Abstract
Francisella tularensis is pathogenic for many mammalian species including humans, causing a spectrum of diseases called tularemia. The highly virulent Type A strains have associated mortality rates of up to 60% if inhaled. An attenuated live vaccine strain (LVS) is the only vaccine to show efficacy in humans, but suffers several barriers to licensure, including the absence of a correlate of protection. An immunoproteomics approach was used to survey the repertoire of antibodies in sera from individuals who had contracted tularemia during two outbreaks and individuals from two geographical areas who had been vaccinated with NDBR Lot 11 or Lot 17 LVS. These data showed a large overlap in the antibodies generated in response to tularemia infection or LVS vaccination. A total of seven proteins were observed to be reactive with 60 % or more sera from vaccinees and convalescents. A further four proteins were recognised by 30–60 % of the sera screened. These proteins have the potential to serve as markers of vaccination or candidates for subunit vaccines.
Keywords: Francisella tularensis, live vaccine strain, vaccine, immunoproteomics, tularemia
INTRODUCTION
Tularemia is a disease in primates caused by the Gram-negative facultative intracellular bacterium, Francisella tularensis. F. tularensis has received increasing attention in the last decade due to its potential for use as a bioweapon (Kortepeter and Parker, 1999; Dennis et al. 2001b). Several subspecies exist, with the most clinically relevant subspecies denoted holarctica and tularensis, commonly known as Type B and A strains, respectively (Sjostedt, 2001). The subspecies tularensis (Type A) is endemic only to North America. Mortality rates of up to 60% have been reported for untreated human cases of disseminated infection caused by Type A strains of the pathogen (DIENST, Jr., 1963). The subspecies holarctica (Type B), endemic to both Europe and North America, is associated with lower mortality rates. Type B strains are responsible for almost all European cases of tularemia (Sjostedt, 2007).
A live vaccine strain (LVS) was derived in the 1950’s from a Soviet strain type B, S15, and protects humans to some degree against subsequent exposure to Type A strains of the pathogen (Hornick and Eigelsbach, 1966). Human LVS vaccination studies were conducted under the Operation Whitecoat (OW) program in the 1950’s. These data showed that LVS administered by scarification was 25–100% effective against aerosol challenge with SCHU S4 (Hornick and Eigelsbach, 1966). All vaccinees were shown to seroconvert to an undefined set of Francisella antigens, but no immunologic correlation was established with the protective status of the host. When LVS replaced killed bacteria as the vaccine at the United States Army Medical Research Institute for Infectious Diseases (USAMRIID), the incidence of respiratory infections among at-risk personnel was significantly reduced (Burke, 1977; Eigelsbach et al. 1967).
Due to renewed concerns regarding the threat of bioterrorism, there has been an increased interest in licensing a tularemia vaccine for general use. However, both the absence of a correlate of protection and the unknown mechanisms of attenuation are significant barriers to LVS licensure. Recent studies using the murine model of tularemia show that adaptive host defense against F. tularensis is likely mediated by both cell mediated immunity (CMI) and humoral immunity (Tarnvik, 1989; Elkins et al. 2003; Kirimanjeswara et al. 2008). Although CMI is thought to be the most essential mechanism in host defense against Type A Francisella, specific antibody responses are mounted during natural Francisella infections or following vaccination (Saslaw and CARHART, 1961; Carlsson et al. 1979; Viljanen et al. 1983; Dennis et al. 2001). The humoral immune response will therefore serve as a facile means of screening sera for markers of successful LVS vaccination.
Ethical considerations prevent a repeat of human LVS vaccine efficacy studies, such as those conducted under OW. Instead, we sought to compare the repertoire of antibodies generated by humans in response to natural tularemia infection with Type A or Type B strains and vaccination with NDBR lot 11 LVS or DVC lot 17 LVS (escalating dose study). Patients that recover from types A and B Francisella infections are rarely reported to show signs of disease following a second exposure, and therefore could be considered a group that is protected from further challenge. This affords the opportunity to compare the repertoire of antibodies between infected, but presumably protected individuals, and vaccinated volunteers whose protective status is unknown.
MATERIALS AND METHODS
Sera used in this study
Four distinct collections of human sera were used in this study, including sera from two groups of human tularemia patients, LVS vaccinated laboratory personnel and clinical trial subjects immunized with LVS (Table 1). For each study group, informed consent was obtained by the study directors prior to serum collection.
Table 1.
Characteristics of sera from study cohorts
| Type | Sample reference number | Date of diagnosis, if known | Date or length of time post infection/vacci nation serum drawn | Route | Serum pairing reference number | Origin |
|---|---|---|---|---|---|---|
| Type B convalescent | 1651 | Unknown | 42 months | Intradermal | NA | Sweden |
| Type B convalescent | 1653 | Unknown | 17 months | Intradermal | NA | Sweden |
| Type B convalescent | 1657 | Unknown | 44 months | Intradermal | NA | Sweden |
| Type B convalescent | 1661 | Unknown | 18 months | Intradermal | NA | Sweden |
| Type B convalescent | 1663 | Unknown | 18 months | Intradermal | NA | Sweden |
| Type B convalescent | 1671 | Unknown | 18 months | Intradermal | NA | Sweden |
| Type B convalescent | 1673 | Unknown | 18 months | Intradermal | NA | Sweden |
| Type B convalescent | 1679 | Unknown | 42 months | Intradermal | NA | Sweden |
| Type B convalescent | 1683 | Unknown | 18 months | Intradermal | NA | Sweden |
| Type B convalescent | 1687 | Unknown | 17 months | Intradermal | NA | Sweden |
| Type B convalescent | 1691 | Unknown | 30 months | Intradermal | NA | Sweden |
| Type B convalescent | 1693 | Unknown | 17 months | Intradermal | NA | Sweden |
| Type A convalescent | f0703 | Unknown | 08/02/2006 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0709 | 2005 | 22/02/2006 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0711 | Unknown | 24/05/2006 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0715 | Unknown | 14/03/2006 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0722 | Unknown | 04/04/2005 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0723 | Unknown | 04/04/2005 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0753 | 2006 | 21/11/2007 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0754 | 2007 | 21/11/2007 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0756 | 2006 | 21/12/2007 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0757 | 2006 | 21/12/2007 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0758 | 2007 | 05/01/2008 | Presumed inhalation | NA | NE, USA |
| Type A convalescent | f0759 | 1999 | 16/01/2008 | Presumed inhalation | NA | NE, USA |
| NDBR Lot 11 Vaccinee | 201 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Vaccinee | 202 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Vaccinee | 203 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Vaccinee | 204 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Vaccinee | 205 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Vaccinee | 206 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Vaccinee | 207 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Vaccinee | 208 | Not applicable | 42 days | Scarification | NA | Sweden |
| NDBR Lot 11 Control | 111 | Not applicable | Control | Scarification | NA | Sweden |
| NDBR Lot 11 Control | 112 | Not applicable | Control | Scarification | NA | Sweden |
| NDBR Lot 11 Control | 113 | Not applicable | Control | Scarification | NA | Sweden |
| NDBR Lot 11 Control | 114 | Not applicable | Control | Scarification | NA | Sweden |
| NDBR Lot 11 Control | 115 | Not applicable | Control | Scarification | NA | Sweden |
| DVC Lot 17 Vaccinee | 200162501 | Not applicable | 2 | Scarification (109 cfu/mL) | 02855 | USA |
| DVC Lot 17 Vaccinee | 200161436 | Not applicable | 10 | Scarification (109 cfu/mL) | 02855 | USA |
| DVC Lot 17 Vaccinee | 200139305 | Not applicable | 2 | Scarification (105 cfu/mL) | 02816 | USA |
| DVC Lot 17 Vaccinee | 200162408 | Not applicable | 10 | Scarification (105 cfu/mL) | 02816 | USA |
| DVC Lot 17 Vaccinee | 200162457 | Not applicable | 2 | Scarification (109 cfu/mL) | 02858 | USA |
| DVC Lot 17 Vaccinee | 200161482 | Not applicable | 10 | Scarification (109 cfu/mL) | 02858 | USA |
| DVC Lot 17 Vaccinee | 200161191 | Not applicable | 10 | Scarification (107 cfu/mL) | 02840 | USA |
| DVC Lot 17 Vaccinee | 200162938 | Not applicable | 10 | Scarification (107 cfu/mL) | 02838 | USA |
| DVC Lot 17 Vaccinee | 200139359 | Not applicable | 2 | Scarification (105 cfu/mL) | 02807 | USA |
| DVC Lot 17 Vaccinee | 200162272 | Not applicable | 10 | Scarification (105 cfu/mL) | 02807 | USA |
| DVC Lot 17 Vaccinee | 200139603 | Not applicable | 2 | Scarification (107 cfu/mL) | 02832 | USA |
| DVC Lot 17 Vaccinee | 200162794 | Not applicable | 10 | Scarification (107 cfu/mL) | 02832 | USA |
| DVC Lot 17 Vaccinee | 200208090 | Not applicable | 10 | Scarification (103 cfu/mL) | 02875 | USA |
The Type B convalescent sera were from patients diagnosed with tularemia in Sweden, where the disease is considered endemic. In total, sera were available from 12 tularemia patients and 3 healthy individuals with no history of tularemia. The Swedish patients were infected with type B strains and the route of infection for the majority of these patients was intradermal.
The Type A convalescent sera were a subset from a total of 59 subjects with tularemia reported on Martha’s Vineyard between 2000 and 2006. Approximately 60 % of cases were thought to be due to inhalation of the bacterium (Feldman et al. 2003; Matyas et al. 2007). In this study, sera from the first physician visit were available from 12 confirmed Type A tularemia patients.
Two sets of sera from separate human LVS vaccinations were studied. In the first set, at- risk laboratory workers in Sweden were immunized with LVS NDBR101 Lot 11. The set was comprised of five sets of paired pre- and post-vaccination samples and an additional three post-vaccination serum samples. NDBR lot 11 was prepared as per the vial instructions. Briefly, the vaccine preparation was reconstituted in 2.0 ml of water to give a concentration of 2.5 × 109 CFU/ml. A droplet of approximately 20 μL (containing ~ 5 × 107 CFU) was administered by scarification using a bifurcated needle to puncture the skin.
The second human vaccinee serum set was from subjects vaccinated with DVC lot 17 LVS (Pasetti et al. 2008), obtained from a Phase I clinical trial carried out at the Baylor College of Medicine, Houston, TX. The vaccine used was manufactured at Cambrex Bio Science, Baltimore, MD, under contract with DynPort Vaccine Company LLC (DVC). The vaccine was administered as described previously (El Sahly et al. 2009). Briefly, the lyophilized vaccine was reconstituted with 0.25 ml of sterile water for injection yielding a vaccine concentration of 1.6×109 CFU/ml. The study design and administration of the vaccine were described in detail previously (El Sahly et al. 2009), with dosages of 103, 105, 107 and 109 CFU/ml administered by scarification with a bifurcated needle. Five paired sera (pre- and 42 days post-vaccination) and three unpaired sera (post-vaccination) were provided.
Two-dimensional polyacrylamide gel electrophoresis (2D PAGE) Western blotting
The protein antigen used in Western blotting experiments was an LPS deficient mutant, SCHU S4 ΔwbtI, to prevent interfering immunoreactivity towards the O-antigen of LPS. Bacterial proteins were extracted as described previously, using a method that solubilizes a broad range of cytoplasmic and membrane associated proteins (Twine et al. 2005). Briefly, the SCHU S4 ΔwbtI mutant was grown in modified Cysteine Heart Agar (CHA) for 24–36 h at 37 °C within a BioSafety (BS) Level 3 containment facility. Bacteria were harvested from plates and lysed using a solution of 5 M urea, 2 M thiourea, 1% DTT, 4% CHAPS, 0.5% ASB-14, as described in our earlier work (Twine et al. 2005). Proteins were separated using immobilized pH gradient strips (IPG), linear pH 4–7, 17 cm (Bio-Rad, Hercules, CA) or linear pH 6–11 (GE Healthcare, Piscataway, NJ), using 100 μg of protein/gel. Much of our previous work with murine sera showed few if any proteins with a pI <4 or >7 to be reactive with sera from LVS immunized BALB/c or C57/BL6 mice. Given the limited amounts of sera available from clinical samples, therefore, the analyses in this study were initially confined to pH 4–7 (Twine et al. 2010). One serum sample from each study set was also screened against antigen separated in the pH range 6–11. Immunoblotting was carried out according to methods previously published by others (Mansfield, 1995) and described in our own work (Twine et al. 2010).
Identification of immunoreactive proteins
Protein spots corresponding to identified areas of immunoreactivity on Western blots were excised from protein stained 2D-PAGE gels and tryptically digested, as described previously (Twine et al. 2010; Twine et al. 2006). The in-gel digests were analyzed by nano-liquid chromatography-MS/MS (Twine et al. 2010). The peaklist files of MS2 spectra of the excised protein spots were searched against a database (2008.03.10) with 11947 entries consisting of the NCBI reference genomes for seven strains of Francisella (NCBI ids: NC_006570, NC_007880, NC_008245, NC_008369, NC_008601, NC_009257, NC_009749) using MASCOT™ (version 2.2.03, Matrix Science, London, UK) for protein identification, as detailed previously (Twine et al. 2010).
RESULTS
Human serum antibody response is directed towards a relatively small number of proteins
The details of sera used in this study are shown in Table 1. Over 95 % of the type B tularemia patients, had the ulceroglandular form of tularemia. Sera from each of twelve type B tularemia patients and from three individuals with no history of tularemia were screened by 2D-Western blotting in the pH range 4–7. Representative Western blots are shown in Figure 1a and b (The complete series of Western blots are shown in Figure S1), with a total of 31 identified immunoreactive proteins (Table 2, Figure 1i). Of the three control sera, drawn from volunteers with no history of tularemia, one serum showed low reactivity with the Chaperonin GroEL. Sera from all 12 tularemia infected individuals showed intense reactivity with the Chaperonin GroEL (FTT_1696). The protein dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex (FTT_0077) was immunoreactive with 11 of the 12 patient sera screened but none of the control sera. Due to limited volumes or sera, a single serum sample from patient 1671 was screened against antigen separated in the pH range 6–11 (Figure 2a). Some background interference was observed in the low MW region of the blot, and a single area of immunoreactivity was observed in the basic high molecular weight region. This protein was not able to be identified and is indicated by arrows in Figure 2e. Further to this, the proteins 50S ribosomal protein L1/L12 (FTT_0143), hypothetical membrane protein (FTT_1778c), and acetyl CoA carboxylase (FTT_0472) were immunoreactive with 8 or more patient sera screened and with none of the control sera. Of note, the proteins FTT_1778c and FTT_0143 focus to discrete spots on 2D-PAGE within close proximity of one another. In some cases the immunoreactivity of these proteins was intense and it was not always possible to discern which individual protein was immunoreactive. In these cases, we have indicated that both proteins were immunoreactive and reported the overall intensity of immunoreactivity. These data are also represented visually as a matrix of immunoreactive proteins, as shown in Figure 3a, with the shading indicating the comparative intensity of the observed immunoreactivity for each spot, as measured by densitometry.
Figure 1. Representative Two-dimensional Western blots probed with sera from tularemia patients and LVS vaccinees.
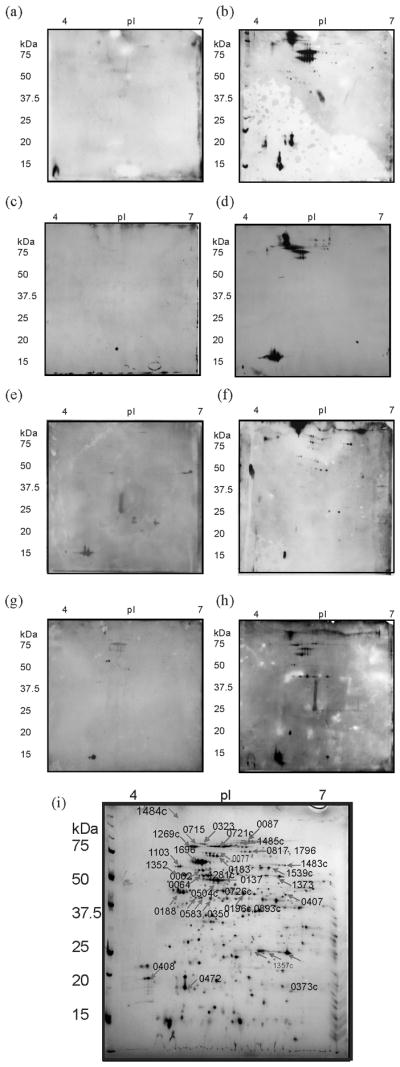
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera as follows (a) Control 1, (b) Type B tularemia patient serum number 1671, (c) Type A tularemia patient serum number MV758, (d) Type A tularemia patient serum number MV756, (e) NDBR lot 11 LVS vaccinee control serum number 110d0, (f) NDBR lot 11 LVS vaccinee day 42 post-vaccination serum number 201d42, (g) DVC lot 17 LVS vaccine pre-vaccination serum number 200139603 (paired id 02832), (h) DVC lot 17 LVS vaccine day 42 post-vaccination serum number 2001362794 (paired id 02832). The complete set of Western blot images are shown in supplementary data Figures S1–3. (i) Two-dimensional gel resolving the SCHU S4 proteome. Protein stained 2D-gel, separating SCHU S4 protein lysates in the pH range 4–7, used for alignment of 2D Western blots and identification of immunoreactive proteins. Identified proteins are annotated with their locus tags, and listed in full in Table 2.
Table 2. Immunoreactive proteins.
A summary of proteins reactive with sera from human tularemia patients (Type A and B) or LVS vaccinees.
| Study | |||||||
|---|---|---|---|---|---|---|---|
| Locus tag | Protein Name | Control sera (n−13) | Vaccine NDBR lot 11 LVS (n=8) | Vaccine DVC lot 17 LVS (n=8) | Type B (n=12) | Type A (n=12) | Previously reported |
| FTT_0060 | ATP synthase subunit B | - | - | - | 5 | 1 | [27], [40] |
| FTT_0062 | ATP synthase subunit A | - | 1 | - | 5 | 1 | [38], [39], [40] |
| FTT_0064 | F0F1 ATP synthase subunit beta | - | - | - | 6 | - | [38], [41] |
| FTT_0077 | Dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex | - | 8 | 7 | 11 | 8 | [29, 30] [27, 38], [39], [40] |
| FTT_0087 | Aconitate hydratase | 1 | - | 1 | 4 | - | [29], [30], [38] |
| FTT_0137 | Elongation factor Tu (EF-Tu) | 1 | - | - | 1 | 4 | [25], [29],[38], [39], [40] |
| FTT_1486c | Hypothetical lipoprotein | - | - | - | 1 | 1 | |
| FTT_0143 | 50S ribosomal protein L7/L12 | 10 | 8 | 6 | 8 | 10 | [25], [29],[39], [40] |
| FTT_0183c | 30S ribosomal protein S1 | 3 | 7 | 5 | 7 | - | [38], [39], [40] |
| FTT_0188 | Cell division protein | - | - | - | 1 | 4 | [29], [38], [39], [40] |
| FTT_0191 | Peptide chain release factor 2 | - | - | - | - | 1 | |
| FTT_0196c | Glutamine synthetase | - | 4 | 2 | - | - | [27], [40] |
| FTT_0307 | Glutamyl-tRNA synthetase | - | - | - | 1 | - | |
| FTT_0323 | Elongation factor G (EF-G) | - | 1 | 1 | 3 | 2 | [38], [39], [40] |
| FTT_0350 | DNA-directed RNA polymerase alpha subunit | - | 5 | 3 | 4 | - | [38], [40] |
| FTT_0373c | Nucleoside diphosphate kinase | - | - | - | - | 1 | [25, 40] |
| FTT_0380c | Glutamate dehydrogenase | - | - | - | - | 1* | |
| FTT_0407 | Aminomethyltransferase | - | 1 | - | - | - | [40] |
| FTT_0408 | Glycine cleavage system H protein | - | 2 | - | 3 | - | |
| FTT_0472 | Acetyl-CoA carboxylase, biotin carboxyl carrier protein subunit | 1 | 6 | 3 | 8 | 7 | [25], [27], [38], [39] |
| FTT_0504c | Succinyl-CoA synthetase subunit beta | - | 2 | - | - | - | [30] |
| FTT_0535c | Malate dehydrogenase | - | 1* | - | - | - | [25] |
| FTT_0583 | Outer membrane associated protein, fopA | - | 8 | 3 | 1 | 6 | [29], [27], [38], [39] |
| FTT0609 | peptidase, M24 family protein | - | 1* | - | - | - | |
| FTT_0673c | Hypothetical protein FTT0673c | - | - | - | 3 | - | |
| FTT_0715 | Chitinase family 18 protein | - | - | - | 1 | - | [38], [39], [40] |
| FTT_0721c | Peroxidase/catalase | 4 | 5 | 5 | 6 | 7 | [25], [29], [30], [38], [39], [40], [41] |
| FTT_0726c | Glycerophosphoryl diester phosphodiesterase family protein | - | 6 | 3 | - | - | [29] |
| FTT_0817 | Threonyl-tRNA synthetase | - | 1 | - | - | - | [39], [40] |
| FTT_0893 | Phosphoribosylaminoimid azole synthetase | - | 5 | 2 | - | - | |
| FTT_1103 | Hypothetical lipoprotein | - | - | - | 1 | - | [29], [27], [38], [39], [40], [41] |
| FTT_1201c | Oxidoreductase, short-chain dehydrogenase family protein | - | 1* | - | - | - | [25], [28] |
| FTT_1269c | Chaperone protein dnaK | 1 | 5 | 7 | 5 | 8 | [25], [29], [30], [27], [38], [39], [40], [41], |
| FTT_1281c | Sigma-54 modulation protein | - | - | - | 1 | - | [25], [40], |
| FTT_1314c | Type IV pili fiber building block protein | 1 | 2 | - | 4 | - | [27], [40] |
| FTT_1352 | Hypothetical protein FTT1352 | 1 | - | 4 | 2 | - | |
| FTT_1357c | Intracellular growth locus, subunit C | - | 3 | 3 | - | - | [40], [41] |
| FTT_1365c | Fructose-bisphosphate aldolase | - | - | - | 3 | - | |
| FTT_1368c | Glyceraldehyde-3-phosphate dehydrogenase | - | - | - | 3 | - | [25], [40] |
| FTT_1373 | 3-oxoacyl-[acyl carrier protein] synthase III | - | - | - | 4 | 2 | [38] |
| FTT1455c | Sugar transamine/perosamine synthetase | - | - | - | - | * | |
| FTT_1483c | Dihydrolipoamide dehydrogenase | 3 | - | - | 1 | - | [29] |
| FTT_1484c | Pyruvate dehydrogenase, E2 component | - | 4 | 3 | 3 | 10 | [29, 30], [27], [38] |
| FTT_1485c | Pyruvate dehydrogenase subunit E1 | - | - | - | - | 2 | [30] |
| FTT_1696 | Chaperone protein, groEL | 2 | 4 | 6 | 12 | 9 | [29], [27], [38], [39], [41] |
| FTT_1778c | Hypothetical membrane protein FTT1778c | 10 | 8 | 6 | 8 | 5 | [27], [38], [39] |
indicates protein identified from limited screen of the proteome separated in the pH range 6–11.
Figure 2. Two-dimensional Western blots probed with sera from selected tularemia patients and LVS vaccinees.
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 6–11. Blots were probed with 1:500 dilution of human sera as follows (a) Type B tularemia patient serum number 1671 (b) Type A tularemia patient serum number MV711 (c) NDBR lot 11 LVS vaccinee control serum number 208d42 (d) DVC lot 17 LVS vaccine pre-vaccination serum number 200208090
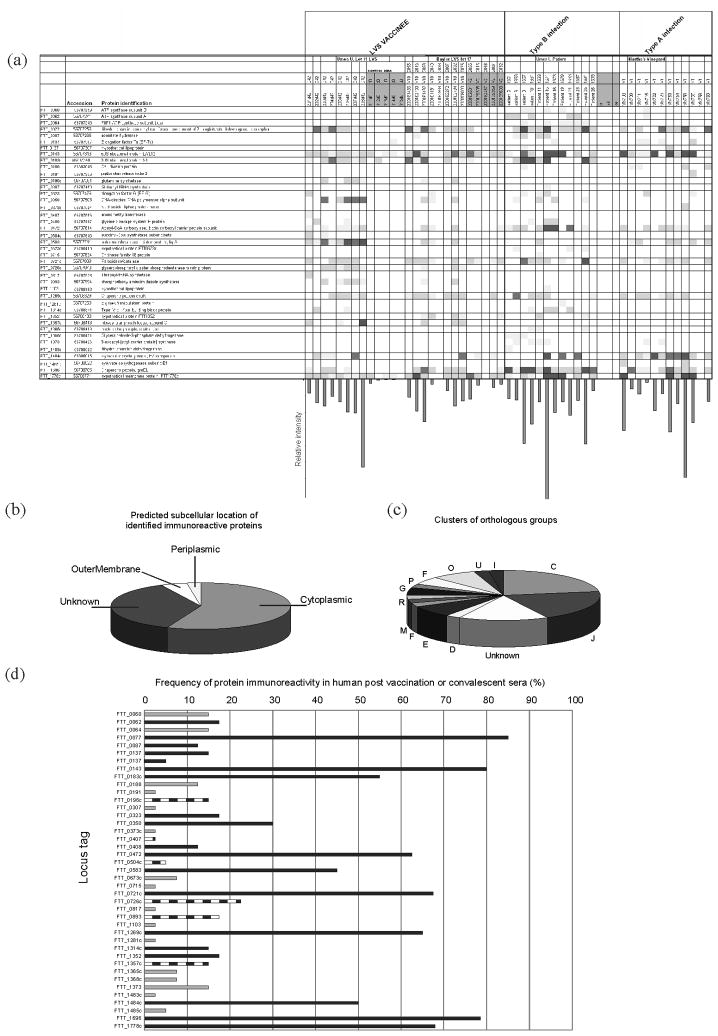
Figure 3. Summary of immunoreactive proteins.
(a) Listed are the identified immunoreactive proteins from all datasets, and their relative intensity values, observed for each serum screened. The bar chart below this matrix shows the total intensity of the immunoreactive proteins for each serum sample screened. (b) Shown are the predicted subcellular locations for the identified immunoreactive proteins. These were determined using the PSORT1b algorithm, as described in the methods. (c) Shown is the Clusters of Orthologous Group classification of the identified immunoreactive proteins. Control sera, from volunteers with no history of tularemia, or from volunteers pre-LVS vaccination are indicated by grey shading at the top of the chart. Groups indicated by letter code are as follows: C Energy production and conversion, J Translation, ribosomal structure and biogenesis, D Cell division and chromosome partitioning, E Amino acid transport and metabolism, F Nucleotide transport and metabolism, M Cell envelope biogenesis, outer membrane, R General function, G Carbohydrate transport and metabolism, P Inorganic ion transport and metabolism, O Posttranslational modification, protein turnover, chaperones, I Lipid metabolism. (d) Bar chart representation of the frequency with which each immunoreactive protein was observed in the post vaccination and convalescent sera screened. Square fill indicates immunoreactive proteins that were only observed to react with sera from tularemia patients. Grey fill indicates proteins that were observed to be immunoreactive only with sera from LVS vaccinees. Black fill indicates immunoreactive proteins that were observed to be reactive with sera from both tularemia patients and vaccinees.
Figure 1c and d show representative Western blots probed with sera from patients recovering from Type A tularemia (complete series of Western blots are shown in Figure S2). A total of 19 proteins were identified as immunoreactive with sera from one or more of the Type A tularemia patients (Figure 1i, Table 2). Serum number 711 was used to probe antigen separated in the pH range 6–11 (Figure 2b) and two very weakly reactive areas were found to correspond to protein spots on equivalent 2D-PAGE, stained for proteins. These were identified as glutamate dehydrogenase (FTT_0380c) and sugar transamine/perosamine synthetase (FTT_1455c), as indicated in the protein stained gel image shown in Figure 2e. No single protein was observed to be immunoreactive with all Type A tularemia patient sera screened, although the proteins pyruvate dehydrogenase E2 (FTT_1484c) and ribosomal protein L7/L12 (FTT_0143) were observed to be reactive with ten of the twelve sera studied. In addition, the proteins dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase (FTT_0077) and chaperonin protein DnaK (FTT_1269c) were reactive with 8 of the total 12 sera.
Representative Western blots probed using sera from LVS vaccinated Swedish laboratory workers (LVS NDBR101 Lot 11) are shown in Figure 1e and f, with the total set of blots shown in Figure S3. Across all eight post-vaccination serum samples screened, a total of 22 immunoreactive proteins were identified (Table 2, Figure 1i). Four proteins were observed to be immunoreactive to some degree with all of the post-vaccination sera: dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex (FTT_0077), 50S ribosomal protein L7/L12 (FTT_0143), outer membrane protein FopA (FTT_0583) and hypothetical protein (FTT_1778c). Of the pre-vaccination sera, one individual showed no detectable immunoreactivity. The remaining four sera showed weak immunoreactivity with the proteins 30S ribosomal protein S1 (FTT_0183c), 50S ribosomal protein L7/L12 (FTT_0143) and hypothetical protein (FTT_1778c). In addition, a single serum sample (# 208) was screened against antigen separated in the basic pH range (Figure 2c). Three weakly immunoreactive regions were aligned with protein spot trains on equivalent 2D-PAGE and identified as peptidase, M24 family protein (FTT_0609), malate dehydrogenase (FTT_0535c) and oxidoreductase, short-chain dehydrogenase family protein (FTT1201c) (Figure 2e).
The second human vaccinee serum set was from humans subjects vaccinated with a new CGMP formulation of LVS (DVC lot 17) and was obtained from a Phase I clinical trial carried out at the Baylor College of Medicine, Houston, TX. The study design and administration of the vaccine was described in detail previously (El Sahly et al. 2009), with dosages of 103, 105, 107 and 109 CFU/mL administered by scarification. Five sets of sera (paired pre- and 42 days post-vaccination) and three unpaired sera (post-vaccination) were provided (Table 1). Representative Western blots probed using sera from vaccinees receiving LVS lot 17 are shown in Figure 1g and h, with the total set of blots shown in Figure S4. Blots probed with sera from humans vaccinated with the new lot of LVS (DVC lot 17) showed immunoreactivity with a total of 18 proteins (Table 2, Figure 1i). For the post-vaccination sera, no single protein was reactive with all sera; however, the proteins dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex (FTT_0077) and chaperonin dnaK (FTT_1269c) were reactive with seven of the eight post LVS vaccination sera. It is also interesting to note that the outer membrane protein, FopA (FTT_0583), was reactive with only three post-vaccination sera. The pre-vaccination sera showed no or weak reactivity towards the proteins dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex (FTT_0077), 30S ribosomal protein S1 (FTT_0183c), catalase (FTT_0721c), 50S ribosomal protein L7/L12 (FTT_0143), and hypothetical protein (FTT_1778c). The proteins 50S ribosomal protein L7/L12 (FTT_0143), and hypothetical protein (FTT_1778c) showed some reactivity with four of the five pre-vaccination sera screened. The escalating vaccine dose did not appear to influence the repertoire of immunoreactive proteins, with the exception of post-vaccination sera from one subject (# 200162938), vaccinated with 107 CFU, which showed no detectable immunoreactivity. In the basic pH region (Figure 2d), the single serum sample (#200208090) screened showed reactivity towards two spot trains, corresponding to unidentified proteins.
Characteristics of the immunoproteomics dataset
The repertoire of immunoreactive proteins catalogued for each serum sample screened in this study is shown graphically in Figure 3a, which also illustrates the relative intensity of each observed immunoreactive spot. The bar chart below the matrix of immunoreactive proteins in Figure 3a shows the sum of the relative intensity values for the identified immunoreactive proteins. With the exception of the NDBR lot 11 LVS vaccinees, the mean total relative intensity in identified immunoreactive spots was similar for each group of sera screened. One subject from each of NDBR lot 11 LVS vaccinees, Type A and type B tularemia patients, showed a markedly higher total intensity of immunoreactive proteins than other subjects in the study. In contrast, two other sera drawn 42 days post-vaccination with 105 CFU DVC lot 17 LVS, also showed comparatively low immunoreactivity. The greatest total relative intensity of identified immunoreactive proteins was observed for the vaccination dose of 109 CFU.
The properties of the reactive proteins were examined according to computationally predicted features to determine whether a particular type of protein was overrepresented. First, we used the PSORT1b algorithm, an algorithm that predicts the subcellular localization of proteins based upon amino acid sequences (Gardy et al. 2005; Gardy et al. 2003; Rey et al. 2005). Figure 3b shows graphically that the vast majority of identified immunoreactive proteins were predicted to be cytoplasmic in location (56 %), with an additional 36 % of the proteins of unknown location. The remaining proteins were predicted to localize to various locations, including the outer membrane and periplasm. Secondly, we classified the identified proteins according to the Clusters of Orthologous Groups (http://www.ncbi.nlm.nih.gov/COG/) where the identified proteins were grouped according to predicted function. Figure 3c shows that 23 % of the identified proteins are predicted to be involved in energy production and conversion, 20 % are predicted to be involved in translation and 15 % to be of unknown function.
Figure 3d shows a graphical representation of the frequency with which each immunoreactive protein was identified, regardless of experimental group. Of note, the protein dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex (FTT_0077) was reactive with 70 % of all sera screened. By contrast, the outer membrane protein FopA (FTT_0583) was observed to be reactive with sera from all LVS NDBR lot 11 vaccinees, but less than half of the subjects from other groups. From this graph, and the matrix of immunoreactive proteins in Figure 3a, eleven proteins were identified as commonly reactive antigens, reactive with both patient and vaccinee sera, with a minimum frequency of 30 %. These included dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex (FTT_0077), 50S ribosomal protein L7/L12 (FTT_0143), 30S ribosomal protein S1 (FTT_0183), DNA-directed RNA polymerase alpha subunit (FTT_0350), Acetyl-CoA carboxylase (FTT0472), Outer membrane associated protein, FopA (FTT_0583), Peroxidase/catalase (FTT_0721c), Chaperone protein DnaK (FTT_1269c), Pyruvate dehydrogenase E2 component (FTT_1484c), Chaperone protein groEL (FTT1696), and Hypothetical membrane protein (FTT_1778c).
DISCUSSION
There is a need for a safe and effective tularemia vaccine, which can be licensed for general use to address potential bioterrorism threats. LVS has been successfully used in Europe and the USA to protect tularemia researchers against infection with Type A strains (Oyston and Quarry, 2005; Conlan, 2004; Titball and Oyston, 2003). During OW and subsequent studies, there have been reports of seroconversion to undefined Francisella antigens (Hornick et al. 1966). However, no correlation between the antibody titer to protein antigens in humans and level of protection against challenge with virulent F. tularensis was observed (Saslaw and CARHART, 1961; Hornick and Eigelsbach, 1966; Saslaw et al. 1961; Saslaw et al. 1961; Saslaw et al. 1961). In the past decade, a handful of studies have surveyed the repertoire of murine antibodies generated in response to LVS vaccination (Havlasova et al. 2005; Sundaresh et al. 2007a; Eyles et al. 2007) and human tularemia infection (Havlasova et al. 2002; Janovska et al. 2007). These data provide insights into the antigens recognized by the immune system after natural infection with Francisella strains differing in virulence, or following vaccination with LVS. We observed that the repertoire of proteins reactive with sera from individuals recovering from natural Type A and B infection showed a great deal of overlap, as shown in Figure 3a & d. This is interesting, given that the most common route of infection for Type A Francisella in the Martha’s Vineyard patients is inhalational (Matyas et al. 2007), whereas the most common route of infection in the European type B infected group is presumed to be arthropod-borne intradermal (Sjostedt, 2007). Therefore, the antibody repertoire generated in response to natural infection with Type A or B strains of Francisella has a large degree of similarity, despite differences in bacterial strains and routes of infection. This was also reported in an earlier study that used a proteome microarray to survey the humoral immune response to tularemia infection (Sundaresh et al. 2007). In this study and our own, subtle differences in the immunoproteomic profiles when screened against the same Francisella antigen, however, were also observed. For example, 80 % of Type A tularemia convalescent sera showed reactivity towards the protein pyruvate dehydrogenase E2 component (FTT_1484c). By contrast, sera from type B tularemia patients showed reactivity towards the same protein in less than 50 % of patients analyzed. In addition, sera from type B tularemia patients showed reactivity with a greater repertoire of proteins, with a total of 31 proteins observed, compared to 18 with sera from Type A patients. Of note, a number of immunoreactive proteins were commonly observed across our own work and the reported studies (Table 2). A small number of the immunoreactive proteins were reactive with the majority of sera screened, and many of the antigenic proteins were also observed to be reactive with sera from our own work, for example the immunoreactive proteins Pyruvate dehydrogenase E2 component (FTT_1484), dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase component (FTT_077), Chaperonin protein GroEL (FTT_1696), Acetyl-CoA carboxylase (FTT_0472), Hypothetical protein (FTT_1441) and 50S ribosomal protein L7/L12 (FTT_0143) (Janovska et al. 2007; Havlasova et al. 2002; Sundaresh et al. 2007). It is difficult, however, to draw conclusions regarding the significance of these observations, given that the exact date and route of infection for each patient is unknown, as is the longevity of the circulating anti-Francisella antibodies. However, one study showed that the majority of patients lacked demonstrable antibody titres 25 years after infection (Ericsson et al. 1994). In relation to this, a recent study reported the repertoire of immunoreactive proteins in the sera of a laboratory worker, accidentally infected with Type A Francisella, did not markedly change over a period of 16 years (Janovska et al. 2007). The one exception was a single immunoreactive protein that was observed to be reactive two years after infection but not at later time points (FTT_0918).
The reactive antigens were not evenly distributed across the proteome. For example, in terms of predicted subcellular location, cytoplasmic proteins were by far overrepresented. This may result from a bias introduced by the gel-based immunoproteomics approach, which is known to have limited capability to resolve very large, small or hydrophobic proteins, even when combined with detergent solutions designed to enhance the solubilization of hydrophobic proteins. A recently developed alternative is the proteome chip, where cell-free expressed proteins immobilized on microarray style chips are probed with immune sera (Sundaresh et al. 2007; Eyles et al. 2007). This approach was used to screen sera from LVS vaccinated mice and did not show the same bias towards cytoplasmic proteins. However, this approach too has limitations, including potential improper protein folding and lack of post-translational modifications of many proteins (Felgner et al. 2009). For many laboratories, the cost of this proteome chip approach can also be prohibitive. Aside from possible limitations of any experimental approach, it may also be that certain functional categories of proteins are selectively recognized by the human immune system. The characteristics of the identified antigenic proteins that allow them to be selectively recognized by the immune system have not been identified.
From this work, no single protein was observed to be immunoreactive with all sera screened. However, eleven proteins were observed to react with at least 30% of the sera screened and are denoted ‘commonly reactive proteins’. Together, reactivity with a combination of these proteins could potentially be predictive of vaccinees protected from challenge with virulent strains. What is still lacking, however, is the ability to conclusively correlate the identity of observed immunoreactive antigens in humans with the protective status of the host. In an attempt to address the issue of the protective status of the host, we chose to screen sera from patients recovering from either Type A or B tularemia. Since the incidence of re-infection is extremely low, these individuals may be assumed to be protected against re-infection. With this assumption, antigens common to patient and vaccinee sera are more likely to serve as potential correlates of protection.
Further to this, ethical considerations preventing LVS vaccine efficacy studies in humans mean reliance upon animal models to bridge efficacy to humans based on correlates of protection. Several immunoproteomics studies of the murine humoral response to LVS vaccination have been reported (Eyles et al. 2007) and eleven antigens in the current study have been reported previously in mice (Table 2). As other animal models of tularemia are developed and characterized, there exist opportunities to correlate the profile of immunoreactive proteins generated by LVS vaccination with the protective status of the host animal. Immunoproteomics studies by our group using sera from tularemia animal models including non-human primates are currently underway.
In addition to increasing understanding of the humoral immune response to tularemia and tularemia vaccination, the identified immunoreactive proteins may be used to design and develop protein subunit based tularemia vaccine candidates.
Supplementary Material
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from patients recovering from type B tularemia. Patient identifiers are indicated above each blot image.
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from patients recovering from type A tularemia. Patient identifiers are indicated above each blot image.
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from volunteers vaccinated with NDBR lot 11. Patient identifiers are indicated above each blot image.
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from volunteers vaccinated with DVC lot 17 LVS. Patient identifiers are indicated above each blot image.
Acknowledgments
This work was supported by Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, Contract No. HHSN266200500041C and in part by the National Research Council, Canada. The authors thank Drs Freyja Lynn, Vicki Pierson, Kristin Debord, Patrick Sanz (National Institutes of Allergy and Infectious Diseases), Melinda Tibbals (EMMES Corporation), Dr J. Wayne Conlan, Luc Tessier and Marianne Savicky (National Research Council, Canada), and Gretchen Stup and Dr Shannon Martin (DynPort Vaccine Company) for their contributions throughout this work.
Funding statement
This work has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, Contract No. HHSN266200500041C and in part by the National Research Council, Canada. This contract was awarded to the DynPort Vaccine Company, who administered funds. National Research Council was a subcontractor of this award. All research described was funded by NIH; no funding was received directly from DynPort Vaccine Company outside the NIH award. The DynPort Vaccine Company was in no way a sponsor of this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
Footnotes
Conflict of interest statement
Two listed authors (L.A. Wolfraim, R.V. House) are employees of the DynPort Vaccine Company, a commercial entity. This company administered a contract from the National Institutes of Health, as detailed in the financial disclosure. The company was not a sponsor of this study and the authors had no financial contribution outside the federally awarded funds. The authors, therefore, do not believe there to be any competing interests. The other authors are or were employees of the National Research Council Canada and Umea University, and have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Carlsson HE, Lindberg AA, Lindberg G, Hederstedt B, Karlsson KA, Agell BO. Enzyme-linked immunosorbent assay for immunological diagnosis of human tularemia. J Clin Microbiol. 1979;10(5):615–621. doi: 10.1128/jcm.10.5.615-621.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan JW. Vaccines against Francisella tularensis--past, present and future. Expert Rev Vaccines. 2004;3(3):307–314. doi: 10.1586/14760584.3.3.307. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001a;285(21):2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- DIENST FT., Jr Tularemia: a perusal of three hundred thirty-nine cases. J La State Med Soc. 1963;115:114–127. [PubMed] [Google Scholar]
- Eigelsbach HT, Hornick RB, Tulis JJ. Recent studies on live tularemia vaccine. Med Ann Dist Columbia. 1967;36(5):282–286. [PubMed] [Google Scholar]
- El Sahly HM, Atmar RL, Patel SM, Wells JM, Cate T, Ho M, Guo K, Pasetti MF, Lewis DE, Sztein MB, Keitel WA. Safety, reactogenicity and immunogenicity of Francisella tularensis live vaccine strain in humans. Vaccine. 2009;27(36):4905–4911. doi: 10.1016/j.vaccine.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 2003;5(2):135–142. doi: 10.1016/s1286-4579(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Ericsson M, Sandstrom G, Sjostedt A, Tarnvik A. Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J Infect Dis. 1994;170(1):110–114. doi: 10.1093/infdis/170.1.110. [DOI] [PubMed] [Google Scholar]
- Eyles JE, Unal B, Hartley MG, Newstead SL, Flick-Smith H, Prior JL, Oyston PC, Randall A, Mu Y, Hirst S, Molina DM, Davies DH, Milne T, Griffin KF, Baldi P, Titball RW, Felgner PL. Immunodominant Francisella tularensis antigens identified using proteome microarray. Crown Copyright 2007 Dstl. Proteomics. 2007;7(13):2172–2183. doi: 10.1002/pmic.200600985. [DOI] [PubMed] [Google Scholar]
- Feldman KA, Stiles-Enos D, Julian K, Matyas BT, Telford SR, III, Chu MC, Petersen LR, Hayes EB. Tularemia on Martha’s Vineyard: seroprevalence and occupational risk. Emerg Infect Dis. 2003;9(3):350–354. doi: 10.3201/eid0903.020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner PL, Kayala MA, Vigil A, Burk C, Nakajima-Sasaki R, Pablo J, Molina DM, Hirst S, Chew JS, Wang D, Tan G, Duffield M, Yang R, Neel J, Chantratita N, Bancroft G, Lertmemongkolchai G, Davies DH, Baldi P, Peacock S, Titball RW. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc Natl Acad Sci USA. 2009;106(32):13499–13504. doi: 10.1073/pnas.0812080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, Ester M, Brinkman FS. PSORTb v. 2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21(5):617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- Gardy JL, Spencer C, Wang K, Ester M, Tusnady GE, Simon I, Hua S, deFays K, Lambert C, Nakai K, Brinkman FS. PSORT-B: Improving protein subcellular localization prediction for Gram-negative bacteria. Nucleic Acids Res. 2003;31(13):3613–3617. doi: 10.1093/nar/gkg602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlasova J, Hernychova L, Brychta M, Hubalek M, Lenco J, Larsson P, Lundqvist M, Forsman M, Krocova Z, Stulik J, Macela A. Proteomic analysis of anti-Francisella tularensis LVS antibody response in murine model of tularemia. Proteomics. 2005;5(8):2090–2103. doi: 10.1002/pmic.200401123. [DOI] [PubMed] [Google Scholar]
- Havlasova J, Hernychova L, Halada P, Pellantova V, Krejsek J, Stulik J, Macela A, Jungblut PR, Larsson P, Forsman M. Mapping of immunoreactive antigens of Francisella tularensis live vaccine strain. Proteomics. 2002;2(7):857–867. doi: 10.1002/1615-9861(200207)2:7<857::AID-PROT857>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hornick RB, Dawkins AT, Eigelsbach HT, Tulis JJ. Oral tularemia vaccine in man. Antimicrobial Agents Chemother(Bethesda) 1966;6:11–14. doi: 10.1128/AAC.6.1.11. [DOI] [PubMed] [Google Scholar]
- Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live Tularemia vaccine. Bacteriol Rev. 1966b;30(3):532–538. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovska S, Pavkova I, Hubalek M, Lenco J, Macela A, Stulik J. Identification of immunoreactive antigens in membrane proteins enriched fraction from Francisella tularensis LVS. Immunol Lett. 2007 doi: 10.1016/j.imlet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kirimanjeswara GS, Olmos S, Bakshi CS, Metzger DW. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol Rev. 2008;225:244–255. doi: 10.1111/j.1600-065X.2008.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortepeter MG, Parker GW. Potential biological weapons threats. Emerg Infect Dis. 1999;5(4):523–527. doi: 10.3201/eid0504.990411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield MA. Rapid immunodetection on polyvinylidene fluoride membrane blots without blocking. Anal Biochem. 1995;229(1):140–143. doi: 10.1006/abio.1995.1391. [DOI] [PubMed] [Google Scholar]
- Matyas BT, Nieder HS, Telford SR., III Pneumonic tularemia on Martha’s Vineyard: clinical, epidemiologic, and ecological characteristics. Ann NY Acad Sci. 2007;1105:351–377. doi: 10.1196/annals.1409.013. [DOI] [PubMed] [Google Scholar]
- Oyston PC, Quarry JE. Tularemia vaccine: past, present and future. Antonie Van Leeuwenhoek. 2005;87(4):277–281. doi: 10.1007/s10482-004-6251-7. [DOI] [PubMed] [Google Scholar]
- Pasetti MF, Cuberos L, Horn TL, Shearer JD, Matthews SJ, House RV, Sztein MB. An improved Francisella tularensis live vaccine strain (LVS) is well tolerated and highly immunogenic when administered to rabbits in escalating doses using various immunization routes. Vaccine. 2008;26(14):1773–1785. doi: 10.1016/j.vaccine.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Acab M, Gardy JL, Laird MR, deFays K, Lambert C, Brinkman FS. PSORTdb: a protein subcellular localization database for bacteria. Nucleic Acids Res. 2005;33(Database issue):D164–D168. doi: 10.1093/nar/gki027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslaw S, CARHART S. Studies with tularemia vaccines in volunteers. III. Serologic aspects following intracutaneous or respiratory challenge in both vaccinated and nonvaccinated volunteers. Am J Med Sci. 1961;241:689–699. [PubMed] [Google Scholar]
- Saslaw S, Eigelsbach HT, PRIOR JA, WILSON HE, CARHART S. Tularemia vaccine study. II Respiratory challenge. Arch Intern Med. 1961a;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- Saslaw S, Eigelsbach HT, WILSON HE, PRIOR JA, CARHART S. Tularemia vaccine study. I Intracutaneous challenge. Arch Intern Med. 1961c;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- Saslaw S, Eigelsbach HT, WILSON HE, PRIOR JA, CARHART S. Tularemia vaccine study. I Intracutaneous challenge. Arch Intern Med. 1961b;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- Sjostedt A. Family XVII. FRANCISELLACEAE Genus I. Francisella. In: Brenner DJ, editor. Bergery’s Manual of Systemic Bacteriology. New York: Springer; 2001. [Google Scholar]
- Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann NY Acad Sci. 2007;1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- Sundaresh S, Randall A, Unal B, Petersen JM, Belisle JT, Hartley MG, Duffield M, Titball RW, Davies DH, Felgner PL, Baldi P. From protein microarrays to diagnostic antigen discovery: a study of the pathogen Francisella tularensis. Bioinformatics. 2007;23(13):i508–i518. doi: 10.1093/bioinformatics/btm207. [DOI] [PubMed] [Google Scholar]
- Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11(3):440–451. [PubMed] [Google Scholar]
- Titball RW, Oyston PC. A vaccine for tularaemia. Expert Opin Biol Ther. 2003;3(4):645–653. doi: 10.1517/14712598.3.4.645. [DOI] [PubMed] [Google Scholar]
- Twine SM, Mykytczuk NC, Petit M, Tremblay TL, Conlan JW, Kelly JF. Francisella tularensis proteome: low levels of ASB-14 facilitate the visualization of membrane proteins in total protein extracts. J Proteome Res. 2005;4(5):1848–1854. doi: 10.1021/pr050102u. [DOI] [PubMed] [Google Scholar]
- Twine SM, Petit MD, Fulton KM, House RV, Conlan JW. Immunoproteomics analysis of the murine antibody response to vaccination with an improved Francisella tularensis live vaccine strain (LVS) PLoS ONE. 2010;5(4):e10000. doi: 10.1371/journal.pone.0010000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine SM, Petit MD, Shen H, Mykytczuk NC, Kelly JF, Conlan JW. Immunoproteomic analysis of the murine antibody response to successful and failed immunization with live anti-Francisella vaccines. Biochem Biophys Res Commun. 2006;346(3):999–1008. doi: 10.1016/j.bbrc.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Viljanen MK, Nurmi T, Salminen A. Enzyme-linked immunosorbent assay (ELISA) with bacterial sonicate antigen for IgM, IgA, and IgG antibodies to Francisella tularensis: comparison with bacterial agglutination test and ELISA with lipopolysaccharide antigen. J Infect Dis. 1983;148(4):715–720. doi: 10.1093/infdis/148.4.715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from patients recovering from type B tularemia. Patient identifiers are indicated above each blot image.
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from patients recovering from type A tularemia. Patient identifiers are indicated above each blot image.
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from volunteers vaccinated with NDBR lot 11. Patient identifiers are indicated above each blot image.
100 μg of SCHU S4 ΔwbtI (O-antigen negative) was used as the antigen, with first dimension separation in the pH range 4–7. Blots were probed with 1:500 dilution of human sera from volunteers vaccinated with DVC lot 17 LVS. Patient identifiers are indicated above each blot image.