Summary
Serotonin 2C receptors (5-HT2CRs) expressed by pro-opiomelanocortin (POMC) neurons of hypothalamic arcuate nucleus regulate food intake, energy homeostasis and glucose metabolism. However, the cellular mechanisms underlying the effects of 5-HT to regulate POMC neuronal activity via 5-HT2CRs have not yet been identified. In the present study, we found the putative transient receptor potential C (TRPC) channels mediate the activation of a subpopulation of POMC neurons by mCPP (a 5-HT2CR agonist). Interestingly, mCPP-activated POMC neurons were found to be a distinct population from those activated by leptin. Together, our data suggest that 5-HT2CR and leptin receptors are expressed by distinct subpopulations of arcuate POMC neurons and that both 5-HT and leptin exert their actions in POMC neurons via TRPC channels.
Introduction
Serotonin (5-hydroxytriptamine, 5-HT) is a neurotransmitter that regulates food intake, energy expenditure, and glucose homeostasis via actions within the central nervous system (Giorgetti and Tecott, 2004; Heisler et al., 2003). Compounds that stimulate the release and/or inhibit the reuptake of 5-HT are potential pharmaceutical targets for the treatment of obesity (Halford et al., 2010; Smith et al., 2010). Indeed, d-fenfluramine (d-Fen) in combination with phenteramine (Fen/Phen) was widely prescribed and was clinically effective to combat obesity. However, the use of serotonergic therapeutics resulted in an increased risk for pulmonary hypertension and ultimately resulted in the withdrawal of d-Fen from the market in 1997 (Connolly et al., 1997). Given the paucity of effective drugs to combat obesity (Astrup, 2010; Pollack, Oct 24 2010, Oct 28 2010), it is imperative to understand the cellular and molecular mechanisms of drugs known to be effective in decreasing food intake and body weight in humans. Understanding the beneficial anorexigenic (appetite-suppressing) effects of anti-obesity serotonergic therapeutics from the adverse cardiopulmonary side effects should be critical to developing novel and safe anti-obesity medications.
Recent studies have established that the 5-HT2C receptors (5-HT2CRs) are key mediators of the ability of 5-HT and drugs like d-Fen to regulate food intake and body weight. For example, global deletion of 5-HT2CRs results in hyperphagia and obesity (Nonogaki et al., 1998; Tecott et al., 1995). Additionally, mice lacking 5-HT2CRs develop insulin resistance and glucose intolerance (Nonogaki et al., 1998). 5-HT2CRs also contribute to the anorexigenic effects of d-Fen (Vickers et al., 1999). Recently, we have found that the anorexigenic effects of d-Fen are mediated in part by the 5-HT2CRs expressed by pro-opiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus (Heisler et al., 2002). Notably, hyperphagia/obesity and insulin resistance observed in 5-HT2CR null mice was normalized when 5-HT2CRs were re-expressed only in POMC neurons (5-HT2CR/POMC mice) (Xu et al., 2010a; Xu et al., 2008). Several groups have also demonstrated that POMC neurons are activated by 5-HT2CR agonists which results in the release of α-melanocyte stimulating hormone (α-MSH) to activate the anorexigenic central melanocortin pathway (Heisler et al., 2002; Heisler et al., 2006; Lam et al., 2008).
Similar to serotonin, the adipose-derived peptide leptin exerts some of its effects by directly activating POMC neurons (Al-Qassab et al., 2009; Balthasar et al., 2005; Cowley et al., 2001; Hill et al., 2010; Hill et al., 2008) Recently, the transient receptor potential C (TRPC) channel was found to underlie the inward currents activated by leptin (Qiu et al., 2010). Importantly, the acute effect of leptin to activate POMC neurons mirrors that of serotonin, but it is unclear whether leptin and serotonin share a similar signaling mechanism leading to the activation of arcuate POMC neurons. Moreover, recent evidence suggests that 5-HT2CR agonists inhibit a GABAB-activated G protein-gated inwardly rectifying K+ (GIRK) conductance in POMC neurons (Qiu et al., 2007). However, it is unclear whether inhibition of a GIRK conductance contributes to the 5-HT2CR induced activation of POMC neurons or underlies the effects of 5-HT2CRs on energy homeostasis. Thus, while available data highlight the importance of 5-HT2CRs in POMC neurons to the metabolic effects of serotonin in the brain, the cellular mechanisms involved in the 5-HT2CR-induced activation of POMC neurons remain undefined.
Results
5-HT2CRs activate a distinct population of POMC neurons
In acute hypothalamic slice preparations from POMC-hrGFP transgenic mice (Parton et al., 2007), we examined the effect of the 5-HT2CR agonist, m-chlorophenylpiperazine (mCPP; 4 μM), on the membrane potential of POMC neurons (Heisler et al., 2002). POMC neurons were identified by hrGFP signals under a fluorescent microscope (Figure 1B). Alexa Fluor 594 was added to the intracellular pipette solutions (Figure 1C) for real-time confirmation that hrGFP-positive neurons were targeted for recording (Figure 1D) and for post hoc identification of neuroanatomical location of the recorded cells (Figure 1E).
Figure 1. mCPP depolarizes POMC-hrGFP neurons.
(A) Brightfield illumination of POMC-hrGFP neuron during acquisition of a whole-cell recording (arrow indicates the targeted cell). Scale bar = 10 μm. (B) The same neuron under fluorescent (FITC) illumination to identify POMC-hrGFP signal. (C) Image shows the complete dialysis of Alexa Fluor 594 from the intracellular pipette at the end of the recording. (D) Image illustrates colocalization of Alexa Fluor 594 with the GFP expression in the same POMC-hrGFP neuron. (E) Post hoc identification of recorded neuron within the arcuate nucleus. Scale bar = 100 μm. (F) Current-clamp recording demonstrates that mCPP (4 μM) depolarizes some POMC-hrGFP neurons. Continuous recordings were interrupted to apply current step pulses as indicated (arrows). Dashed line indicates the resting membrane potential. (G) Current-clamp recording demonstrates that mCPP still depolarizes POMC-hrGFP neurons in the presence of TTX (1 μM). (H) Bar graph summarizing the averaged changes in membrane potential of POMC-hrGFP neurons. Numbers in parentheses indicate number of cells tested. Changes in membrane potential were not affected by TTX, but were significantly attenuated by TRPC channel blockers (SKF96365 & 2-APB), by replacing external Na+ and Ca2+ (in Ca2+ free/Choline), and by a PLC blocker (U73122). Data are presented in mean ± SEM and * indicates P<0.05.
We recorded from 59 POMC-hrGFP neurons in control artificial cerebrospinal fluid (ACSF) bath solutions. Similar to several previous reports (Claret et al., 2007; Cowley et al., 2001; Hill et al., 2008; Williams et al., 2010), in current clamp mode POMC neurons had a resting membrane potential of -52.2 ± 0.8 mV. Application of mCPP depolarized 15 of 59 POMC-hrGFP cells by 5.5 ± 0.4 mV (n=15; Figure 1F). Typically, the depolarization started gradually within 1 min of mCPP application, reached a maximal membrane potential deflection within 2 min, and was reversed upon washout of mCPP ~5 min later. mCPP did not affect the membrane potential in 43 of the remaining cells, while 1 cell was hyperpolarized by -5 mV. For some experiments, tetrodotoxin (TTX, 1 μM) was added to the bath solution to block action potential (AP)-dependent presynaptic activity from afferent neurons that may affect the membrane potential of postsynaptic neurons targeted for recording. In the presence of TTX, application of mCPP (4μM) resulted in a depolarization from rest in 5 of 15 POMC-hrGFP neurons (5.2 ± 0.4 mV; n=5; Figure 1G). The remaining 10 cells were unaffected by mCPP (0.4 ± 0.3 mV; n=10), indicative of a direct membrane depolarization independent of AP-mediated synaptic transmission. Responses of POMC neurons to mCPP are summarized in Table 1.
Table 1.
Rostrocaudal distribution of mCPP-activated and mCPP-inhibited POMC-hrGFP neurons.
| Region | Recorded cells | mCPP-activated cells (%) | mCPP-inhibited cells (%) |
|---|---|---|---|
| RCA | 14 | 3(21.4%) | 1 (7.1 %) |
| Arc1 | 14 | 5 (35.7 %) | 0 (0 %) |
| Arc2 | 26 | 7 (26.9 %) | 0 (0 %) |
| Arc3 | 5 | 0 (0 %) | 0 (0 %) |
|
| |||
| Total | 59 | 15 (25.4%) | 1 (1.7%) |
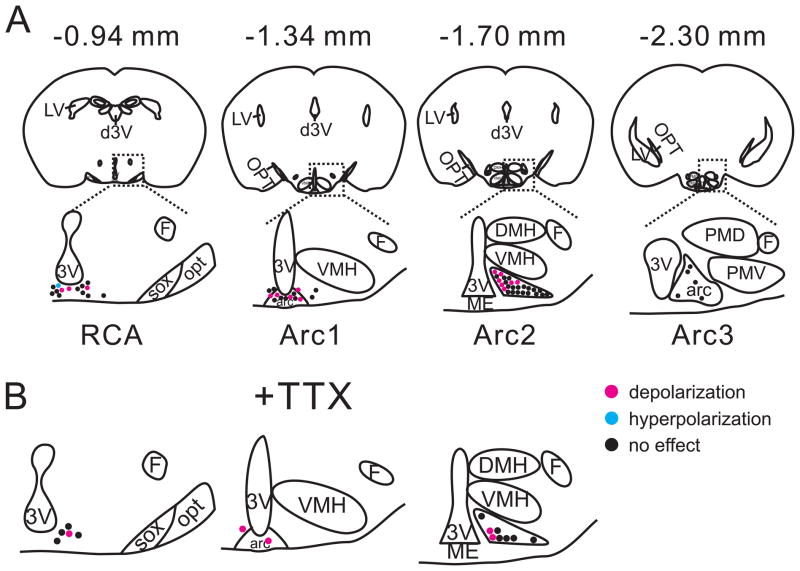
Subsequent to recording, slices were fixed and examined for their location in the rostrocaudal and mediolateral extent of the arcuate nucleus with respect to their responses to mCPP (Figure 2). The illustrations in Figure 2A demonstrate that mCPP-depolarized POMC neurons were located adjacent to the midline and the 3rd ventricle. Moreover, the majority of mCPP-depolarized POMC neurons were located between coronal brain sections corresponding to levels −1.30 mm and −1.70 mm from bregma along the rostrocaudal axis (Paxinos and Franklin, 2001). This distribution pattern was conserved when the experiments were performed in the presence of TTX (Figure 2B) or in neurons from 5-HT2CR/POMC mice (Figure S1). These results suggest that there is a distinct distribution of POMC neurons that are activated by 5-HT2CR agonists.
Figure 2. mCPP-activated POMC neurons are located in distinct areas of arcuate nucleus.
(A) Representative drawings at four rostrocaudal levels of the mouse hypothalamus illustrate the location of mCPP-activated POMC-hrGFP neurons (red dots). (B) Drawings at three rostrocaudal levels of the mouse hypothalamus demonstrate the location mCPP-activated POMC-hrGFP neurons (red dots) in the presence of TTX. 3V: 3rd ventricle, arc: arcuate nucleus, d3V: dorsal 3rd ventricle, DMH: dorsomedial hypothalamic nucleus, F: fornix, LV: lateral ventricle, ME: median eminence, opt: optic tract, PMD: premammillary nucleus, dorsal part, PMV: premammillary nucleus, ventral part, RCA: retrochiasmatic area, sox: supraoptic decussation, VMH: ventromedial hypothalamic nucleus.
GIRK channels do not contribute to POMC neuron activation by 5-HT2CRs
A recent report suggested that 5-HT2C receptors blunt a GABAB-activated GIRK conductance in POMC neurons (Qiu et al., 2007). Therefore, we hypothesized that 5-HT2C receptors blunt GABAB-activated GIRK currents in POMC neurons ultimately leading to an activation of POMC neurons. To confirm the effects of mCPP on the GABAB-dependent outward currents, POMC neurons were voltage-clamped at a membrane potential of −40 mV, and changes in whole-cell current in response to bath application of 50 μM baclofen, a specific GABAB receptor agonist, was monitored in the presence of 0.5 μM TTX (Figures 3A and 3B). Similar to previous reports (Cruz et al., 2004; Qiu et al., 2007), application of 50 μM baclofen resulted in an outward current of +17.6 ± 2.7 pA (n=14). The baclofen-induced current (I1) was readily reversible upon washout, and the baclofen-induced outward current was repeatable in subsequent applications such that baclofen resulted in a second GIRK current (I2) of similar magnitude (I2/I1 = 91.2 ± 5.9 %; n=6) (Figures 3A and 3C). Notably, the specific GABAB receptor antagonist, CGP54626 (2 μM), completely suppressed the baclofen-activated GIRK currents in POMC neurons (Figure 3C), which is consistent with previous reports in the midbrain (Cruz et al., 2004). Moreover, as previously reported, pretreatment with mCPP prior to the second baclofen application significantly decreased I2 resulting in a reduced average ratio of I2/I1 (47.8 ± 5.6 %; n=4) (Figures 3B and 3C). These data support a role of mCPP to suppress the baclofen-induced GIRK currents in POMC neurons, however it remains unclear if GIRK channels are involved in the mCPP-induced excitation of POMC neurons.
Figure 3. POMC-hrGFP neuron depolarization by mCPP is not mediated by the inhibition of GIRK channels.
(A) Voltage clamp recordings of membrane currents at a holding potential of −40 mV. TTX (0.5 μM) was present in the bath solution throughout the recording. Successive application of baclofen (50 μM) elicited outward GIRK currents with similar amplitudes. (B) Pretreatment with mCPP prior to the second application of baclofen significantly reduced the second GIRK amplitudes. (C) Bar graph summarizing the averaged ratio of first and second GIRK current amplitudes (I2/I1). Data are presented in mean ± SEM and ** indicates P<0.01. Note that GABAB-activated GIRK currents are almost completely blocked by CGP54626 (2 μM). (D) Bath application of CGP54626 (2 μM) failed to affect the membrane potential of 9 POMC-hrGFP neurons tested. (E) mCPP depolarized some POMC-hrGFP neurons from GIRK1 knockout mice. Continuous recordings were interrupted to apply current step pulses as indicated (arrows) (a). mCPP decreased voltage deflections in response to hyperpolarizing current steps as shown at the bottom (b). Current versus voltage (I–V) plot from same neuron illustrating a characteristic decrease in input resistance subsequent to mCPP application. Shown are responses before (control) and during mCPP application. Note that the input resistance was decreased by mCPP (c).
GIRK currents contribute to the resting membrane potential of several types of neurons (Cruz et al., 2004; Luscher et al., 1997). Thus, in order to determine if inhibition of GABAB-activated GIRK currents may contribute to the mCPP-induced depolarization in POMC neurons we examined the effect of the GABAB antagonist CGP54626 on the resting membrane potential of POMC neurons. Perfusion of CGP54626 (2 μM) failed to alter the membrane potential of all POMC neurons tested (−53.1 ± 1.9 mV in control vs. −53.5 ± 1.9 mV in CGP54626, n=9) (Figure 3D) suggesting that GABAB receptors do not constitutively activate GIRK channels nor contribute to a ‘leak’ GIRK conductance in POMC neurons. Therefore an mCPP-induced suppression of a GABAB activated GIRK conductance may not result in the cellular activation of POMC neurons. However, these data do not eliminate the possibility that mCPP may modulate a GIRK conductance independent of GABAB activity.
Previous reports suggest GIRK1 and/or GIRK2 subunits are largely responsible for GIRK currents in the brain (Koyrakh et al., 2005; Luscher and Slesinger, 2010). Thus, to further examine the contribution of GIRK channel subunits on resting membrane potential in POMC neurons, we generated POMC-hrGFP mice with global deletion of either GIRK1 or GIRK2. The average membrane potential of POMC-hrGFP neurons from GIRK1 knockout mice was −47.0 ± 0.6 mV (n=16), which was significantly depolarized compared to wildtype mice (−53.3 ± 1.5 mV, n=14, p < 0.05; Figure S2A). In contrast, the average resting membrane potential of POMC-hrGFP neurons from GIRK2 knockout mice was similar to control POMC-hrGFP neurons (−51.3 ± 0.9 mV, n=7, p > 0.05 vs. wildtype). These results suggest that GIRK channels, which contain GIRK1 subunits, are constitutively active at rest in POMC neurons and contribute to the resting membrane potential of POMC neurons. In support of this, POMC neurons from GIRK1 knockout mice had a significantly higher input resistance as determined by hyperpolarizing current steps (1,514 ± 118 MΩ in GIRK1 knockout vs. 1,142 ± 76 MΩ in wildtype mice) (Figure S2B). POMC neurons from GIRK2 knockout mice had a slightly higher input resistance (1,382 ± 112 MΩ), but the difference was not significant.
We next examined the requirement of GIRK1 or GIRK2 subunits in the baclofen-induced hyperpolarization of the membrane potential of POMC neurons. Baclofen hyperpolarized 11 of 14 (78.6 %) POMC-hrGFP neurons from wildtype mice by −15.1 ± 2.1 mV (from −54.3 ± 1.7 mV in control to −69.4 ± 2.4 mV in baclofen, n=11; Figure S2C). The hyperpolarization was accompanied by a 40.8 ± 6.2 % decreased input resistance with a reversal potential of −91.2 ± 1.6 mV, supportive of K+ as the major cation responsible for the membrane hyperpolarization (Figures S2D and S2E). In GIRK1 knockout mice, baclofen hyperpolarized 2 of 16 (12.5 %) POMC-hrGFP neurons (hyperpolarized by −8 mV and −9 mV), while the remaining neurons were unchanged in response to baclofen (Figure S2F). In GIRK2 knockout mice, baclofen hyperpolarized 4 of 7 (57.1 %) POMC-hrGFP neurons by −4.0 ± 0.7 mV (from −51.8 ± 1.1 mV in control to −55.8 ± 1.8 mV in baclofen, n=4) (Figure S2G). These results support a key role of GIRK1 subunits, but not GIRK2, in both constitutively active and GABAB-activated GIRK currents in POMC neurons (Figures S2H and S2I).
We next determined the requirement of GIRK1 subunits in the mCPP induced depolarization of POMC-hrGFP neurons in GIRK1 knockout mice (Figure 3E). Perfusion of mCPP depolarized the membrane potential of 6 of 18 (33.3 %) POMC-hrGFP neurons from GIRK1 knockout mice by 5.2 ± 0.3 mV (n=6), which was similar to the effect of mCPP observed in POMC neurons from wildtype mice. Together, these data suggest that inhibition of constitutively active GIRK channels (Chen and Johnston, 2005) is not responsible for the mCPP-induced excitation of POMC neurons.
Activation of inward conductance underlies POMC neuron depolarization by 5-HT2CRs
In order to further determine the conductance involved in the mCPP-induced depolarization, POMC neurons from wildtype mice were monitored for changes in input resistance and neuronal excitability. In current clamp configuration, continuous recordings of membrane potential were interrupted by hyperpolarizing rectangular current steps (500 ms; −10 to −50 pA; arrows in Figures 1F and 1G). In control ACSF, the whole-cell input resistance of POMC neurons was 1,323 ± 60 MΩ (n=59), similar to previous reports (Hill et al., 2010; Hill et al., 2008). The mCPP-induced depolarization of POMC neurons was accompanied by a reversible 17.1 ± 1.5 % decrease in whole-cell input resistance. Specifically, the input resistance was reduced from 1,330 ± 135 MΩ in control ACSF to 1,095 ± 107 MΩ in mCPP (n=15) (Figures 4A-4C). Extrapolation of the linear slope conductance in control and mCPP-containing ACSF revealed a reversal potential (Er) of −27.3 ± 3.4 mV (n=15) for the depolarization (Figure 4B). The whole-cell input resistance of mCPP-activated cells was also decreased in the presence of TTX (22.6 ± 3.0 %; from 1,364 ± 408 MΩ in control ACSF +TTX to 1,023 ± 266 MΩ in mCPP +TTX; n=5; Er =−25.7 ± 5.0 mV) (Figure 4C) and in POMC neurons recorded from 5-HT2CR/POMC mice (23.3 ± 5.3 %, from 1,384 ± 196 MΩ in control ACSF to 1,066 ± 185 MΩ in mCPP; n=5; Er=−28.0 ± 3.7 mV). Therefore, the mCPP-induced depolarization of POMC neurons is concomitant with an activated conductance with a reversal potential indicative of a putative mixed-/nonselectivecation channel.
Figure 4. mCPP decreases input resistance of POMC-hrGFP neurons by activating TRPC channels.
(A) Current clamp recording from a POMC-hrGFP neuron shows decreased voltage deflection and increased action potential frequency after mCPP application (upper and middle traces), and its recovery (lower trace). Hyperpolarizing current steps were applied as shown at the bottom (500 ms; −10 to −50 pA). (B) Current versus voltage (I–V) plot from same neuron illustrating a characteristic decrease in input resistance subsequent to mCPP application. Shown are responses before (control) and during mCPP application. Note that the input resistance was decreased by mCPP. (C) Decreased whole-cell input resistance from the neuron groups examined. Decreases in input resistance were not affected by TTX, but were significantly attenuated TRPC channel blockers (SKF96365 & 2-APB), by replacing external Na+ and Ca2+ (in Ca2+ free/Choline), and by a PLC blocker (U73122). Data are presented in mean ± SEM and ** indicates P<0.01. (D) mCPP failed to depolarize POMC-hrGFP neurons in the presence of SKF96365. (E) mCPP failed to depolarize when external Na+ and Ca2+ were replaced. 1 μM TTX was added to the external solution.
Some POMC neurons were transiently monitored in voltage-clamp in order to better assess changes in membrane conductance. Current-voltage relationships were examined by applying voltage ramps (−130 mV to 10 mV in 1.4 s, 100 mV/s) from a holding potential of −50 mV in 9 neurons which were depolarized in response to mCPP (Figure S3A). Application of mCPP resulted in an inward current at −50 mV (−7.5 ± 1.1 pA; n=9; Figure S3C). Extrapolation of the linear portion of the slope conductance was used to determine the whole-cell membrane conductance and reversal potential (Figure S3D). The membrane conductance was increased by 22.5 ± 3.4 % (from 0.9 ± 0.1 nS in control ACSF to 1.1 ± 0.2 nS in mCPP, n=9) with a reversal potential of −27.2 ± 5.4 mV (n=9). Moreover, when Cs+ was used as the major cation in the recording pipette, which blocks most leak potassium conductances including GIRK channels (Davila et al., 2003), the mCPP induced inward current was still observed in arcuate POMC neurons (−14.5 ± 4.2 pA, n=3). Collectively, these data suggest that mCPP activates a mixed-/nonselective-cation whole-cell conductance independent of afferent inputs which results in a direct membrane depolarization in arcuate POMC neurons.
5-HT2CRs activate POMC neurons via PLC-dependent TRPC channel activation
Leptin-induced inward currents in POMC neurons have recently been attributed to the activation of TRPC channels (Qiu et al., 2010). Given the electrophysiological properties of the mCPP-activated current observed in the present study, we hypothesized that TRPC channels may also mediate the acute effects of mCPP on POMC neurons. To directly assess the role of TRPC channels in the mCPP-dependent depolarization of POMC neurons, we used the TRPC channel antagonists, SKF96365 (100 μM) and 2-APB (100 μM) (Qiu et al., 2010).
Preapplication of SKF96365 completely prevented the depolarization of POMC neurons by mCPP in all neurons examined (−0.1 ± 0.1 mV, n=11; Figures 1H and 4D). Similarly, 11 out of 12 neurons were unresponsive to mCPP when pretreated with 2-APB (0.1 ± 0.2 mV; n=12; Figure 1H). The remaining neuron was depolarized by 2 mV, however the whole-cell input resistance remained unchanged throughout the recording. Previous reports suggested that TRPC channels are selectively permeable to both Na+ and Ca2+ (Clapham et al., 2001), thus we replaced extracellular NaCl with equimolar choline chloride. Extracellular CaCl2 was also replaced with equimolar MgCl2 and 0.1 mM EGTA, which has previously been shown to greatly decrease the permeable cations through the TRPC channel (Qiu et al., 2010). Ion replacement of extracellular Na+ and Ca2+ resulted in a failure of mCPP to depolarize all POMC neurons tested (0.1 ± 0.1 mV, n=12; Figures 1H and 4E). Similarly, no change in input resistance was observed in the presence of mCPP in all three conditions (Figure 4C). These pharmacological and ion substitution experiments suggest the involvement of TRPC channels in the mCPP-induced POMC neuronal activation.
TRPC channels may be activated by PLC and Gq protein-coupled receptors (GqPCRs) (Strubing et al., 2001). Since 5-HT2CRs are coupled to Gq proteins, we predicted that mCPP may activate the TRPC channel via the Gq-phospholipase C (PLC) signaling pathway. We tested this hypothesis using the PLC inhibitor, U73122. Preapplication of U73122 (5 μM) prevented the depolarization of POMC neurons by mCPP in all neurons examined (−0.2 ± 0.2 mV, n=12; Figures 1H and 4D). Thus, mCPP-induced POMC neuronal depolarization involves PLC-dependent activation of TRPC channels. The distribution of mCPP-treated POMC-hrGFP neurons for these experiments is illustrated in Figure S4.
Serotonin and leptin activate distinct population of POMC neurons
Serotonin and leptin both inhibit food intake and regulate energy balance and both activate TRPC channels to excite POMC neurons. We recently reported that there is a functional segregation of the acute effects of leptin and insulin in POMC neurons (Williams et al., 2010). Our current data suggest that serotonin and leptin share common signaling mechanisms (TRPC channels) in order to modify POMC neuronal activity. Thus, it is formally possible that 5-HT and leptin target the same POMC neurons. To further delineate whether POMC neurons could respond to both serotonin and leptin, identified POMC cells were next assessed for effects of leptin and serotonin on membrane potential following successive application of both compounds.
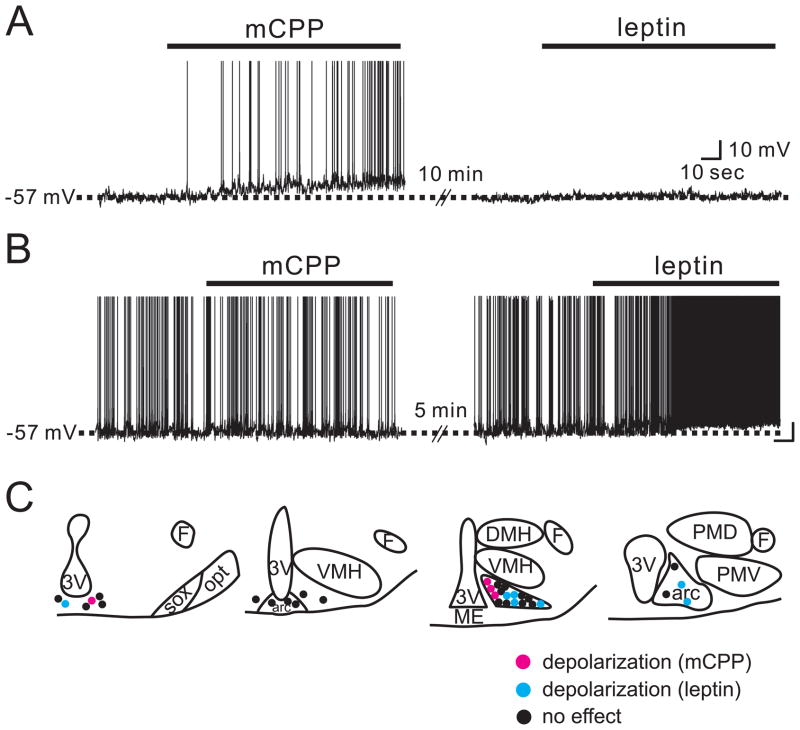
Application of mCPP depolarized 25% of arcuate POMC neurons and was readily reversed upon washout (Figure 1). Subsequent application of mCPP resulted in a depolarization that was 51.0 ± 9.9 % (n=6) of the first depolarization and suggests that although the response is smaller and maybe subject to desensitization, TRPC channels can be activated during subsequent applications. Following washout of mCPP, neurons were examined for the effects of leptin on membrane potential in 32 cells. Perfusion of mCPP depolarized 4 of 32 POMC neurons (5.8 ± 0.9 mV, n=4). The remaining 28 neurons were unresponsive to mCPP (0.1 ± 0.1 mV; n=28). After washout of the mCPP-induced depolarization, subsequent perfusion of leptin (100 nM) failed to alter the membrane potential in each of the neurons previously depolarized by mCPP (0.2 ± 0.3 mV, n=4; Figure 5A). However, 7 of 28 cells that did not initially respond to mCPP were subsequently depolarized in response to leptin (4.8 ± 0.4 mV, n=7; Figure 5B). The leptin-induced depolarization was accompanied by a 21.7 ± 2.8 % decrease in input resistance (from 1,290 ± 137 MΩ in control ACSF to 1,006 ± 102 MΩ in leptin; n=7) with a reversal potential of −28.6 ± 3.7 mV. The remaining 21 cells were unresponsive to leptin (0.2 ± 0.2 mV, n=21). These results indicate that mCPP and leptin activate distinct subpopulations of arcuate POMC neurons (Figure 5C). Interestingly, when compared to the distribution of mCPP-activated POMC neurons, leptin-activated cells were located more laterally in the arcuate nucleus than the mCPP responsive neurons in a similar distribution pattern of leptin-activated POMC neurons previously reported (Williams et al., 2010).
Figure 5. POMC-hrGFP neurons are activated by either mCPP or leptin, but not by both.
(A) In 4 POMC-hrGFP neurons depolarized by mCPP, subsequent application of leptin did not change the membrane potential. (B) Leptin depolarized the membrane potential in 7 out of 28 POMC-hrGFP neurons that were not depolarized by mCPP. (C) Drawings at four rostrocaudal levels of the mouse hypothalamus demonstrate the location mCPP-activated (red dots) and leptin-activated (blue dots) POMC-hrGFP neurons. See Figure 2 for abbreviations.
To further investigate the segregation of the acute leptin and serotonin effects on POMC-hrGFP neurons, we specifically labeled leptin receptor (LepR)-expressing POMC neurons using a transgenic approach. We generated POMC::LepR-cre::tdtomato (PLT) reporter mice (see Experimental Procedures). These PLT mice enabled identification of neurons expressing POMC-hrGFP (green), LepR-cre::tdtomato (red), and POMC-hrGFP::LepR-cre::tdtomato (green/red) in the arcuate nucleus (Figures 6A-1 and 6B-1). POMC neurons from PLT mice were then examined for the acute effects of leptin and mCPP as measured by whole-cell patch clamp electrophysiology.
Figure 6. mCPP and leptin activates distinct population of POMC-hrGFP neurons.
(A-1) LepR-positive POMC-hrGFP neurons with both red and green fluorescence were targeted for electrophysiological characterization with recording pipettes containing Alexa Fluor 350. (A-2) Leptin depolarized 11 out of 16 LepR-positive POMC-hrGFP neurons. (B-1) LepR-negative POMC-hrGFP neurons with green fluorescence only were targeted for electrophysiological characterization. (B-2) mCPP depolarized 5 out of 11 LepR-negative POMC-hrGFP neurons. Scale bar = 10 μm. (C) Drawings at three rostrocaudal levels of the mouse hypothalamus demonstrate the location leptin-activated LepR-positive POMC-hrGFP neurons (red dots). Note that mCPP did not activate any of these neurons. (D) Drawings at three rostrocaudal levels of the mouse hypothalamus demonstrate the location mCPP-activated LepR-negative POMC-hrGFP neurons (red dots). Note that leptin did not activate any of these neurons. See Figure 2 for abbreviations.
As expected, leptin failed to alter the membrane potential of POMC-hrGFP (green) neurons that did not express the leptin receptor reporter (−0.1 ± 0.1 mV; n=10, Figure 6D, lower panels). In current-clamp configuration, 11 of 16 (68.7 %) POMC-hrGFP::LepR-cre::tdtomato (green/red) neurons from PLT mice were depolarized in response to leptin (5.4 ± 0.4mV, n=11, Figure 6A-2 and 6C, lower pannels). Consistent with previous studies and results in the present study, the leptin-induced depolarization was accompanied by a 21.4 ± 2.7% decrease in input resistance (1,709 ± 143MΩ in control ACSF to 1,349 ± 142 MΩ in leptin, n=11). Moreover, extrapolation of the linear slope conductance revealed a reversal potential of −28.8 ± 1.8 mV. The membrane potential of the remaining POMC-hrGFP::LepR-cre::tdtomato (green/red) neurons were either hyperpolarized (−8 mV, n=1) or remained unchanged (0.8 ± 0.5 mV, n=4) in response to leptin. Interestingly, 5 of 11 POMC-hrGFP neurons not expressing leptin receptors (green cells) were depolarized by 5.1 ± 0.4 mV in response to mCPP (Figures 6B-2 and 6D, upper panels). The mCPP-induced depolarization was accompanied by a 19.8 ± 5.1 % decrease in input resistance (from 1,428 ± 252 MΩ resistance in control ACSF to 1,174 ± 251 MΩ in mCPP) with a reversal potential of −31.1 ± 4.5 mV. However, none of the POMC-hrGFP::LepR-cre::tdtomato (green/red) neurons from PLT mice responded to mCPP (0.1 ± 0.2 mV, n=20; Figure 6C, upper panels). Responses of POMC-hrGFP neurons with or without leptin receptors to mCPP and leptin are summarized in Table 2. Together, these data support the hypothesis that the acute effects of leptin and serotonin are functionally segregated in distinct arcuate POMC neurons.
Table 2.
mCPP exclusively depolarizes LepR-negative POMC-hrGFP neurons and leptin exclusively depolarizes LepR-positive POMC-hrGFP neurons.
| POMC-hrGFP/LepR-positive | POMC-hrGFP/LepR-negative | |||
|---|---|---|---|---|
| mCPP | Leptin | mCPP | Leptin | |
| Depolarized | 0 (0 %) | 11 (68.7%) | 5 (45.5 %) | 0 (0 %) |
| Hyperpolarized | 0 (0 %) | 1 (6.3 %) | 0 (0 %) | 0 (0 %) |
| No response | 20 (100%) | 4 (25 %) | 6 (54.5 %) | 10 (100%) |
|
| ||||
| Recorded | 20 | 16 | 11 | 10 |
Discussion
In the present study, we found that about 25% of POMC neurons are depolarized by the 5-HT2CR agonist, mCPP via activation of TRPC channels. Additionally, these data suggest that the mCPP induced activation of POMC neurons is independent of GIRK channel activity. We also compared the activation of POMC neurons by mCPP and leptin, and found that mCPP-activated and leptin-activated POMC neurons comprised distinct populations. The segregation of mCPP- and leptin-activated POMC neurons was further confirmed by the use of a transgenic mouse model to identify the acute effects of serotonin and leptin on POMC neurons that either express or do not express leptin receptors. Our results demonstrate that serotonin and leptin, key anorexigenic signals, activate distinct subpopulations of POMC neurons via activation of TRPC channels.
The arcuate nucleus of the hypothalamus is one of the most studied regions in the brain as it relates to neuronal regulation of feeding and metabolism. Arcuate POMC neurons release α-MSH that activates downstream melanocortin receptors (MC3R/MC4R) resulting in decreased food intake. Arcuate neuropeptide Y (NPY) neurons release agouti-related peptide (AgRP) which antagonizes the action of α-MSH on MC3R/MC4R and increases food intake. Thus, α-MSH and AgRP reciprocally regulate the central melanocortin pathway to modulate energy balance and glucose homeostasis. The anorexigenic effect of d-Fen is mediated by the activation of POMC neurons through 5-HT2CR and subsequent activation of melanocortin pathway (Heisler et al., 2002; Xu et al., 2010b). On the other hand, 5-HT1BR agonists hyperpolarize NPY neurons which decrease the frequency of inhibitory postsynaptic currents (IPSCs) onto POMC neurons resulting in the activation of the central melanocortin pathway by indirectly increasing α-MSH release from POMC neurons and directly decreasing AgRP release (Heisler et al., 2006). Of note, disturbances in the regulation of food intake and insulin sensitivity found in 5-HT2CR null mice are normalized by the re-expression of 5-HT2CR in POMC neurons (Xu et al., 2010a; Xu et al., 2008). Moreover, the activation of POMC neurons by 5-HT2CRs underlies these observations since mCPP did not depolarize POMC neurons from 5-HT2CR null mice; rather depolarizing POMC neurons from mice which selectively expressed 5-HT2CR in POMC neurons (Figure S1; Xu et al., 2010a). Therefore the melanocortin pathway is a key mediator through which serotonin regulates metabolism.
A recent study first suggested the role of hypothalamic GIRK channels in regulating food intake and body weight (Perry et al., 2008). In the current study, we demonstrated a major role of GIRK1 subunits in both constitutively active and GABAB-activated GIRK channel in POMC neurons. POMC neurons in GIRK1 knockout mice showed a significant (~6 mV) depolarization of the resting membrane potential and impaired hyperpolarizing response to baclofen. The GIRK1/2 heteromultimer is the neuronal GIRK channel prototype (Luscher and Slesinger, 2010), and this would be the first example where an electrophysiological phenotype was observed in GIRK1 knockout but not GIRK2 knockout neurons. Since GIRK1 alone cannot form a functional channel (Hedin et al., 1996; Kennedy et al., 1996; Kennedy et al., 1999; Krapivinsky et al., 1995; Ma et al., 2002), it suggests that GIRK1 is interacting with GIRK3 or GIRK4 to form the GIRK channel in POMC neurons. Although the physiological significance of GIRK1 subunit in POMC neurons remains to be determined, given the role of GABA release from NPY neurons in energy balance and the presence of GABAergic inputs to POMC neurons from adjacent NPY neurons (Cowley et al., 2001; Tong et al., 2008), GIRK channels may be the postsynaptic target to mediate the observed metabolic phenotypes.
TRPC channels are a family of the larger TRP channels, and further classified into 4 subfamilies (TRPC1, TRPC4/5, TRPC3/6/7, and TRPC2) (Clapham et al., 2001). TRPC channels are known to have numerous physiological functions (Clapham et al., 2001; Freichel et al., 2005), including a role of TRPC3 in motor coordination (Hartmann et al., 2008) and TRPC5 in fear conditioning (Riccio et al., 2009) in brain. Interestingly, recent evidence suggests an emerging role for TRPC channels in the regulation of energy homeostasis by leptin (Qiu et al., 2010) and now serotonin. We found an involvement of PLC in 5-HT2CR depolarization of POMC neurons. It has been shown that TRPC3/6/7 are activated by diacylglycerol (DAG) (Hofmann et al., 1999; Trebak et al., 2003), but it is not clear if these channels are also activated by DAG generated from PLC-protein kinase C (PKC) signaling pathway in native systems. On the other hand, TRPC4/5 has been shown to be activated by PLC and Gq protein-coupled receptors (GqPCRs) (Strubing et al., 2001). Thus TRPC4/5 are potential molecular candidates mediating the mCPP-induced depolarization of POMC neurons. This hypothesis is supported by the single cell reverse transcription polymerase chain reaction (RT-PCR) data performed in mouse POMC neurons suggesting that the most prevalent subunit in POMC neurons was TRPC5, and this was followed by TRPC1, 4 and 7 (Qiu et al., 2010). Moreover, the leptin-mediated inward currents were shown to be dependent on PLCγ which is a possible downstream signaling molecule of PI3K pathway. Although these data suggest potential compositions of the TRPC channel involved in the 5-HT2CR acute activation of POMC neurons, the identity of the TRPC channel remains undefined.
The current study suggests that TRPC channels may be a common ion channel mediating the acute effects of the two potent anorexigenic signals, leptin (Qiu et al., 2010) and serotonin. Although leptin and serotonin share a common target of cellular activation, TRPC channels, it was unclear if the acute effects of serotonin and leptin are observed in a similar subpopulation of arcuate POMC neurons. It is possible that 5-HT2CR and leptin receptor activate different intracellular signaling pathways within the same neuron. For instance, 5-HT2CR has been shown to activate PLC-PKC-IP3-dependent signaling pathways while leptin receptor activates PI3K-dependent downstream pathways both resulting in activation of TRPC channels. An alternative possibility is that POMC neurons activated by 5-HT2CR and leptin receptor are anatomically segregated in the arcuate nucleus. This possibility was recently demonstrated for the acute effects of leptin and insulin, as at least 2 functionally heterogeneous groups of arcuate POMC neurons (Williams et al., 2010). We found in the present study that mCPP and leptin activate distinct subpopulations of POMC neurons (Figures 5 and 6). Our results support the model of a diversity of POMC neuronal populations suggesting that there are at least 3 functionally heterogeneous groups of POMC neurons.
Intriguingly, deletion of leptin receptors selectively in POMC neurons does not significantly alter food intake (Balthasar et al., 2005; Hill et al., 2010). However recent evidence suggests reactivation of 5-HT2CR selectively in POMC neurons blunts the hyperphagia characterisctic of 5-HT2CR null mouse (Xu et al., 2008). Together with the current study suggesting that 5-HT2CR and LepRs both activate POMC neurons via a TRPC conductance (Qiu et al., 2010), these data suggest a segregation of the metabolic effects of leptin and serotonin in arcuate POMC neurons. In support of these data, we now demonstrate via the use of a novel transgenic line (PLT mice) that the acute effects of leptin and serotonin are segregated in POMC neurons.
Our results also indicate that mCPP-activated and leptin-activated POMC neuronal subpopulations may modify the activity of POMC neurons which project to different brain regions and activate melanocortin pathways of distinct functions. We previously reported a divergence of melanocortin pathways in controlling food intake and energy expenditure (Balthasar et al., 2005). MC4Rs in paraventricular hypothalamus and amygdala were responsible for the regulation of food intake while those in other unidentified brain regions were responsible for energy expenditure. It is currently unclear which areas each subpopulation of POMC neurons projects to, but the possibility of differential projection by mCPP- or leptin-activated POMC neurons will be an exciting focus of future studies.
In conclusion, our results provide a cellular mechanism for the ability of 5-HT to activate POMC neurons. This may prove to be useful in the design of rational pharmaceutical strategies to combat obesity and diabetes. We also provided evidence that POMC neurons are activated by either 5-HT2CR or leptin receptor alone, but not by both. Our results further highlight the functional heterogeneity of POMC neurons regulating energy balance.
Experimental Procedures
Mice
Male (4–16 weeks old) pathogen-free POMC-hrGFP mice (Parton et al., 2007; Ramadori et al., 2008) expressing humanized Renilla green fluorescent protein (hrGFP) under the transcriptional control of the Pomc gene were used for all experiments so that we may identify POMC neurons. 5-HT2CR null mice (Xu et al., 2008) were crossed with POMC-hrGFP mice for some experiments. These mice were then crossed with POMC-cre mice to specifically activate 5-HT2CRs in POMC neurons. GIRK1 or GIRK2 knockout mice were crossed with POMC-hrGFP mice for some experiments. To identify POMC neurons with or without leptin receptors, we first generated LepR reporter mice by mating LepR-cre mice (Scott et al., 2009) with the tdtomato reporter mouse (Jackson Laboratory, #007908). LepR-cre::tdtomato reporter mice were subsequently mated with POMC-hrGFP mice to produce POMC::LepR-cre::tdtomato (PLT) mice. All mice used in this study were housed in a light – dark (12 h on/off; lights on at 7:00 A.M.) and temperature-controlled environment with food and water available ad libitum in the University of Texas Southwestern Medical Center Facility. All experiments were performed in accordance with the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals, as well as with those established by the University of Texas Institutional Animal Care and Use Committee.
Electrophysiology
Whole-cell patch-clamp recordings from POMC-hrGFP neurons maintained in hypothalamic slice preparations and data analysis were performed as previously described (Hill et al., 2008). Briefly, 4- to 16-week-old male mice were deeply anesthetized with i.p. injection of 7% chloral hydrate and transcardially perfused with a modified ice-cold artificial CSF (ACSF) (described below), in which an equiosmolar amount of sucrose was substituted for NaCl. The mice were then decapitated, and the entire brain was removed, and immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) ACSF (126 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 5mM glucose). A brain block containing the hypothalamus was made. Coronal sections (250 μm) were cut with a Leica VT1000S Vibratome and then incubated in oxygenated ACSF at room temperature for at least 1 h before recording. Slices were transferred to the recording chamber and allowed to equilibrate for 10–20 min before recording. The slices were bathed in oxygenated ACSF (32°C–34°C) at a flow rate of ~2 ml/min. The pipette solution for whole-cell recording was modified to include an intracellular dye (Alexa Fluor 594 or Alexa Fluor 350) for whole-cell recording: 120 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, and 2 mM MgATP, 0.03 mM Alexa Fluor 594 or Alexa Fluor 350 hydrazide dye, pH 7.3. K-glucoate was replaced by equimolar Cs-gluconate for some experiments. Epifluorescence was briefly used to target fluorescent cells, at which time the light source was switched to infrared differential interference contrast imaging to obtain the whole cell recording (Zeiss Axioskop FS2 Plus equipped with a fixed stage and a QuantEM:512SC electron-multiplying charge-coupled device camera). Electrophysiological signals were recorded using an Axopatch 700B amplifier (Molecular Devices), low-pass filtered at 2–5 kHz, and analyzed offline on a PC with pCLAMP programs (Molecular Devices). Recording electrodes had resistances of 2.5–5 MΩ when filled with the K-gluconate internal solution. Input resistance was assessed by measuring voltage deflection at the end of the response to a hyperpolarizing rectangular current pulse steps (500 ms of −10 to −50 pA). Membrane potential values were compensated to account for junction potential (−8 mV).
mCPP (4 μM, Aldrich) and leptin (100 nM; provided by A. F. Parlow, through the National Hormone and Peptide Program) were added to the ACSF for specific experiments. Solutions containing mCPP or leptin were typically perfused for 2–4 min. A drug effect was required to be associated temporally with peptide application, and the response had to be stable within a few minutes. A neuron was considered depolarized or hyperpolarized if a change in membrane potential was at least 2 mV in amplitude. After recording, slices were fixed with 4% formalin in PBS at 4°C overnight. After washing in PBS, slices were mounted onto slides, covered in Vectashield (Vector Laboratories), and coverslipped to reduce photo-oxidation during visualization with fluorescent light. Cells were then visualized with ApoTome imaging system (Imager Z1; Zeiss) to identify post hoc the anatomical location of the recorded neuron.
Drugs
TTX, SKF96365, 2-APB, baclofen and CGP54626 were obtained from Tocris. U73122 was obtained from Calbiochem. All solutions were made according to manufacturer’s specifications. Stock solutions of SKF96365, 2-APB, CGP54626, U73122 were made by dissolution in DMSO (Sigma). The concentration of DMSO in the external solution was <0.1 %. Stock solutions of leptin were made by dissolution in D-PBS (Gibco). Stock solutions of mCPP, TTX, and baclofen were made by dissolution in de-ionized water.
Data analysis
Statistical data are expressed as mean ± SEM, where n represents the number of cells studied. The significance of differences between was evaluated using unpaired 2-tailed Student’s t test with a confidence level of P < 0.05(*) or P<0.01 (**).
Supplementary Material
Highlights.
5-HT2CRs excite arcuate POMC neurons independent of altering GIRK channel activity.
Arcuate POMC 5-HT2CRs activate a Na+/Ca2+ permeable mixed-cation conductance.
5-HT2CR-induced excitation of arcuate POMC neurons requires a putative TRPC channel.
5-HT2CR- and LepR-activated arcuate POMC neurons are anatomically segregated.
Acknowledgments
We thank Dr. Jefferey Friedman (Rockfeller University) for kindly providing us with the LepR-cre mice. This work was supported by grants to J.-W.S. (American Diabetes Association), K.W. (R01MH061933, P50DA011806), K.W.W. (K01DK087780), and J.K.E. (R01DK53301, R01DK088423, and RL1DK081185). This work was also supported by PL1 DK081182 and UL1RR024923.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A. Drug management of obesity-efficacy versus safety. N Engl J Med. 2010;363:288–290. doi: 10.1056/NEJMe1004076. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci. 2003;23:5693–5697. doi: 10.1523/JNEUROSCI.23-13-05693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel M, Vennekens R, Olausson J, Stolz S, Philipp SE, Weissgerber P, Flockerzi V. Functional role of TRPC proteins in native systems: implications from knockout and knock-down studies. J Physiol. 2005;567:59–66. doi: 10.1113/jphysiol.2005.092999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Tecott LH. Contributions of 5-HT2C receptors to multiple actions of central serotonin systems. Eur J Pharmacol. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol. 2010;6:255–269. doi: 10.1038/nrendo.2010.19. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin KE, Lim NF, Clapham DE. Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron. 1996;16:423–429. doi: 10.1016/s0896-6273(00)80060-4. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JM, et al. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Kennedy ME, Nemec J, Clapham DE. Localization and interaction of epitope-tagged GIRK1 and CIR inward rectifier K+ channel subunits. Neuropharmacology. 1996;35:831–839. doi: 10.1016/0028-3908(96)00132-3. [DOI] [PubMed] [Google Scholar]
- Kennedy ME, Nemec J, Corey S, Wickman K, Clapham DE. GIRK4 confers appropriate processing and cell surface localization to G-protein-gated potassium channels. J Biol Chem. 1999;274:2571–2582. doi: 10.1074/jbc.274.4.2571. [DOI] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O’Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33:715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Maryland Heights, MO: Academic Press; 2001. [Google Scholar]
- Perry CA, Pravetoni M, Teske JA, Aguado C, Erickson DJ, Medrano JF, Lujan R, Kotz CM, Wickman K. Predisposition to late-onset obesity in GIRK4 knockout mice. Proc Natl Acad Sci U S A. 2008;105:8148–8153. doi: 10.1073/pnas.0803261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A. No FDA Approval for New Diet Pill. The New York Times; Oct 24, 2010. p. A27. [Google Scholar]
- Pollack A. FDA Rejects Qnexa, a Third Weight-Loss Drug. The New York Times; Oct 28, 2010. p. A1. [Google Scholar]
- Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Ronnekleiv OK, Kelly MJ. Serotonin 5-hydroxytryptamine 2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol Pharmacol. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Vazquez G, Bird GS, Putney JW., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT2C receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Berglund ED, Sohn JW, Holland WL, Chuang JC, Fukuda M, Rossi J, Williams KW, Jones JE, Zigman JM, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010a;13:1457–1459. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Lauzon DA, Anderson JG, Balthasar N, Heisler LK, Zinn AR, Lowell BB, Elmquist JK. A serotonin and melanocortin circuit mediates D-fenfluramine anorexia. J Neurosci. 2010b;30:14630–14634. doi: 10.1523/JNEUROSCI.5412-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.