Abstract
Background
Ossification of the posterior longitudinal ligament (OPLL) in the thoracic spine produces myelopathy. This is often progressive and is not affected by conservative treatment. Therefore, decompressive surgery is usually chosen.
Objective
To conduct a stress analysis of the thoracic OPLL.
Methods
The three-dimensional finite element spinal cord model was established. We used local ossification angle (LOA) for the degree of compression of spinal cord. LOA was the medial angle at the intersection between a line from the superior posterior margin at the cranial vertebral body of maximum OPLL to the top of OPLL with beak type, and a line from the lower posterior margin at the caudal vertebral body of the maximum OPLL to the top of OPLL with beak type. LOA 20°, LOA 25°, and LOA 30° compression was applied to the spinal cord in a preoperative model, the posterior decompressive model, and a model for the development of kyphosis.
Results
In a preoperative model, at more than LOA 20° compression, high stress distributions in the spinal cord were observed. In a posterior decompressive model, the stresses were lower than in the preoperative model. In the model for development of kyphosis, high-stress distributions were observed in the spinal cord at more than LOA 20° compression.
Conclusions
Posterior decompression was an effective operative method. However, when the preoperative LOA is more than 20°, it is very likely that symptoms will worsen. If operation is performed at greater than LOA 20°, then correction of kyphosis by fixation of instruments or by forward decompression should be considered.
Keywords: Myelopathy, Finite-element method, Local ossification angle, Ossification of the posterior longitudinal ligament, Spine surgery, Thoracic spinal cord, Kyphosis
Introduction
Ossification of the posterior longitudinal ligament (OPLL) in the thoracic spine produces myelopathy due to anterior spinal cord compression. This is often progressive and is not affected by conservative treatment. Therefore, decompressive surgery is usually chosen. Numerous publications have described various operative methods resulting in variable postoperative outcomes that are not always satisfactory. There is little discussion in these papers on the results of preoperative evaluation. Matsuyama et al. classified thoracic OPLL into two groups according to the type of ossification: continuous or mixed-type OPLL with a flat shape (flat type) or segmental OPLL with sharp protrusions located behind the disc spaces (beak type). In this report, cases with neurologic deterioration were patients with OPLL of the beak type.1
We used a three-dimensional finite-element method (3D-FEM) to analyze the stress distribution on the spinal cord under three compression levels of thoracic OPLL with a rigid, narrow trapezium body (beak type). These levels correspond to the preoperative model, the posterior decompressive model, and the model for development of kyphosis. For the local ossification angle (LOA), we used the degree of compression of the spinal cord employed by Ito et al.2 to analyze postoperative results.
Methods
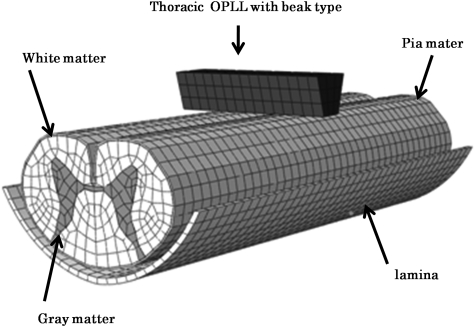
The ABAQUS (Valley Street, Providence, RI, USA) 6.9-3 standard finite-element package was used for FEM simulation. The 3D-FEM spinal cord model for this study consisted of gray matter, white matter, and pia mater. In order to simplify calculation in the model, the denticulate ligament, dura, and nerve root sheaths were not included. The pia mater was included since it has been demonstrated that spinal cord with and without pia mater shows significantly different mechanical behavior.3 The spinal cord was assumed to be symmetrical about the mid-sagittal plane, such that only half the spinal cord required reconstruction and the whole model could be integrated by mirror image. The model simulated thoracic OPLL with the beak type. The lamina model was established by measuring thoracic computed tomography myelographs. The rigid narrow trapezium body was used to simulate thoracic OPLL with the beak type (Fig. 1). Thoracic OPLL of the beak type was located at the longitudinal center of the spinal cord.
Figure 1.
The three-dimentional finite-element model of spinal cord, lamina, and thoracic OPLL with beak type.
The spinal cord consists of three distinct materials: white matter, gray matter, and pia mater. The mechanical properties (Young's modulus and Poisson's ratio) of the gray and white matter were determined using data obtained by the tensile stress strain curve and stress relaxation under various strain rates.4 The mechanical properties of pia mater were obtained from the literature.5 The mechanical properties of lamina and OPLL were stiff enough for the spinal cord to be pressed. Based on the assumption that no slippage occurs at the interfaces of white matter, gray matter, and pia mater, these interfaces were glued together. There are no data on the friction coefficient between bone and spinal cord. The contact between lamina and spinal cord was assumed to be frictionless due to the lubricating effect of fluid. The coefficient of friction between OPLL with beak type and the spinal cord was glue at the contact interfaces and frictionless next to the part in contact. To simulate the axial injury model of the spinal cord, the nodes at the bottom and top of the spinal cord model were constrained in all directions. The spinal cord, lamina, and OPLL with beak type were symmetrically meshed with 20-node elements. The total number of isoperimetric 20-node elements was 11 095 and the total number of nodes was 64 724.
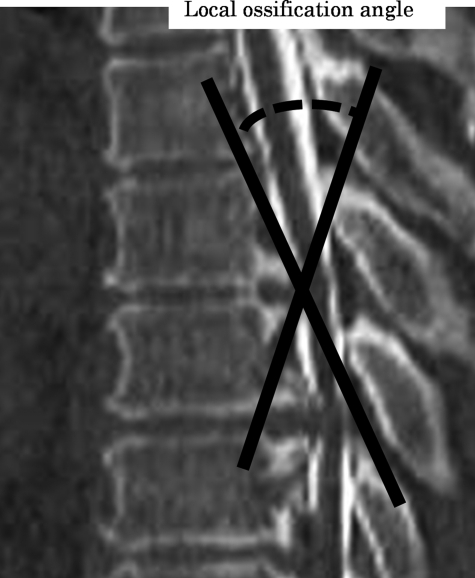
For the preoperative model, lamina was fixed in all directions to support the spinal cord from behind and then anterior static compression was applied to the spinal cord by OPLL with the beak type. For the posterior decompressive model, the lamina was shifted back so as not to contact the spinal cord under the application of anterior static compression. For the developmental model of kyphosis, the spinal cord was shifted forward approximately 50° to both lateral sides from the posterior center of the spinal cord. Tokuhashi et al. evaluated the effectiveness of spinal decompression in the presence or absence of echo-free space (i.e. space between the ventral side of the spinal cord and OPLL) using an intra-operative ultrasonogram. The 50° angle was used to measure the axial view of spinal cord in negative echo-free space, which was worsened by paralysis.6 The LOA used in this analysis was defined as follows: the medial angle at the intersection between a line from the superior posterior margin at the cranial vertebral body of maximum OPLL to the top of OPLL with beak type, and a line from the lower posterior margin at the caudal vertebral body of the maximum OPLL to the top of OPLL with beak type (Fig. 2). Anterior static compression was applied to the spinal cord by OPLL with the beak type. First, in the preoperative model, LOA 20° compression was applied to the spinal cord. This was followed by LOA 25° and LOA 30° compression. Second, using LOA 20°, LOA 25°, and LOA 30° compression to represent the posterior decompressive model, lamina was shifted back so as not to contact the spinal cord. Finally, under LOA 20°, LOA 25°, and LOA 30° compression to represent the model for development of kyphosis, the posterior surface of the spinal cord was shifted forward. In total, nine different compression combinations ware evaluated. In each cross section, the average von Mises stress was recorded.
Figure 2.
The LOA was as follows: LOA was the medial angle at the intersection between a line from the superior posterior margin at the cranial vertebral body of maximum OPLL to the top of OPLL with beak type and a line from the lower posterior margin at the caudal vertebral body of the maximum OPLL to the top of OPLL with beak type.
Results
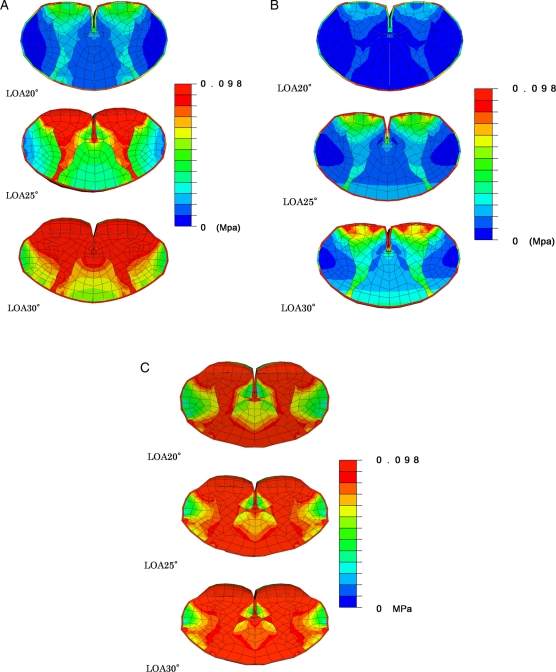
In the preoperative model, stresses were very low using LOA 20° compression. These were confined to gray matter and to anterior funiculus. At LOA 25° compression, the stresses on gray matter, anterior funiculus, lateral funiculus, and posterior funiculus all increased. At LOA 30° compression, high-stress distributions were observed in the spinal cord pressed by OPLL of the beak type (Fig. 3A).
Figure 3.
Cross-sectional view of stress distributions after static compression corresponding to LOA 20°, 25°, and 30° of the spinal cord was applied to thoracic OPLL with beak type in the preoperative model (A), posterior decompressive model (B), and model for the development of kyphosis (C).
In the posterior decompressive model, the stresses from LOA 20° compression were lower than stresses from LOA 20° in the preoperative model and were confined to the anterior funiculus. At LOA 25° compression, the stresses increased on gray matter and the anterior funiculus. At LOA 30° compression, stress distributions were observed in gray matter, anterior funiculus, and posterior funiculus (Fig. 3B).
In the model for development of kyphosis, the stresses on gray matter, anterior funiculus, and posterior funiculus increased using LOA 20° compression. At LOA 25° and LOA 30° compression, high-stress distributions were observed in the spinal cord pressed by OPLL of the beak type (Fig. 3C).
Discussion
Conservative treatment for myelopathy associated with thoracic OPLL is mostly ineffective. Surgical treatments are therefore usually chosen. However, since physiological kyphosis is present in the thoracic spine, it is sometimes difficult to obtain good results for decompression of the spinal cord through laminectomy alone via the posterior approach. Yamasaki et al. reported a case that deteriorated neurologically following laminectomy. The neurological status improved upon stabilization of the fusion using instruments in a second operation.7 Decompression via the anterior approach is also technically difficult because of adhesion between the dura and ossified ligament, a narrow visual field requiring a longitudinal section of sternum in the upper thoracic area, and the necessity for an antero-lateral approach by transversectomy of the middle-lower thoracic region.8–11 No standard surgical procedure was established in these reports. The poor surgical outcome can be explained by several factors: (a) the presence of physiologic kyphosis in the upper and middle thoracic spine may impair the effects of posterior decompression; (b) the thoracic spinal cord is the watershed area of spinal circulation and is therefore vulnerable to intra-operative ischemic damage; (c) thoracic kyphosis can readily progress by incorrect positioning of the patient during operation or by laminectomy.1
Although various operative methods and postoperative results have been reported, only a few authors have inferred the likely prognosis following preoperative evaluation. Matsuyama et al. reported that cases with neurologic deterioration after laminectomy were patients with OPLL of the beak type. In such patients, a subtle alteration in spinal alignment during posterior decompression procedures can increase spinal cord compression, leading to the deterioration of symptoms.1 Therefore, a potential increase in kyphosis following laminectomy should be avoided by fixation with a temporary rod. However, the beak type or flat types are visual classifications. Ito et al. reported that more or less than LOA 28° gave good postoperative results. They recommended anterior decompression for cases less than LOA 28°, or posterior decompression with fixation of instrumentation when LOA was more than 28°.2
Based on this prior knowledge, we conducted stress analysis using LOA of the thoracic OPLL with beak type in a preoperative model, a postoperative model, and a model for the development of kyphosis. We analyzed changes in stress using FEM, and tested thoracic OPLL with beak type.
The overall goal of this study was to develop a 3D-FEM spinal cord model that simulates the clinical situation. Similar to previous studies by Kato et al.12–14 and Xin-Feng, Li et al.,15–16 bovine spinal cord was used in the model for the current analysis since it was impossible to obtain fresh human spinal cord. The mechanical properties of spinal cord used in our study were similar to earlier reports.4,5 Li and Dai noted that it was reasonable to make use of the mechanical properties of bovine spinal cord because the brain and spinal cord of cattle and humans show similar injury changes.15 For the purpose of this study we also assumed the mechanical properties of spinal cord from the two species were similar. Cecilia et al.3 reported on the division of spinal cord into pia mater and, white and gray matter. These workers showed that the presence of pia mater had a significant effect on spinal cord deformation. Interestingly, our results from the preoperative model and the model for development of kyphosis showed that stress at the center of the spinal cord increased, thus confirming that pia mater was needed to simulate the clinical situation.
When LOA was 20° in the preoperative model, intra-spinal stress began to increase. When LOA was 25° in this model, the stress to gray matter and anterior funiculus also increased. At LOA of 30°, the stress to gray matter, anterior funiculus, lateral funiculus, and posterior funiculus increased even further. From these results, we conclude that symptoms may be aggravated when LOA is greater than 20°.
In the posterior decompressive model, the intra-spinal stress decreased. Even when LOA was 30°, stress increased slightly in the anterior horn, anterior funiculus, and posterior funiculus. From this stress analysis, we conclude that posterior decompression was an effective operative method. If the vertebral body is fixed in this situation, stable decompression is obtained. We assume this posterior decompressive model corresponds to the model representing fixation of instruments.
In the model for development of kyphosis, even when LOA was 20°, stress increased in the anterior horn, anterior funiculus, posterior horn, and posterior funiculus. When LOA was 25°, high-stress distributions were observed in the spinal cord. From this analysis we conclude that when LOA is more than 20°, the increased stress leads to instability that can favor the development of kyphosis and result in the progression of paralysis.
Conclusion
We conducted the stress analyses using LOA of the thoracic OPLL with beak type in a preoperative model, postoperative model, and a model for development of kyphosis.
When the preoperative LOA is more than 20°, it is very likely that symptoms will worsen. If operation is performed at greater than LOA 20°, then correction of kyphosis by fixation of instruments or by forward decompression should be considered.
References
- 1.Matsuyama Y, Yoshihara H, Tsuji T, Sakai Y, Yukawa Y, Nakamura H. Surgical outcome of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine: implication of the type of ossification and surgical options. J Spinal Disord Tech 2005;18:492–7 [DOI] [PubMed] [Google Scholar]
- 2.Ito K, Yukawa Y, Horie Y, Kato F. Surgical treatment for ossification of posterior longitudinal ligament in thoracic spine: Influence of local ossification angle. Rinsyo Seikeigeka (Japanese) 2008;43:539–42 [Google Scholar]
- 3.Cecilia P, Jon S, Richard M. The importance of fluid-structure interaction in spinal trauma models. J Neurotrauma 2011;28:113–25 [DOI] [PubMed] [Google Scholar]
- 4.Ichihara K, Taguchi T, Sakuramoto I, Kawano S, Kawai S. Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg 2003;993 suppl:278–85 [DOI] [PubMed] [Google Scholar]
- 5.Tunturi AR. Elasticity of the spinal cord, pia and denticulate ligament in the dog. J Neurosurg 1978;48:975–9 [DOI] [PubMed] [Google Scholar]
- 6.Tokuhashi Y, Matsuzaki H, Oda H, Uei H. Effectiveness of posterior decompression for patients with ossification of the posterior longitudinal ligament in the thoracic spine: usefulness of the ossification-kyphosis angle on MRI. Spine 2006;31:E26–30 [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki M, Okawa A, Koda M, Goto S, Minami S, Moriya H. Transient paraparesis after laminectomy for thoracic myelopathy due to ossification of posterior longitudinal ligament. Spine 2005;30:343–6 [DOI] [PubMed] [Google Scholar]
- 8.Fujimura Y, Nishi Y, Nakamura M, Toyama Y, Suzuki N. Long-term follow up study of anterior decompression and fusion for thoracic myelopathy resulting from ossification of the posterior longitudinal ligament. Spine 1997;22:305–11 [DOI] [PubMed] [Google Scholar]
- 9.Tomita K, Kawahara N, Baba H, Kikuchi Y, Nishimura H. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine 1990;15:1114–20 [DOI] [PubMed] [Google Scholar]
- 10.Tsuzuki N, Hirabayashi S, Abe R, Saiki K. Staged spinal cord decompression through posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine 2001;26:1623–30 [DOI] [PubMed] [Google Scholar]
- 11.Yonenobu K, Korkusuz F, Hosono N, Ono K. Lateral rhachotomy for thoracic spinal lesions. Spine 1990;15:1121–5 [DOI] [PubMed] [Google Scholar]
- 12.Kato Y, Kataoka H, Ichihara K, Imajo Y, Yaji K, Kawano S, et al. Biomechanical study of cervical flexion myelopathy using a three-dimensional finite element method. J Neurosurg Spine 2008;8(5):436–41 [DOI] [PubMed] [Google Scholar]
- 13.Kato Y, Kanchiku T, Imajo Y, Ichihara K, Kawano S, Hamanaka D, et al. Flexion model simulating spinal cord injury without radiographic abnormality in patients with ossification of the longitudinal ligament: the influence of flexion speed on the cervical spine. J Spinal Cord Med 2009;32(5):555–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato Y, Kanchiku T, Imajo Y, Kimura K, Ichihara K, Kawano S, et al. Biomechanical study of the effect of the degree of static compression of the spinal cord in ossification of the posterior longitudinal ligament. J Neurosurg Spine 2010;12:301–5 [DOI] [PubMed] [Google Scholar]
- 15.Xin-Feng Li, MSc T, Li-Yang Dai. Three-dimensional finite element model of the cervical spinal cord. Spine 2009;34:1140–7 [DOI] [PubMed] [Google Scholar]
- 16.Xin-Feng Li, MSc T, Li-Yang Dai. Acute central cord syndrome. Spine 2010;35:E955–64 [DOI] [PubMed] [Google Scholar]