shanghai Jingcheng Medical Instrument Co., Ltd., Shanghai, China). At last, after urination induced by the train stimulation, residual urine volume was obtained with a 20-ml injector through the cystometry cannula. All these studies on the left side were performed again immediately after the spinal lower motor neuron lesion in both groups.
Observation of animal urinary infection rates
After the urodynamic study the micturition of the dogs was observed, and urinary infections were recorded for the next 3 months.
Statistical analysis
All data are expressed as the mean ± SD. The SPSS11.0® software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. χ2 test was used to compare the differences between groups. The results were considered significant in both groups with P < 0.05.
Results
After the surgical procedure the dogs were fed for 12 months. During this period, one dog was excluded in group A and two dogs were excluded in group B because chronic health problems developed. Data for urodynamic and neural electrophysiology studies were obtained from the seven dogs in group A and six dogs in group B. Three months after the spinal lower motor neuron lesion, another dog died and was excluded from group B.
Neural electrophysiological testing
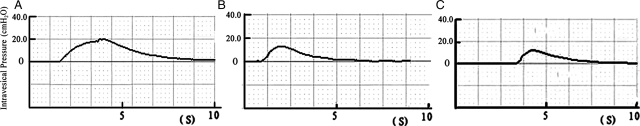
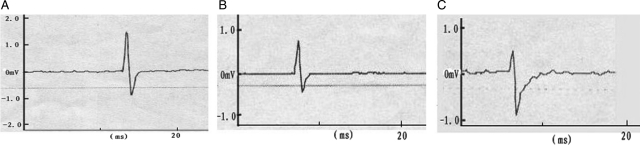
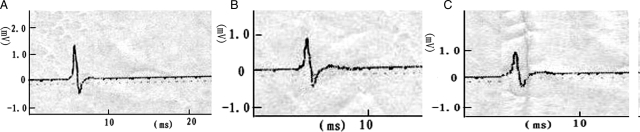
After single stimulation to the left S2 DR in group A before and after the spinal lower motor neuron lesion, we recorded the action potentials at S2 VR. The amplitudes were 0.68 ± 0.11 and 0.60 ± 0.08 mV. The action potential amplitudes of the experimental side were similar (P > 0.05). With stimulation to the S2 DR on the control side, the action potential waveform recorded was similar to the experimental side, but the mean amplitude of the action potentials was 1.21 ± 0.13 mV. The action potentials on right S2 VR were greater than on the left side (Fig. 3) (P < 0.01). The amplitudes of action potentials were recorded during single stimulation to the left L7 DR in group B before and after the spinal lower motor neuron lesion. The amplitudes of the evoked potentials were 0.75 ± 0.10 and 0.67 ± 0.05 mV (not significant). When the right S2 DR was stimulated, the action potential waveform recorded at S2 VR was similar to the left side, but the amplitude of the action potentials was 1.30 ± 0.09 mV. The action potentials of S2 VR on the control side recorded were greater than on the experimental side before and after spinal cord transection (Fig. 4) (P < 0.01). The action potential amplitudes on both the experimental and the control side were similar in these two groups before and after the spinal lower motor neuron lesion (Table 1) (P > 0.05).
Figure 3.
Action potentials were recorded both before and after the spinal lower motor neuron lesion by using the single electrical stimulus to excite S2 DR in group A. The morphology of the tracing recorded on the left side was similar before and after lesion. The amplitude of the action potential on control side was significantly greater than that of the left side. A, control side; B, before lesion; C, after lesion.
Figure 4.
Action potentials were recorded both before and after the spinal lower motor neuron lesion by using the single electrical stimulus to excite S2 DR in group B. The morphology of the tracing recorded on the left side was similar before and after lesion. The amplitude of the action potential on control side was significantly greater than that of the left side. A, control side; B, before lesion; C, after lesion.
Table 1.
Neural electrophysiological testing and urodynamic study in groups A and B before and after the spinal lower motor neuron lesion. In neural electrophysiological testing
| Group A |
Group B |
||||
|---|---|---|---|---|---|
| Left side | Control side | Left side | Control side | ||
| Neural electrophysiological testing (mV) | Before transection | 0.68 ± 0.11 | 1.21 ± 0.13** | 0.75 ± 0.10 | 1.30 ± 0.09** |
| After transection | 0.60 ± 0.08 | 0.67 ± 0.05 | |||
| Bladder pressure (cmH2O) | Before transection | 10.20 ± 1.33 | 19.38 ± 1.02* | 10.91 ± 1.22 | 18.67 ± 1.12* |
| After transection | 9.18 ± 1.22 | 10.00 ± 1.12 | |||
| Qmax (ml/second) | Before transection | 15.30 ± 2.03 | 23.49 ± 3.16* | 14.78 ± 1.06 | 21.68 ± 3.19* |
| After transection | 13.20 ± 1.63 | 13.77 ± 0.98 | |||
| Residual urine volume (ml) | Before transection | 21.00 ± 5.09 | 9.57 ± 3.26* | 20.67 ± 4.18 | 11.00 ± 1.79* |
| After transection | 23.14 ± 5.67 | 22.50 ± 3.83 | |||
*P < 0.05 vs. left side before and after the spinal lower motor neuron lesion. **P < 0.01 vs. left side before and after the spinal lower motor neuron lesion. In the measurement of bladder pressure, Qmax, and residual urine volume.
Urodynamic study
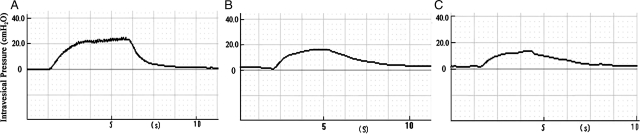
When train stimulation was applied to the left S2 DR before and after the spinal lower motor neuron lesion in group A, the mean elevation of intravesical pressure by artificial physiological reflex arc was 10.20 ± 1.33 and 9.18 ± 1.22 cmH2O, respectively (not significant). When the right S2 DR was stimulated before lesion, the mean elevation of intravesical pressure was 19.38 ± 1.02 cmH2O, which was significantly higher than that of the experimental side (Fig. 5) (P < 0.05). In group B when the left L7 DR was stimulated, the mean intravesical pressure elicited by artificial reflex arc was 10.91 ± 1.22 and 10.00 ± 1.12 cmH2O, respectively, before and after the spinal lower motor neuron lesion. The differences between them were not significant (P > 0.05). When the right S2 DR was stimulated before lesion, the mean elevation of intravesical pressure was 18.67 ± 1.12 cmH2O, obviously higher than that of the left side (Fig. 6) (P < 0.05). Comparing group A with group B before and after the spinal lower motor neuron lesion, the mean elevation of intravesical pressure on two experimental sides was similar (Table 1) (P > 0.05), and that was also similar on both control sides.
Figure 5.
Bladder pressures were recorded both before and after the spinal lower motor neuron lesion by train stimulation to S2 DR. Amplitude of the bladder pressure on left side was similar in group A before and after lesion. The amplitude of the bladder pressure on control side was significantly greater than that of the left side. A, control side; B, before lesion; C, after lesion.
Figure 6.
Bladder pressures were recorded both before and after the spinal lower motor neuron lesion by train stimulation to S2 DR. Amplitude of the bladder pressure on the left side was similar in group B before and after lesion. The amplitude of the bladder pressure on the control side was significantly greater than that on the left side. A, control side; B, before lesion; C, after lesion.
Following train stimulation of left S2 DR, the Qmax in group A was 15.30 ± 2.03 and 13.20 ± 1.63ml/second, respectively, before and after the spinal lower motor neuron lesion; and after train stimulation to right S2 DR before transection, the Qmax was 23.49 ± 3.16 ml/second. After stimulation of the control side the Qmax was remarkable higher than that of the experimental side. In group B after stimulation of left L7 DR the Qmax was 14.78 ± 1.06 and 13.77 ± 0.98 ml/second before and after lesion, and after stimulation of right S2 DR before lesion the Qmax was 21.68 ± 3.19 ml/second, which was also obviously higher than the left side. After the stimulation of the experimental sides in both groups before and after the spinal lower motor neuron lesion the induced Qmax was similar (Table 1) (P > 0.05), and that was also similar on the both control sides.
After left S2 DR-induced urination, the residual urine volume of the bladder in group A was 21.00 ± 5.09 ml before lesion and 23.14 ± 5.67 ml after lesion; after right S2 DR-induced urination, the residual urine volume was 9.57 ± 3.26 ml. In group B, after left L7-induced urination, the residual urine volume of the bladder was 20.67 ± 4.18 and 22.50 ± 3.83 ml before and after the spinal lower motor neuron lesion; after right S2 DR-induced urination, the residual urine volume was 11.00 ± 1.79 ml. The residual urine volume of the bladder in both groups was similar before and after the spinal lower motor neuron lesion (Table 1).
Urinary infection rates
After the spinal lower motor neuron lesion, paraplegia occurred in all the survival dogs. We recorded the surviving dogs' urinary infections for 3 months. The symptoms included frequent urination and cloudy urine or with light red. The sick dogs were found to be in poor spirit and were disinclined to walk. The urine examination revealed white blood cells: 5–10/HP or red blood cells: 2–8/HP. Infections were controlled after administration of 1–3 doses of norfloxacin 0.2 g. In group A three urinary infections were recorded during the 3 months and in group B, eight infections. By χ2 test, the incidence of urinary tract infection (UTI) in group A was significant lower than in group B (χ2 = 4.35, P < 0.05).
Discussion
After SCI, bladder dysfunction affects life quality adversely. Fortunately, some achievements have been made both experimentally and clinically in bladder reconstruction for this type of patients. Xiao et al. confirmed what had previously been reported, that an artificial bladder reflex arc with ‘skin–spinal cord center-bladder’ below the level of SCI could be used to recover controllable micturition in both animal and clinical models. 6–11 Zheng et al.9 and Lin et al.12,13 constructed the ‘Achilles’ tendon (or knee tendon)-bladder reflex arc' below the SCI plane to recover the bladder innervation. Their studies showed that the somatic motor neurons could grow into the autonomic nervous and control the bladder smooth muscle. The methods of artificial bladder reflex arc reconstruction were appropriate for patients whose SCI level was above the sacral nerve. Some complete reflex arc pathways (such as knee-jerk, achilles tendon reflex) still remained below the damage level. Thoracolumbar vertebral fracture and dislocation often led to sacral nerve and below nerve injury, leaving no complete reflex arc. For this reason, Lin et al.1 anastomosed the T11 VR and S2/3 VR above the level of SCI, retained T11 DR, and controlled urination via the triggered abdominal reflex arc. This method could recover the bladder voiding function partly, but this method did not reconstruct the sensation to the bladder. Bladder filling could not be sensed, and so micturition could not be triggered via the physiological sensation.
Vorstman et al.10 and Chuang et al.11 experimented in cats and rats. Spinal nerves were anastomosed on the bladder denervation side. They anastomosed the ventral and DRs of spinal nerves above the injury level and the ventral and the DRs of the sacral nerve below the plane of damage level, respectively. At the same time, they carried out the reconstruction of bladder motor−motor, sensory−sensory nerve function. After the axons have regenerated, the single stimulation to the anastomosed nerve can cause bladder contraction. The mean amplitude of contraction can reach 56% of the normal control.
Zheng et al.2 and Lin et al.3,4 reported anastomosing L5 VR and DR and S2 VR and DR, respectively, and reconstructing physiological urination reflex arc in atonic bladder after SCI. Their neural electrophysiological examinations show that in both VR and DR, the axons of neurons could grow into the distal ends, and the regenerative axons have the ability of conducting the nerve impulse; urodynamic examinations show that electrostimulation of left S2 DR could induce bladder contraction before and after SCI. Although it has been shown that all these reconstruction methods can induce bladder micturition, systematic comparison between the methods has not been conducted. Therefore, we reconstructed the two most important micturition reflex arcs and then established a beagle acute SCI model with S1 level as the highest level of injury to compare the effect of sensory input from above and below the lesions.
The main symptoms of atonic bladder are detrusor muscle weakness, sphincter relaxation, bladder compliance increase, and urinary retention with overflow incontinence. UTI is also a common problem among patients with SCI. The vast majority of people have bacteria in their bladder related to urine stasis. Normally, urine is sterile. In UTI, bacteria travel to the urethra and then to the bladder, resulting in urethritis and cystitis. A severe infection that is not treated may then travel further up the ureters and infect the kidneys.
Our results showed that for the same electric stimulation of the left S2 DR (group A) or L7 DR (group B), action potentials could be recorded in the S2 VR, and the bladder contraction pressure was increased. The differences between the action potential values and the bladder pressure in two groups were not significant. Compared with the control side, the experimental side had lower action potential values and bladder pressure. Our results were similar to those by Chun-Lin Hou and Lin et al. Following the train stimulation of left DR, the Qmax was recorded, and no significant difference was found between the left sides in the two groups. The residual urine volume was measured after stimulation-induced urination, and there was no significant difference between the left sides in two groups. These data indicate that the two methods could both result in partial recovery of bladder micturition function and that the effect of recovery was almost the same. However, the incidence of urinary infections in group A was significantly lower than in group B.
In group B the method of reconstructing artificial reflex arc did not rebuild the sensory nerve, and so animals could not sense bladder filling. Only an active stimulation that activated the somatic nerve-parasympathetic nerve reflex could result in urination. However, the animals displayed frequent urination and dripping, and more frequent urinary tract infections. In group A the artificial physiological reflex arc reconstruction method established both the sensory and the motor nerve root of the bladder; the possible mechanism of the reconstruction micturition may be: the bladder filling stimulates its sensory nerves, the generated nerve impulse by S2 DR to reach across the anastomosis to L7 DR. Then the nerve impulse is received and conducted across the anastomosis by L7 VR to S2 VR and finally causes detrusor contraction, just like inducing bladder micturition via complete physiological micturition reflex arc.2 When bladder pressure reaches 5–15 cmH2O, bladder sensory nerves can produce impulses which can trigger the reflex arc, resulting in urination. This is may be a better approach to control the uroclepsia and UTI complications of atonic bladder after paraplegia.
Conclusions
In conclusion, current data clearly demonstrate that both reconstructing artificial micturition reflex and physiological micturition reflex can induce bladder urination. The comparative incidence of UTI is a preliminary observation that warrants additional study. Future studies will describe in more detail the urodynamic differences between two methods and measure degree of incontinence and determine the factors involved in micturition.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant #30973058), Jiangsu Province Nature Science Foundation (BE2010743), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU (No. IRT-015), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- 1.Lin H, Hou C, Chen A, Xu Z. Innervation of reconstructed bladder above the level of spinal cord injury for inducing micturition by contractions of the abdomen-to-bladder reflex arc. Neurosurgery 2010;66(5):948–52 [PubMed] [Google Scholar]
- 2.Zheng X, Hou C, Chen A. Experimental study on reconstruction of physiological reflex arc after medullary cone injury in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2008;22(4):426–30 [PubMed] [Google Scholar]
- 3.Lin H, Hou C, Zheng X, Xu Z, Wang J. An experimental study of establishment of physiological reflex arc after conus medullary injury in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2008;22(6):719–23 [PubMed] [Google Scholar]
- 4.Lin H, Hou C, Zhen X. Bypassing spinal cord injury: surgical reconstruction of afferent and efferent pathways to the urinary bladder after conus medullaris injury in a rat model. J Reconstr Microsurg 2008;24(8):575–81 [DOI] [PubMed] [Google Scholar]
- 5.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 2005;289(3):F604–10 [DOI] [PubMed] [Google Scholar]
- 6.Xiao CG, de Groat WC, Godec CJ, Dai C, Xiao Q. ‘Skin-CNS-bladder’ reflex pathway for micturition after spinal cord injury and its underlying mechanisms. J Urol 1999;162(3 Pt 1):936–42 [DOI] [PubMed] [Google Scholar]
- 7.Xiao CG, Du MX, Dai C, Li B, Nitti VW, de Groat WC. An artificial somatic-central nervous system-autonomic reflex pathway for controllable micturition after spinal cord injury: preliminary results in 15 patients. J Urol 2003;170(4 Pt 1):1237–41 [DOI] [PubMed] [Google Scholar]
- 8.Xiao CG. Reinnervation for neurogenic bladder: historic review and introduction of a somatic-autonomic reflex pathway procedure for patients with spinal cord injury or spina bifida. Eur Urol 2006;49(1):22–8 [DOI] [PubMed] [Google Scholar]
- 9.Zheng XY, Hou CL, Zhong HB, Xu RS, Chen AM, Xu Z, et al. Reconstructed bladder innervation below the level of spinal cord injury: the knee-tendon to bladder artificial reflex arc. J Spinal Cord Med 2009;32(1):79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vorstman B, Schlossberg SM, Kass L, Devine CJ., Jr Urinary bladder reinnervation. J Urol 1986;136(4):964–9 [DOI] [PubMed] [Google Scholar]
- 11.Chuang DC, Chang PL, Cheng SY. Root reconstruction for bladder reinnervation: an experimental study in rats. Microsurgery 1991;12(4):237–45 [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Hou CL, Zhong G, Xie Q, Wang S. Reconstruction of reflex pathways to the atonic bladder after conus medullaris injury: preliminary clinical results. Microsurgery 2008;28(6):429–35 [DOI] [PubMed] [Google Scholar]
- 13.Lin H, Hou C, Zhen X, Xu Z. Clinical study of reconstructed bladder innervation below the level of spinal cord injury to produce urination by Achilles tendon-to-bladder reflex contractions. J Neurosurg Spine 2009;10(5):452–7 [DOI] [PubMed] [Google Scholar]