Abstract
The design, synthesis and biological evaluation of conformationally constrained coumermycin A1 analogues are reported. Compounds were evaluated against both breast cancer (SKBr3 and MCF7) and prostate cancer (PC3mm2, A549 and HT29) cell lines. Non-noviosylated coumermycin A1 analogues that manifest potent anti-proliferative activity resulting from Hsp90 inhibition are provided, wherein replacement of the stereochemically complex noviose sugar with readily available piperidine rings resulted in ~100 fold increase in anti-proliferative activities as compared to coumermycin A1, producing small molecule Hsp90 inhibitors that exhibit nanomolar activities.
Introduction
Interest in small molecule heat shock protein 90 (Hsp90) inhibitors has exploded during the past decade. Unfortunately, much of this effort has been met with limited success in the clinic.1 Hsp90 exists as a homodimer and contains multiple small molecule binding sites. The N-terminal nucleotide binding site is the most widely studied and inhibitors of this domain have risen to clinical evaluation.2–3 A second small molecule binding site located proximal to the C-terminal dimerization domain has also been identified,4–5 and modulators of this region are gaining enthusiasm as a consequence of the different biological activities manifested by these inhibitors as compared to those that target the N-terminus.
Hsp90 inhibitors exhibit promising anti-cancer properties as proteins associated with malignant growth: including growth factors, kinases, and hormone receptors are dependent upon the Hsp90 protein folding machinery for their maturation and/or activation.6–9 As a molecular chaperone, Hsp90 is responsible for folding these client protein substrates. Consequently, inhibitors of Hsp90 can disrupt multiple signaling cascades simultaneously, resulting in a combinatorial attack on numerous signaling pathways10–11.
Novobiocin (1), a potent inhibitor of bacterial DNA gyrase12, was identified as the first Hsp90 C-terminal inhibitor.13–14 However, its low efficacy against cancer cells (IC50 ~ 700 µM) prevents its use as chemotherapeutic option.4–5 Although novobiocin displays weak activity, the dimeric compound, coumermycin A1 (3) displays a 10-fold greater anti-proliferative activity (IC50 ~ 70 µM) and thus, represents a promising scaffold for the design of more potent Hsp90 inhibitors that target the Hsp90 homodimer.15
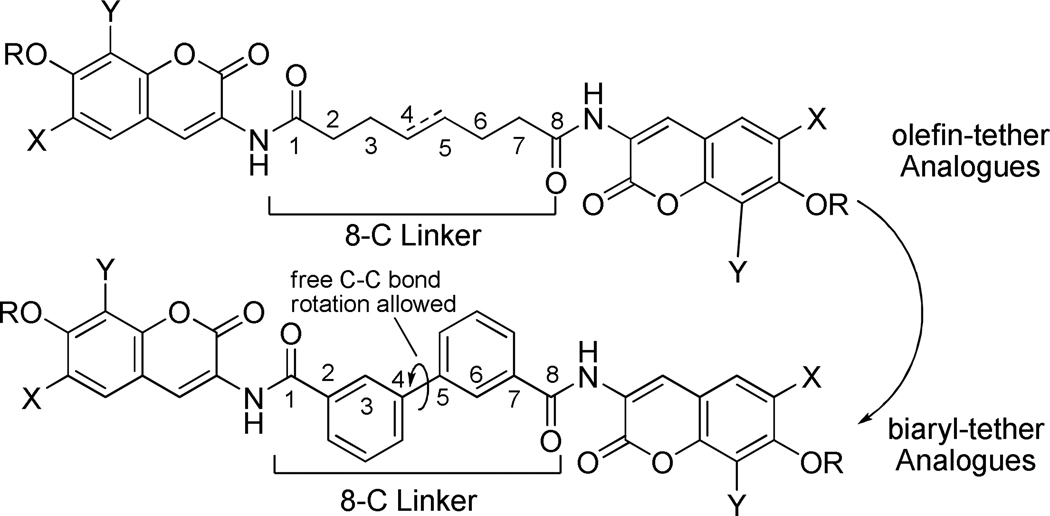
Structural modifications and structure-activity relationships (SAR) for novobiocin 1 have been investigated and have given rise to analogues that manifest nanomolar anti-proliferative activity via Hsp90 inhibition.7,16–22 In contrast, modifications to the coumermycin A1 scaffold have not been similarly pursued. Coumermycin A1 is a homobifunctional dimer; each monomeric unit contains a 3'-substituted noviose sugar and a 4-hydroxy-8-methylcoumarin connected at the 3-position of the coumarin through a 5-methylpyrrole linker. Previous coumermycin A1 analogues exchanged the pyrrole linker for an aryl, heteroaryl or olefin-containing tether that altered both the length and geometry of the linker.23 These analogues retained the noviose sugar and the 8-methyl substituent on the coumarin, which produced compounds that manifested anti-proliferative activities in the low micromolar range. In addition to the modest activity observed for noviose-containing analogues, the synthesis of noviose is laborious and hinders rapid development of SAR24–26.
Recent publications focused on the monomeric inhibitor, novobiocin, have demonstrated that replacement of 8-methyl coumarin with the 8-methoxy coumarin18 and exchange of the stereochemically complex noviose sugar with simple, commercially available heterocycles resulted in a 2- to 20-fold enhancement in anti-proliferative activity.19–20,27 The synthesis of noviose sugar is laborious and requires 11 steps for its preparation. Therefore a series of dimeric Hsp90 inhibitors were designed to contain substituents identified from the optimized monomeric species in an effort to produce a more efficacious class of C-terminal inhibitors. Specifically, we sought to replace the 8-methyl appendage with an 8-methoxy as well as to introduce the 8-methyl-6-methoxy coumarin; and replace the noviose sugar with N-methyl-4-piperdine or N,N-dimethyl ethyl amine. Due to the conformationally flexible nature of the Hsp90 homodimer, the 5-methylpyrrole linker was exchanged for bicyclic, tricyclic, and flexible tethers that could provide occupancy of both binding sites simultaneously via a single inhibitor. The design, synthesis, evaluation and first structure–activity relationships for coumermycin A1 analogues that target Hsp90 are reported herein.
Results and Discussion
Design of New Coumermycin A1 Analogues
To determine structure–activity relationships for coumermycin A1 analogues and to provide more efficacious compounds, we sought to explore three regions of coumermycin A; the coumarin core, the sugar, and the linker, each by systematic evaluation. We chose sugar surrogates based upon previously reported novobiocin analogues,19–20,27 wherein the N-methyl-4-piperidine and N-N-dimethyl ethyl amine substituted coumarins manifested increased anti-proliferative activities against a range of cancer cell lines. Modified coumarins were chosen due to the increased inhibitory activities observed for the corresponding novobiocin derivatives,18–19 specifically 6- and 8-alkoxy substituted and 6,8-disubstituted coumarins were found to be more active than the 8-methyl coumarin present in novobiocin and coumermycin A1. The linkers were modified to determine the optimal distance between the monomeric binding sites and to account for the flexible nature of the chaperone. Although the alkane- and alkene-containing linkers were chosen to determine the distance between these binding sites, which are located adjacent to the dimerization domain15, the biaryl and tricycle containing linkers were chosen for incorporation of the optimal side chain reported for the monomeric species.
The retrosynthesis of coumermycin A1 analogues is depicted in Scheme 1. The sugar-substituted coumarins were prepared as previously described.18–21,23 Coupling of the sugar-substituted amino-coumarins with either the diacid or diacid chloride linker could then be achieved upon exposure to standard amide forming conditions.
Scheme 1.
Retrosynthesis of coumermycin A1 analogues.
Synthesis and Evaluation of Olefin and Saturated-Linkers for Coumermycin A1 Analogues
The olefinic tethers were chosen based upon previously reported coumermycin A1 analogues.23 These linkers varied in length and geometry to identify the optimal distance between the two C-terminal binding sites in the C-2 symmetric, Hsp90 homodimer. Previous synthesis of coumermycin A1 analogues resulted in low yields from the cross-metathesis reaction (9–51%).23 Therefore, linkers 10–12 were prepared first and then subsequently coupled with the corresponding amino-coumarins,10,13 using standard peptide coupling conditions (Scheme 2). The diacid olefin linkers (10–12) were prepared via cross-metathesis of the olefin containing benzyl esters (4–6) followed by hydrolysis. Amino-coumarins (14 or 15) were coupled with the commercially available diacid 13 or diacid linker 10 using EDCI in a mixture of pyridine and methylene chloride, which after solvolysis of the noviose cyclic carbonate, provided coumermycin analogues 16–19 in good yield.
Scheme 2.
Synthesis of noviosylated olefin dimers.
Replacement of the stereochemically complex noviose sugar with simple, commercially available amines were sought as outlined in Scheme 3. These sugar surrogates were chosen based on recent studies that demonstrated these moieties are optimal for the monomeric inhibitors.19–20 The EDCI coupling method employed for the construction of compounds 16–19 was not successful with these derivatives, as the tertiary amines readily protonated and precipitated out of solution. However, dimers 26–36 were successfully prepared utilizing a combination of DCC and DMAP, which promoted the union of amines 22–2527 with olefinic linkers 10–13 in good to moderate yields.28
Scheme 3.
Synthesis of olefinic dimers.
For comparison, saturated dimers (42–44) were prepared by coupling the commercially available diacid chlorides (39–41) with amino-coumarin 22 in excellent yield (Scheme 4). The 8-carbon, cis-olefin containing linker 38, was also prepared for direct comparison to the trans-isomer, 29.
Scheme 4.
Synthesis of saturated- and cis-dimers.
Once synthesized, these coumermycin A1 analogues that contain both olefinic and saturated linkers, were evaluated for anti-proliferative activity against SKBr3 (estrogen receptor negative, Her2 over-expressing breast cancer cells), MCF-7 (estrogen receptor positive breast cancer cells), A549 (human lung adenocarcinoma epithelial), HT29 (Human colon adenocarcinoma grade II), and PC3mm2 (androgen receptor insensitive prostate cancer) cell lines. The anti-proliferative activities provide some insight into the optimal distance between binding sites and provide rationale for subsequent analogue design. As shown in Table 1, the eight-carbon olefinic dimers, 18 and 19, were more efficacious than the analogous six-carbon linkers, 16 and 17, while substitution at the 6-position of the coumarin ring exhibited minimal effect on inhibitory activity. This result was surprising, because for the monomeric inhibitors, the 6-OMe-8-Me (16 and 18) and 8-OMe coumarins (17 and 19) produced compounds that displayed enhanced activity as compared to the 8-Me derivative. These data suggest the dimers may bind in an altered orientation as compared to the monomeric novobiocin analogues, or at a different point in the chaperone cycle.
Table 1.
Anti-Proliferation Activities of Noviosylated Olefin Dimers.
 | |||||
|---|---|---|---|---|---|
| Entry | n | X | Y | SKBr3 | MCF-7 |
| Coumermycin A1a | 5.0 ± 0.1 | 8.8 ± 0.1 | |||
| 16 | 1 | H | OMe | >100b | >100 |
| 17 | 2 | H | OMe | 52.0 ± 7.8 | >100 |
| 18 | 1 | OMe | Me | 105.7 ± 13.2 | 168.0 ± 9.7 |
| 19 | 2 | OMe | Me | 4.1 ± 0.5 | 2.61 ± 0.8 |
| 2021 | 1 | H | Me | >100 | 53.1 ± 7.1 |
| 2121 | 2 | H | Me | 1.5 ± 0.1 | 3.9 ± 0.7 |
Anti-proliferative activities reported from reference 23
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate, all values presented in µM
To determine the optimal distance between the coumarin moieties in non-noviosylated coumermycin A1 dimers (26–36), a series of compounds was prepared to contain an increasing number (6, 8, 10 and 12) of methylene units in the linker. Compounds 26–36 were found to be 10–100 fold more potent than the corresponding noviosylated coumermycin A1 analogues, 16– 19 (Table 2). In the case of 8-methyl coumarin, the 6 and 8-carbon linker dimers (26 and 29) were approximately 2–3 fold more active than the dimer containing a 10-carbon linker (32). Interestingly the 10-carbon dimer, 32, was 10–20 fold more active than any other dimer against prostate cancers, manifesting low nanomolar anti-proliferative activities (~200–400 nM). In general, compounds containing either the 8-OMe/6-OMe or 8-OMe coumarin substitution were found to be more efficacious against prostate cancer cell lines than their 8-Me counterparts.
Table 2.
Anti-Proliferation Activities of non-Noviosylated Olefin Dimers
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | R3 | n | X | Y | SKBr3 | MCF7 | PC3mm2 | A549 | HT29 |
| 26 | 1 | H | Me | 0.18 ± 0.03a | 0.29 ± 0.01 | 7.51 ± 4.38 | 21.48 ± 0.08 | 7.1 ± 1.7 | |
| 29 | 2 | H | Me | 0.15 ± 0.01 | 0.27 ± 0.02 | 4.19 ± 0.53 | 5.54 ± 0.04 | 0.05 ± 0.04 | |
| 32 | 3 | H | Me | 0.89 ± 0.01 | 0.63 ± 0.03 | 0.44 ± 0.13 | 0.22 ± 0.15 | 0.24 ± 0.16 | |
| 35 | 4 | H | Me | 0.51 ± 0.06 | 0.73 ± 0.10 | NT | NT | NT | |
| 27 | 1 | OMe | Me | 0.27 ± 0.01 | 0.56 ± 0.05 | 0.17 ± 0.12 | 1.25 ± 0.03 | NT | |
| 30 | 2 | OMe | Me | 1.10 ± 0.13 | 1.31 ± 0.1 | 4.86 ± 1.3 | 1.44 ± 0.02 | NT | |
| 33 | 3 | OMe | Me | 0.22 ± 0.05 | 0.31 ± 0.05 | 0.38 ± 0.07 | 37.7 ± 5.6 | NT | |
| 28 | 1 | H | OMe | 0.71 ± 0.04 | 1.46 ± 0.2 | 8.63 ± 1.27 | NT | NT | |
| 31 | 2 | H | OMe | 2.22 ± 0.5 | 1.12 ± 0.03 | 0.06 ± 0.01 | 1.22 ± 0.24 | NT | |
| 34 | 3 | H | OMe | 0.37 ± 0.05 | 0.88 ± 0.11 | 0.05 ± 0.02 | 1.21 ± 0.8 | NT | |
| 36 | 1 | H | Me | 0.46 ± 0.02 | 0.84 ± 12 | 15.2 ± 1.82 | 19.4 ± 5.09 | 12.2 ± 0.01 | |
| 38 | cis-isomer | H | Me | >100 | 49.9 ± 2.6 | 32.9 ± 18.2 | 77.6 ± 22.4 | NT | |
Values represent mean ± standard deviation for at least two separate experiments presented in triplicate,all values performed in µM
The effect of saturation and conformational flexibility was evaluated by measurement of the anti-proliferative activity of compounds 42–44. In general, saturated analogues 42–44 were less active than the corresponding trans-olefin containing dimers, which were more active than cis-isomer, 38 (Table 3). It appears as though the trans-olefin can orient the coumarin rings into a more favorable conformation, while the cis-olefin appears to disrupt favorable orientation of the coumarin rings. Since the saturated linker is flexible, it allows the coumarin rings to achieve a favorable conformation, but it also elicits an entropic penalty, manifesting activity that is between the cis- and trans-isomers.
Table 3.
Anti-proliferation Activities of Saturated Linker Dimers
 | ||||||
|---|---|---|---|---|---|---|
| Entry | n | SKBr3 | MCF-7 | PC3mm2 | A549 | HT29 |
| 42 | 1 | 1.26 ± 0.2a | 2.46 ± 0.4 | NT | NT | NT |
| 43 | 3 | 1.19 ± 0.3 | 2.82 ± 0.3 | 13.8 ± 9.81 | 30.4 ± 12.3 | 26.3 ± 2.72 |
| 44 | 5 | 2.84 ± 0.1 | 3.68 ± 0.4 | 10.2 ± 1.81 | 13.2 ± 2.1 | 3.9 ± 1.78 |
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate, all values presented in µM
Synthesis of Biaryl-Tether Coumermycin A1 Analogues
After preparation of the olefin-containing linkers, conformationally constrained analogues were prepared to include a tether that represents the optimal length, contains a pseudotrans double bond, and also includes the biaryl ring system that is present in the monomeric inhibitors. This biaryl system was chosen because it allows rotation between the biaryl rings, resulting in multiple conformations and mimicking the trans double bond found in 29.
Additionally, as shown in Figure 2, inclusion of the biaryl side l places the two coumarin rings at a distance that corresponds to the optimal distance, 8 carbons.16 Although slight conformational flexibility is produced by this motif, π-stacking attributes may also be manifested by these molecules, which may be responsible for the increased inhibitory activities manifested by monomeric species that contain this ring system. To validate this hypothesis, biaryl linkers 57–60 containing various patterns of methoxy substitution, which mimic the substitution pattern of monomeric novobiocin analogues containing the methoxy-substituted biaryl side chain, were prepared. Synthesis of the biaryl linkers commenced with phenols 4529 and 46 (Scheme 5). Conversion of 45 or 46 to the triflate 47 or 48, followed by conversion to the boronic ester,30 allowed subsequent Suzuki coupling with the triflate-containing compounds (47, 48) or with the commercially available iodo-containing compound (49), to afford biaryl diesters 53–56 in good yield.
Figure 2.
Rationale for biaryl-tether analogues.
Scheme 5.
Synthesis of conformationally flexible biaryl linkers.
Diesters 53–56 were then hydrolyzed31 to the corresponding diacids, 57–60, and subsequently converted to diacid chlorides32 before coupling with amino-coumarins 13–15 to produce the biaryl-linked noviose-containing dimers 65–70 upon hydrolysis of the cyclic carbonate (Scheme 6). Diacid chloride 62 was also coupled with amino-coumarins 22 and 25, to give biaryl dimers containing sugar surrogates, 71–73, in excellent yields (Scheme 6).
Scheme 6.
Synthesis of biaryl noviosylated dimers.
Synthesis of Tricyclic-Tether Coumermycin A1 Analogues
To further assess conformational flexibility and optimal coumarin ring geometry, conformationally constrained biaryl analogues were also synthesized. The tricyclic linkers containing varying bridges of 5, 6 or 7 atoms would yield dimers that exhibit decreasing flexibility in their prescribed conformations. The 5- 6- and 7-membered tricyclic tethered linkers (91, 92, and 95) were designed alongside the pseudo cis and trans 6-membered tethered tricycles in an effort to elucidate the orientation by which these molecules bind Hsp90 (Figure 3).
Figure 3.
Rationale for tricyclic-tether coumermycin A1 analogues
Retrosynthetic analysis of the tricyclic-containing coumermycin A1 analogues is depicted in Scheme 7, in which two molecules of the sugar substituted amino-coumarin can be coupled with the tricyclic diacid chloride. Tricyclic tethers 76 and 81–83 were envisioned to be prepared via nucleophilic displacement of methyl 4-(bromomethyl)-3-iodobenzoate or methyl 3-bromo-4-fluorobenzoate with methyl salicylate, followed by an intermolecular Heck-cyclization.33
Scheme 7.
Retrosynthesis of 5- and 6- membered tricyclic-tether analogues.
Preparation of the five–membered tricyclic tether commenced by coupling methyl 3-bromo-4-fluorobenzoate 7434 with methyl salicylate, enlisting sodium carbonate in N-N-dimethylacetamide (DMA), to provide biaryl ether 75 in moderate yield (Scheme 8). Intramolecular Heck-cyclization35 of biaryl ether 75 afforded the 5-membered tricyclic tether, 76, in good yield.
Scheme 8.
Synthesis of 5- and 6-membered tricyclic tether.
Six-membered tethers (81–83) were prepared by coupling o-, m- or p- methyl salicylate with methyl 4-(bromomethyl)-3-iodobenzoate (77)36 to obtain iodo benzyl ethers 78–80, which were subjected to an intra-molecular Heck-cyclization37 to give the 6-membered products, 81–83, in excellent yields. Initially, preparation of the seven-membered tether (90) was approached similarly, but the Heck-cyclization produced an inseparable (5:6) mixture of cyclized and dehalogenated compounds. Consequently, the biaryl bond was constructed first, followed by cyclization to afford the seven-membered tether, 90, as described in Scheme 9.
Scheme 9.
Retrosynthesis of 7-membered tricyclic-tether.
Synthesis of 90 commenced with methyl 3-bromo-2-methoxybenzoate (84),38 which was converted to boronic acid 85 in two steps (Scheme 10). The boronic acid was coupled with methyl 3-iodo-4-(2-methoxy-2-oxoethyl)benzoate (86)39 under standard Suzuki coupling conditions38 to yield triester 87. The aliphatic ester was selectively reduced to alcohol 88, followed by cleavage of the methyl ether to give the free phenol. The aliphatic alcohol was converted to tosylate 89 and subjected to an intramolecular cyclization in the presence potassium carbonate to give the seven-membered product, 90 in good yield, and with only trace amounts of styrene product resulting from elimination.
Scheme 10.
Synthesis of 7-membered tether.
Upon preparation, the 5-, 6-, and 7-membered tricyclic esters were hydrolyzed, converted to the corresponding diacid chlorides 96–100, and coupled with amino-coumarin 10 to provide the requisite dimers 101–105 following hydrolysis (Scheme 11).
Scheme 11.
Synthesis of tricyclic tether noviosylated dimers.
Biological Evaluation Biaryl- and Tricycle-Containing Coumermycin A1 Analogues
After construction of the olefin and alkane linked dimers, analogues containing biaryl linkers with varying methoxy substitution and coumarin scaffolds (65–70) were prepared and subsequently evaluated for anti-proliferative activity (Table 4). To evaluate the effect of the methoxy group, four biaryl linkers (65–70) were synthesized. Among these, the symmetrical (66 and 68) biaryl dimers were found to be more active than the non-symmetrical analogue (67). Analogue 66 (6-Ome, 6’-OMe) exhibited 2-fold greater activity than 68 (5-OMe, 5’-OMe) against breast cancer cell lines, however these molecules were less active against prostate cancer cell lines. Interestingly, the dimer containing the 8-OMe substitution on the coumarin scaffold (70) manifested equal potency against the breast cancer cell lines as the corresponding 8-Me analogue 66, but was 100–150 fold more active against prostate cancer cell lines. Analogue 69 (8-Me and 6-OMe coumarin) was 7–8 fold more active against SKBr3 cell lines and slightly more potent against MCF-7 cell lines than its corresponding 8-Me and 8-OMe coumarin analogues, 66 and 68.
Table 4.
Anti-Proliferation Activities of Biaryl Dimers.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | X | Y | R | R1 | SKBr3 | MCF-7 | PC3mm2 | A549 | HT29 |
| 65 | H | Me | H | H | 0.86 ± 0.14a | 1.26 ± 0.17 | NT | NT | NT |
| 66 | H | Me | 6-OMe | 6’-OMe | 1.16 ± 0.21 | 0.76 ± 0.14 | 36.68 ± 8.1 | 35.4 ± 0.01 | 36.54 ± 12. 7 |
| 67 | H | Me | 6-OMe | 5’-OMe | 28.50 ± 4.4 | 38.0 ± 1.5 | NT | NT | NT |
| 68 | H | Me | 5-OMe | 5’-OMe | 1.95 ± 0.4 | 1.85 ± 0.52 | 12.53 ± 2.0 | 28.90 ± 8.62 | 11.72 ± 1.43 |
| 69 | OMe | Me | 6-OMe | 6’-OMe | 0.11 ± 0.05 | 0.72 ± 0.21 | NT | NT | NT |
| 70 | H | OMe | 6-OMe | 6’-OMe | 0.91 ± 0.12 | 0.88 ± 0.2 | 0.27 ± 0.17 | 0.21 ± 0.08 | 0.27 ± 0.12 |
Values repressent mean ± standard deviation for at least two separate experiments performed in triplicate, all values presented in µM
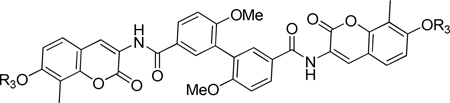
Analogous dimers to the previously described novobiocin monomer analogues with secondary amine-containing sugar replacements (72 and 73) were also evaluated. Interestingly, these compounds were ~10-fold less active than the corresponding noviosylated coumarin-containing (65–70) analogues (Table 6). This trend is opposite to that of the novobiocin series of compounds.19–20 Compounds 71 and 72 also exhibited poor solubility in DMSO, which may contribute to their modest inhibitory activity.
Table 6.
Anti-Proliferation Activities of Non-Noviosylated Biaryl Dimers.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R3 | SKBr3 | MCF-7 | PC3mm2 | A549 | HT29 |
| 71 | 4.98 ± 0.7 | 14.23 ± 2.3 | NT | NT | NT | |
| 72 | 9.50 ± 1.2 | 11.66 ± 1.6 | 52.27 ± 24.3 | 93.45 ± 0.25 | 62.7 ± 18.7 | |
| 73 | OAc | 11.84 ± 0.8a | >100 | NT | NT | NT |
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate, all values presented in µM
As mentioned above, we sought to optimize the linker geometry by synthesizing conformationally constrained tricyclic analogues, with ring sizes containing 5, 6 and 7 atoms (101–105). These tri-cyclic systems allowed the dimers to exhibit increasingly flexible geometries that were dependent on ring size and attachment to the coumarin ring. After synthesis of the tricyclic tether analogues 101–105, they were evaluated for anti-proliferative activity. Among these analogues, the 6- and 7-membered tricyclic tether dimers (102 and 105) were found to be more active than the corresponding 5-membered analogue, 101 (Table 7). Anti-proliferative activity against the SKBr3 breast cancer cell line was similar for both 6- and 7-membered dimers (102 and 105), but against MCF-7 cell lines, the 7-membered analogue (103) was 3-fold more active than the 6-membered analogue (102). The tricyclic constrained analogues (101–105) were less potent than the more flexible biaryl linkers (65–70). These data may indicate that free rotation about the aryl carbon-carbon bond is necessary to orient the methoxy group of the linker and the two coumarin rings into a favorable conformation, since the tricyclic analogues (101–105) are conformationally rigid and lack free rotation about these aryl rings.
Table 7.
Anti-Proliferation Activities of Tricyclic Tether Dimers
 | ||||||
|---|---|---|---|---|---|---|
| Entry | n | amide positions | SKBr3 | MCF-7 | ||
| 101 | 0 | 2,6 | < 100a | < 100 | ||
| 102 | 1 | 4,8 | 60.1 ± 2.8 | 22.0 ± 3.4 | ||
| 103 | 1 | 3,8 | < 100 | < 100 | ||
| 104 | 1 | 2,8 | < 100 | < 100 | ||
| 105 | 2 | 4,10 | 59.9 ± 9.8 | 7.1 ± 1.6 | ||
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate, all values presented in µM
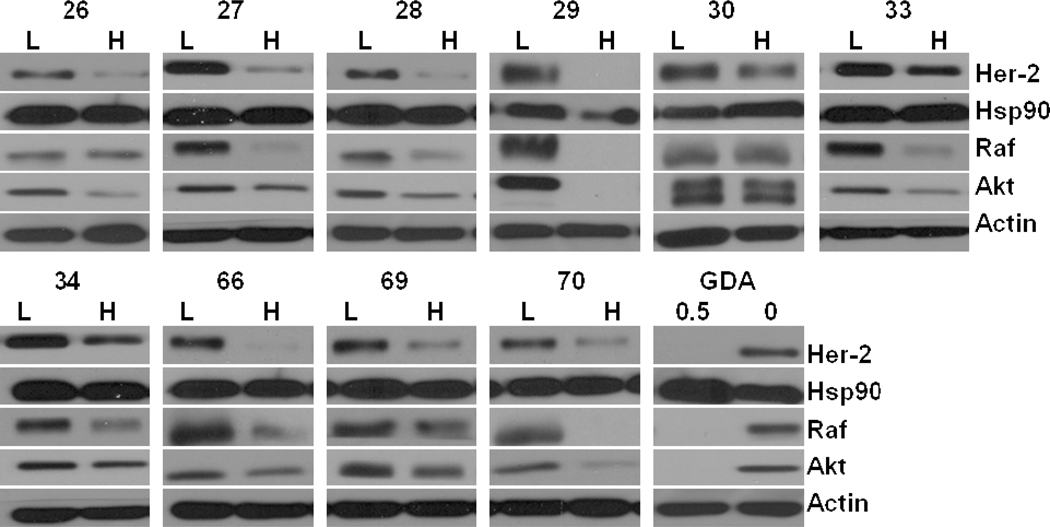
To validate Hsp90 as the target responsible for manifesting the observed anti-proliferative activities exhibited by these molecules, analogues manifesting IC50 values less than 2 µM were evaluated for their ability to induce degradation of Hsp90-dependent client proteins (Her-2, Raf, and Akt). Since actin is not dependent on Hsp90 for its maturation, actin levels should remain constant with an Hsp90 inhibitor and is therefore used as a control.
Figure 4 shows the effect of these compounds on Hsp90 client proteins from MCF-7 breast cancer cell lysates, following a 24 hour incubation with each molecule. Each compound was dosed at two concentrations, H represents a concentration 5-fold higher than the antiproliferative IC50 value, whereas L represents a concentration equal to one half of the observed IC50 value, while geldanamycin (500 nM, 10x the IC50) was used as a positive control and dimethyl sulfoxide (0) as a negative control.
Figure 4.
Western blot analyses induced the Hsp90 client protein degradation in MCF-7 breast cancer cells for coumermycin A1 analogues that target Hsp90. L represents a concentration ½ of the anti-proliferative IC50 value while H represents a concentration 5 times greater than the anti-proliferative IC50 value. GDA (500 nM) represents a positive control, while DMSO (0), vehicle, serves as the negative control.
The majority of the compounds screened by western blot analyses induced degradation of Hsp90 client proteins, while causing no change in actin, which indicates these compounds manifest anti-proliferative activity through Hsp90 inhibition. There were 3 compounds, 31, 32 and 36 (Figure 5) that produced unique client protein profiles at the two concentrations tested. Compounds 31 and 36 appeared to manifest no activity against Hsp90 client proteins, while 32 only induced the degradation of Raf and Akt, but exhibited no effect on Her2. Further studies are needed to determine whether the activity manifested by 32 is dependent upon Hsp90. Prior studies have shown that extracellular Hsp90, which binds Her240–41, can be selectively targeted with non-permeable inhibitors42, but no data has been previously observed for reciprocal activity.
Figure 5.
Western blot analyses of Hsp90 client protein degradation in MCF-7 breast cancer cells for coumermycin A1 Analogues that appear to not target Hsp90. L represents a concentration ½ of the anti-proliferative IC50 value while H represents a concentration 5 times the anti-proliferative IC50 value.
Conclusion
In summary we have prepared both conformationally constrained and flexible coumermycin A1 analogues that manifest nanomolar anti-proliferative activity against breast (SKBr3 and MCF7) and prostate cancer (PC3mm2, A549 and HT29) cell lines. Amongst these analogues were those that contained surrogates for the noviose sugar and varying coumarin substitution. In regards to the tether, the trans-alkene linkers (Table 2) containing 6–8 carbons (26, 29, and 27) represent the most active analogues compared to the longer linkers as well as the corresponding cis-olefinic (38) linker. The biaryl linked dimers (69 and 70) which mimicked the monomeric species, were found to be less active than the dimers that contain a flexible linker. Most of the coumermycin A1 analogues prepared in this article manifested potent anti-proliferative activity that was directly correlated to Hsp90 inhibition, as evidenced by the degradation of Hsp90-dependent client proteins. The most active compounds identified from this study manifest IC50 values ~500-fold more potent than the natural product lead compounds, coumermycin A1.
Experimental Section:General
1H NMR were recorded at 400 or 500 MHz (Bruker DRX-400 Bruker with a H/C/P/F QNP gradient probe) spectrometer and 13C NMR spectra were recorded at 125 MHz (Bruker DRX 500 with broadband, inverse triple resonance, and high resolution magic angle spinning HR-MA) probe) spectrometer; chemical shifts are reported in δ (ppm) relative to the internal reference chloroform-d (CDCl3, 7.27ppm). FAB (HRMS) spectra were recorded with a LCT Premier (Waters corp., Milford MA) spectrometer and IR spectra were recorded on a Magna FT-IR spectrometer (Nicolet Instrument Corporation, Madison, WI, US). The purity of all compounds was determined to be >95% as determined by H1 NMR and 13C NMR spectra, unless otherwise noted. The most active 10 compounds were verified for >95% purity by HPLC analyses.. TLC was performed on glass-backed silica gel plates (Uniplate) with spots visualized by UV light. All solvents were reagent grade, and when necessary, were purified and dried by standard methods. Concentration of solutions after reactions and extractions involved the use of a rotary evaporator operating at reduced pressure.
General procedure for benzyl protection of olefinic acids
K2CO3 (8.28 g, 59.9 mmol), and benzyl bromide (2.84 mL, 23.96 mmol) were added sequentially to a solution of pent-4-enoic acid (2 g, 19.97 mmol) in anhydrous DMF (50 mL). The mixture was stirred at rt for 14 h and quenched by the addition of H2O (80 mL). The aqueous phase was extracted with EtOAc (3 × 80 mL); and the combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified via column chromatography on silica gel (hexanes/ EtOAc, 9/1) to afford benzyl pent-4-enoate (4) as colorless oil (3.65 g, 92%).
benzyl pent-4-enoate (4)
1H NMR (400 MHz, CDCl3) δ 7.36 (m, 5H), 5.84 (ddt, J = 6.2, 10.2, 16.5 Hz, 1H), 5.14 (s, 2H), 5.05 (m, 2H), 2.49 (m, 2H), 2.41 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 173.0, 136.7, 136.1, 128.7, 128.4, 115.7, 66.4, 33.7, 29.0; HRMS (FAB) m/z: [M+H+] for C12H15O2, calcd 191.1072; found, 191.1069.
benzyl hex-5-enoate (5)
Colorless oil, (2.25 g, 96%); 1H NMR (500 MHz, CDCl3) δ 7.36 (m, 5H), 5.78 (ddt, J = 6.7, 10.2, 17.0 Hz, 1H), 5.16 (s, 2H), 5.02 (m, 2H), 2.39 (t, J = 7.5 Hz, 2H), 2.11 (q, J = 7.1 Hz, 2H), 1.77 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 173.0, 137.6, 136.1, 128.6, 128.2, 115.4, 66.1, 33.6, 33.1, 24.1; HRMS (FAB) m/z: [M+H+] for C13H17O2, calcd 205.1229; found, 205.1234.
benzyl hept-6-enoate (6)∷
Colorless oil, (1.87 g, 95%); 1H NMR (400 MHz, CDCl3) δ 7.35 (m, 5H), 5.80 (ddt, J = 6.7, 10.2, 16.9 Hz, 1H), 5.13 (s, 2H), 5.01 (m, 2H), 2.38 (t, J = 7.5 Hz, 2H), 2.08 (m, 2H), 1.68 (m, 2H), 1.44 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 173.6, 138.5, 136.2, 128.5, 128.3, 114.8, 66.2, 34.3, 33.5, 28.4, 24.5; HRMS (FAB) m/z: [M+H+] for C14H19O2, calcd 219.1385; found, 219.1381.General procedure for the cross-metathesis reaction: Grubbs’ second generation catalyst (320 mg, 0.38 mmol, 2 mol %) was added to a solution of benzyl pent-4-enoate 1 (3.6 g, 18.92 mmol) in 10 mL of dichloroethane. The mixture was refluxed for 2 h, then filtered through a plug of silica gel and concentrated. The residue was purified by column chromatography on silica gel (hexanes/EtOAc, 8/1) to provide (E)-dibenzyl oct-4-enedioate 7 (1.8 g, 49%) as a colorless oil.
(E)-dibenzyl oct-4-enedioate (7)
1H NMR (400 MHz, CDCl3) δ 7.35 (m, 10H), 5.46 (m, 2H), 5.12 (s, 4H), 2.41 (m, 4H), 2.33 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 173.0, 136.1, 129.5, 128.7, 128.4, 66.3, 34.2, 27.9; HRMS (FAB) m/z: [M+Na+] for C22H24NaO4: calcd 375.1572, found: 375.1566.
(E)-dibenzyl dec-5-enedioate (8)
Colorless oil, (1.27 g, 62%); 1H NMR (500 MHz, CDCl3) δ 7.35 (m, 10H), 5.39 (m, 2H), 5.12 (s, 4H), 2.35 (m, 4H), 2.04 (dt, J = 9.7, 10.8 Hz, 4H), 1.71 (dt, J = 7.4, 14.5 Hz, 4H); 13C NMR (125 MHz, CDCl3) δ 177.8, 130.6, 130.3, 128.7, 128.3, 66.2, 33.7, 32.0, 24.8; HRMS (FAB) m/z: [M+Na+] for C24H28NaO4, calcd 403.1885; found,403.1883.
(E)-dibenzyl dodec-6-enedioate (9)
Colorless oil, (1.56 g, 54%); 1H NMR (400 MHz, CDCl3) δ 7.34 (m, 10H), 5.38 (m, 2H), 5.12 (s, 4H), 2.38 (dd, J = 12.2, 19.6 Hz, 4H), 2.01 (q, J = 11.2 Hz, 4H), 1.65 (m, 4H), 1.36 (m, 4H); 13C NMR (400 MHz, CDCl3) δ 173.7, 136.2, 130.3, 128.7, 128.3, 66.2, 34.3, 32.3, 29.1, 24.6; HRMS (FAB) m/z: [M+Na+] for C26H32NaO4, calcd 431.2198; found, 431.2202.General procedure for benzyl ester hydrolysis: LiOH (1.97 g, 46.8 mmol) was added to a solution of (E)-dibenzyl oct-4-enedioate 7 (1.65 g, 4.68 mmol) in 40 mL of THF: MeOH: H2O (3:2:2) at rt and stirred for 6 h. The resulting mixture was acidfied to pH ~ 3 with 2N HCl and the white solid was filtered. The product was recrystalized in 30% ethylacetate and hexane to afford acid (E)-oct-4-enedioic acid 10 (0.77 g, 96%) as a colorless amorphous solid.
(E)-oct-4-enedioic acid (10)
1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 2H), 5.44 (t, J = 3.2 Hz, 2H), 2.24 (m, 4H), 2.18 (m, 4H); 13C NMR (100 MHz, DMSO-d6) δ 173.9, 129.3, 33.6, 27.4; HRMS (FAB) m/z: [M-H+] for C8H11O4, calcd 171.0657; found, 171.0655.
(E)-dec-5-enedioic acid (11)
Colorless amorphous solid, (0.66 g, 92%); 1H NMR (500 MHz, DMSO-d6) δ 11.99 (s, 2H), 5.38 (d, J = 3.6 Hz, 2H), 2.19 (t, J = 7.4 Hz, 4H), 1.94 (m, 4H), 1.56 (m, 4H); 13C NMR (125 MHz, DMSO-d6) δ 174.6, 126.3, 63.7, 33.8, 31.9; HRMS (FAB) m/z: [M-H+] for C10H15O4, calcd 199.0970; found, 199.0969.
(E)-dodec-6-enedioic acid (12)
Colorless amorphous solid, (0.46 mg, 89%) 1H NMR (500 MHz, DMSO-d6) δ 11.96 (br s, 2H), 5.37 (t, J = 3.6 Hz, 2H), 2.18 (t, J = 7.4 Hz, 4H), 1.95 (m, 4H), 1.48 (m, 4H), 1.31 (m, 4H); 13C NMR (125 MHz, DMSO-d6) δ 174.4, 129.9, 33.5, 31.7, 28.5, 24.0; HRMS (FAB) m/z: [M-H+] for C12H19O4, calcd 227.1283; found, 227.1277.
General Procedure for peptide coupling of noviosylated olefin dimers
N-(3-(Dimethylamino)propyl)-N’-ethylcarbodiimide hydrochloride (176 mg, 0.92 mmol) was added to a solution of amino-coumarin 15 (164 mg, 0.38 mmol) and commercially available (E)-hex-3-enedioic acid (22 mg, 0.15 mmol) in CH2Cl2 containing 30% pyridine at rt. The resulting solution was stirred for 14 h, concentrated, and the residue purified by column chromatography on silica gel (CH2Cl2/acetone, 8/1) to afford the amides as colorless amorphous solids.
Et3N (10% total volume) was added dropwise to a solution of above cyclic carbonate diamides in methanol. The resulting mixture was stirred for 14 h, and concentrated. The residue was purified by column chromatography on silica gel (CH2Cl2/ MeOH, 19/1) to yield the olefin linked noviosylated dimer 16 (74% in two steps) as a colorless amorphous solid.
(E)-N1-(7-((2R,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-6-methoxy-8-methyl-2-oxo-2H-chromen-3-yl)-N6-(7-((2S,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-6-methoxy-8-methyl-2-oxo-2H-chromen-3-yl)hex-3-enediamide (16)
1H NMR (500 MHz, CDCl3) δ 8.52 (s, 2H), 6.77 (s, 2H), 5.86 (t, J = 4.5 Hz, 2H), 5.13 (d, J = 4.8 Hz, 2H), 4.17 (dd, J = 3.5, 6.6 Hz, 2H), 4.01 (t, J = 3.7 Hz, 2H), 3.82 (s, 6H), 3.48 (s, 6H), 3.46 (s, 6H), 3.13 (d, J = 6.7 Hz, 2H), 2.34 (s, 6H), 1.29 (s, 6H), 1.28 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 171.3, 158.9, 149.5, 146.6, 143.3, 127.5, 124.5, 122.6, 121.2, 115.5, 106.7, 102.8, 83.5, 78.3, 70.5, 68.6, 60.7, 56.1, 40.9, 26.4, 24.7, 9.9; IR (KBR) νmax 3400, 3286, 2972, 2931, 1703, 1681, 1529, 1385, 1250, 1114, 1084, 952, 770 cm−1; HRMS (FAB) m/z: [M+Na+] for C44H54N2NaO18, calcd 921.3269; found, 921.3239. ν
(E)-N1-(7-((2R,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methoxy-2-oxo-2H-chromen-3-yl)-N6-(7-((2S,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methoxy-2-oxo-2H-chromen-3-yl)hex-3-enediamide (17)
Colorless amorphous solid (81% in two steps). 1H NMR (500 MHz, DMSO-d6) δ 9.69 (s, 2H), 8.53 (s, 2H), 7.38 (d, J = 8.9 Hz, 2H), 7.18 (d, J = 8.9 Hz, 2H), 5.72 (t, J = 4.2 Hz, 2H), 5.48 (d, J = 2.2 Hz, 2H), 5.31 (d, J = 4.5 Hz, 2H), 5.05 (d, J = 6.1 Hz, 2H), 3.98 (m, 2H), 3.88 (m, 2H), 3.84 (s, 6H), 3.49 (s, 6H), 3.27 (d, J = 9.3 Hz, 4H), 3.27 (d, J = 9.3 Hz, 2H), 1.24 (s, 6H), 1.06 (s, 6H). 13C NMR (125 MHz, DMSO-d6) δ 170.8, 157.4, 150.8, 147.4, 143.7, 135.6, 126.9, 124.9, 122.6, 122.3, 114.4, 112.4, 99.2, 83.3, 78.0, 70.9, 67.5, 61.2, 61.1, 28.6, 22.9; IR (KBR) νmax 3400, 3342, 3286, 2972, 2931, 1703, 1681, 1529, 1435, 1385, 1298, 1114, 1089, 950, 770 cm−1; HRMS (FAB) m/z: [M+Na+] for C42H50N2NaO18, calcd 893.2956; found, 893.2952.
(E)-N1-(7-((2R,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-6-methoxy-8-methyl-2-oxo-2H-chromen-3-yl)-N8-(7-((2S,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-6-methoxy-8-methyl-2-oxo-2H-chromen-3-yl)oct-4-enediamide (18)
Colorless amorphous solid (84% in two steps). 1H NMR (500 MHz, CDCl3) δ 8.41 (s, 2H), 6.66 (s, 2H), 5.52 (t, J = 3.5 Hz, 2H), 5.10 (d, J = 4.5 Hz, 2H), 4.12 (dd, J = 3.5, 7.1 Hz, 2H), 4.00 (t, J = 3.5 Hz, 2H), 3.76 (s, 6H), 3.45 (s, 6H), 3.11 (d, J = 7.1 Hz, 2H), 2.43 (d, J = 6.7 Hz, 4H), 2.33 (m, 4H), 2.24 (s, 6H), 1.25 (s, 12H). 13C NMR (125 MHz, CDCl3) δ 172.3, 159.0, 149.4, 146.5, 143.2, 129.9, 124.3, 122.5, 120.9, 115.4, 106.4, 96.0, 83.6, 78.3, 70.5, 68.5, 60.7, 55.9, 36.8, 27.8, 26.7, 24.4, 9.7; IR (KBR) νmax 3440, 3398, 3313, 2974, 2933, 1714, 1686, 1627, 1529, 1465, 1389, 1120, 1066, 950, 769 cm−1; HRMS (FAB) m/z: [M+Na+] for C46H58N2NaO18, calcd 949.3582; found, 949.3589.
(E)-N1-(7-((2R,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methoxy-2-oxo-2H-chromen-3-yl)-N8-(7-((2S,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methoxy-2-oxo-2H-chromen-3-yl)oct-4-enediamide (19)
Colorless amorphous solid (69% in two steps). 1H NMR (400 MHz, CD3OD) δ 8.40 (s, 2H), 7.07 (d, J = 8.9 Hz, 2H), 7.00 (d, J = 8.9 Hz, 2H), 5.46 (t, J = 3.5 Hz, 2H), 5.38 (d, J = 2.4 Hz, 2H), 4.03 (dd, J = 3.4, 9.1 Hz, 2H), 3.99 (t, J = 3.4 Hz, 2H), 3.77 (s, 6H), 3.45 (s, 6H), 3.21 (d, J = 9.2 Hz, 2H), 2.38 (t, J = 6.6 Hz, 4H), 2.29 (m, 4H), 1.22 (s, 6H), 1.03 (s, 6H). 13C NMR (125 MHz, DMSO-d6) δ 171.3, 156.8, 150.1, 142.6, 134.9, 128.5, 123.4, 121.1, 113.5, 111.6, 98.4, 82.7, 77.3, 77.4, 70.1, 67.0, 60.5, 60.2, 35.3, 27.7, 27.0, 21.7; IR (KBR) νmax 3645, 3518, 3329, 2968, 2931, 2833, 1709, 1682, 1604, 1526, 1464, 1361, 1280, 1049, 1031, 950, 798 cm−1; HRMS (FAB) m/z: [M+Na+] for C44H54N2NaO18, calcd 921.3269; found, 921.3256. This material was determined to be 98.3% pure (Retention time = 2.174) by HPLC (Phenomenex Luna C-18, 5 micron, 10 ×250 mm column eluting with 49% CHCl3 49% MeOH and 2% H2O, flow rate 5.0 mL/min.
Gemneral Procedure for peptide coupling of non-noviosylated olefin dimers
N, N’-Dicyclohexylcarbodiimide (290 mg, 1.4 mmol), followed by 4-(N,N-dimethylamino)pyridine (137 mg, 1.12 mmol) and two drops of DMF were added simultaneously to a solution of (E)-hex-3-enedioic acid (40 mg, 0.28 mmol) in THF (3 mL) at rt. The mixture was stirred for 15 min, before adding amino coumarin 22 (295 mg, 0.7 mmol) in THF (2 mL). The resulting reaction mixture was stirred at 50 °C for 14 h, quenched with water, extracted with DCM (3 × 15 mL), and combined organic layers were washed with saturated NaCl, dried over anhydrous Na2SO4, filtered and concentrated. The crude residue was purified through silica gel column chromatography (CH2Cl2/MeOH/Et3N, 90/9/1) to give compound 26 (108 mg, 57%) as a colorless amorphous solid.
(E)-N1,N6-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)hex-3-enediamide (26)
1H NMR (500 MHz, CDCl3) δ 8.61 (s, 2H), 8.10 (s, 2H), 7.27 (d, J = 8.7 Hz, 2H), 6.85 (d, J = 8.7 Hz, 2H), 5.94 (t, J = 3.9 Hz, 2H), 4.45 (m, 2H), 3.29 (d, J = 5.5 Hz, 4H), 2.64 (m, 4H), 2.34 (m, 4H), 2.32 (s, 6H), 2.31 (s, 6H), 2.01 (m, 4H), 1.90 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 169.6, 159.3, 157.1, 149.6, 127.7, 125.6, 124.8, 121.2, 115.3, 113.2, 110.6, 72.6, 52.5, 46.4, 41.3, 30.9, 8.5; IR (KBr) νmax 3380, 3231, 3010, 2925, 2597, 1716, 1685, 1600, 1525, 1467, 1353, 1103 cm−1; HRMS (FAB) m/z: [M+H+] for C38H45N4O8, calcd 685.3237; found, 685.3222. This material was determined to be ~95% pure (Retention time = 2.137) by HPLC analysis on autosampler (Agilent TOF/AgilentA3B1C3.m method with 49% CHCl3 49% MeOH and 2% H2O, flow rate 5.0 mL/min.
(E)-N1,N6-bis(6-methoxy-8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)hex-3-enediamide (27)
Colorless amorphous solid (40 mg, 59%); 1H NMR (500 MHz, CD3OD) δ 8.51 (s, 2H), 6.74 (s, 2H), 5.80 (t, J = 3.7 Hz, 2H), 4.18 (m, 2H), 3.22 (d, 2H), 3.78 (s, 6H), 2.81 (m, 4H), 2.30 (m, 4H), 2.30 (s, 6H), 2.25 (s, 6H), 1.93 (m, 8H), 1.82 (4, 2H). 13C NMR (125 MHz, CD3OD) δ 170.3, 156.1, 150.2, 146.4, 140.6, 127.5, 124.8, 122.4, 121.1, 118.8, 115.0, 106.5, 55.9, 52.4, 45.3, 40.8, 30.8, 9.4; IR (KBR) νmax 3274, 2937, 2848, 1708, 1689, 1604, 1521, 1457, 1386, 1080, 772 cm−1; HRMS (FAB) m/z: [M+H+] for C40H49N4O10, calcd 745.3449; found, 745.3418. This material was determined to be ~97.3% pure (Retention time = 2.049) by HPLC analysis on autosampler (Agilent TOF/AgilentA3B1C3.m method with 49% CHCl3 49% MeOH and 2% H2O, flow rate 5.0 mL/min
(E)-N1,N6-bis(8-methoxy-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)hex-3-enediamide (28)
Colorless amorphous solid (34 mg, 44%); 1H NMR (500 MHz, CDCl3) δ 8.62 (s, 2H), 8.13 (s, 2H), 7.16 (d, J = 8.8 Hz, 2H), 6.93 (dd, J = 8.7, 17.2 Hz, 2H), 5.93 (m, 2H), 4.48 (m, 2H), 3.98 (s, 6H), 3.29 (dd, J = 1.6, 3.9 Hz, 4H), 2.81 (m, 4H), 2.46 (m, 4H), 2.41 (s, 6H), 2.14 (m, 4H), 1.98 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 169.7, 158.6, 151.9, 144.4, 137.8, 127.7, 124.3, 122.6, 121.9, 114.8, 113.6, 73.5, 61.7, 52.2, 45.9, 41.3, 30.4; IR (KBR) νmax 3377, 2943, 2881, 1701, 1691, 1604, 1518, 1460, 1357, 1205, 1059, 972 cm−1; HRMS (FAB) m/z: [M+H+] for C38H45N4O10, calcd 717.3136; found, 717.3135.
(E)-N1,N8-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)oct-4-enediamide (29)
Colorless amorphous solid (87 mg, 53%) 1H NMR (500 MHz, CDCl3+CD3OD) δ 8.46 (s, 2H), 7.16 (d, J = 8.7 Hz, 2H), 6.75 (d, J = 8.7 Hz, 2H), 5.49 (dd, J = 9.4, 12.9 Hz, 2H), 4.42 (m, 2H), 2.61 (m, 4H), 2.41 (m, 8H), 2.31 (m, 4H), 2.30 (s, 6H), 2.16 (s, 6H), 1.94 (m, 4H), 1.84 (m, 4H). 13C NMR (125 MHz, CDCl3+CD3OD) δ 172.2, 159.2, 156.6, 149.3, 129.8, 125.5, 125.2, 121.0, 114.8, 113.1, 110.3, 71.5, 51.7, 45.6, 36.8, 29.8, 27.9, 8.0; IR (KBR) νmax 3335, 3085, 3043, 2923, 2852, 1703, 1681, 1604, 1523, 1377, 1097, 771 cm−1; HRMS (FAB) m/z: [M+H+] for C40H49N4O8, calcd 713.3550; found, 713.3564. This material was determined to be ~100% pure (Retention time = 2.137) by HPLC analysis on autosampler (Agilent TOF/AgilentA3B1C3.m method with 49% CHCl3 49% MeOH and 2% H2O, flow rate 5.0 mL/min
(E)-N1,N8-bis(6-methoxy-8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)oct-4-enediamide (30)
Colorless amorphous solid (45 mg, 61%); 1H NMR (500 MHz, CDCl3) δ 8.56 (s, 2H), 6.76 (s, 2H), 5.59 (t, J = 3.5 Hz, 2H), 4.26 (m, 2H), 3.84 (s, 6H), 2.98 (m, 4H), 2.47 (m, 12H), 2.31 (s, 6H), 2.06 (m, 12H), 1.97 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 172.0, 159.1, 150.2, 146.8, 143.5, 130.0, 124.1, 122.6, 120.5, 115.3, 106.5, 56.0, 52.2, 45.3, 37.2, 37.1, 30.6, 28.1, 9.7; IR (KBR) νmax 3323, 2933, 2850, 1716, 1685, 1533, 1465, 1389, 1220, 1190, 771 cm−1; HRMS (FAB) m/z: [M+H+] for C42H53N4O10, calcd 773.3762; found, 773.3774.
(E)-N1,N8-bis(8-methoxy-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)oct-4-enediamide (31)
Colorless amorphous solid (27 mg, 49%); 1H NMR (500 MHz, CDCl3) δ 8.61 (s, 2H), 8.06 (s, 2H), 7.13 (d, J = 8.8 Hz, 2H), 6.90 (d, J = 8.9 Hz, 2H), 5.61 (t, J = 3.4 Hz, 2H), 4.42 (m, 2H), 3.98 (s, 6H), 2.71 (m, 4H), 2.50 (t, J = 6.6 Hz, 4H), 2.45 (m, 4H), 2.31 (m, 4H), 2.31 (s, 6H), 2.04 (m, 4H), 1.91 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 171.8, 158.8, 152.1, 144.1, 137.5, 130.1, 124.3, 122.4, 121.9, 114.7, 113.4, 74.4, 61.6, 52.7, 46.3, 37.3, 31.1, 28.2; IR (KBR) νmax 3374, 2948, 2880, 1704, 1690, 1604, 1522, 1465, 1362, 1227, 1067, 972, 773 cm−1; HRMS (FAB) m/z: [M+H+] for C40H49N4O10, calcd 745.3449; found, 745.3434. This material was determined to be ~93.3% pure (Retention time = 2.180) by HPLC analysis on autosampler (Agilent TOF/AgilentA3B1C3.m method with 49% CHCl3 49% MeOH and 2% H2O, flow rate 5.0 mL/min
(E)-N1,N10-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)dec-5-enediamide (32)
Colorless amorphous solid (47 mg, 77%); 1H NMR (500 MHz, CDCl3) δ 8.65 (s, 2H), 7.99 (s, 2H), 7.28 (d, J = 8.7 Hz, 2H), 6.86 (d, J = 8.7 Hz, 2H), 5.47 (t, J = 3.7 Hz, 2H), 4.47 (m, 2H), 2.68 (m, 4H), 2.42 (m, 8H), 2.35 (s, 6H), 2.32 (s, 6H), 2.12 (m, 4H), 2.05 (m, 4H), 1.93 (m, 4H), 1.82 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 172.4, 159.4, 157.0, 149.5, 130.5, 125.6, 124.6, 121.4, 115.4, 113.4, 110.6, 72.2, 52.4, 46.3, 37.0, 32.0, 30.8, 25.1, 8.5; IR (KBR) νmax 3328.9, 2935, 2786, 1708, 1676, 1604, 1527, 1371, 1265, 1099, 769 cm−1; HRMS (FAB) m/z: [M+H+] for C42H53N4O8, calcd 741.3863; found, 741.3863.
E)-N1,N10-bis(6-methoxy-8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)dec-5-enediamide (33)
Colorless amorphous solid (54 mg, 70%); 1H NMR (500 MHz, CDCl3) δ 8.63 (s, 2H), 8.07 (s, 2H), 6.79 (s, 2H), 5.46 (t, J = 3.7 Hz, 2H), 4.22 (m, 2H), 3.86 (s, 6H), 2.77 (m, 4H), 2.43 (t, J = 7.5 Hz, 4H), 2.35 (s, 6H), 2.29 (s, 6H), 2.11 (m, 8H), 1.93 (m, 8H), 1.84 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 172.5, 159.2, 150.5, 147.1, 143.6, 130.5, 124.0, 122.6, 120.8, 115.1, 106.5, 78.5, 56.1, 53.6, 46.2, 37.0, 32.1, 31.9, 25.1, 9.8; IR (KBR) νmax 3325, 2939, 2849, 1708, 1686, 1521, 1465, 1387, 1085, 1010, 772 cm−1; HRMS (FAB) m/z: [M+H+] for C44H57N4O10, calcd 801.4075; found, 801.4058.
(E)-N1,N10-bis(8-methoxy-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)dec-5-enediamide (34)
Colorless amorphous solid (24 mg, 42%); 1H NMR (500 MHz, CDCl3) δ 8.65 (s, 2H), 8.02 (s, 2H), 7.16 (d, J = 8.8 Hz, 2H), 6.91 (d, J = 8.8 Hz, 2H), 5.46 (tt, J = 1.4, 3.8 Hz, 2H), 4.47 (m, 2H), 3.99 (s, 6H), 2.78 (t, J = 10.1 Hz, 4H), 2.44 (m, 4H), 2.42 (t, J = 7.4 Hz, 4H), 2.38 (s, 6H), 2.11 (m, 8H), 1.96 (m, 4H), 1.81 (p, J = 7.2, 14.5 Hz, 4H); 1H NMR (125 MHz, CDCl3) δ 172.5, 158.9, 151.9, 144.4, 137.8, 130.5, 124.1, 122.5, 122.0, 114.9, 113.5, 61.6, 52.3, 46.0, 37.0, 31.9, 30.6, 30.1, 25.1; IR (KBR) νmax 3379, 29439, 2864, 1718, 1697, 1647, 1607, 1521, 1460, 1369, 1280, 1034, 968, 767 cm−1; HRMS (FAB) m/z: [M+H+] for C42H53N4O10, calcd 773.3762; found, 773.3757. This material was determined to be ~93.5% pure (Retention time = 2.353) by HPLC analysis on autosampler (Agilent TOF/AgilentA3B1C3.m method with 49% CHCl3 49% MeOH and 2% H2O, flow rate 5.0 mL/min
(E)-N1,N12-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)dodec-6-enediamide (35)
Colorless amorphous solid (54 mg, 68%) 1H NMR (500 MHz, CDCl3) δ 8.62 (s, 2H), 7.98 (s, 2H), 7.24 (d, J = 8.6 Hz, 2H), 6.83 (d, J = 8.7 Hz, 2H), 5.41 (t, J = 3.7 Hz, 2H), 4.45 (m, 2H), 2.65 (m, 4H), 2.42 (t, J = 7.5 Hz, 4H), 2.37 (m, 4H), 2.32 (s, 6H), 2.30 (s, 6H), 2.02 (m, 8H), 1.90 (m, 4H), 1.73 (m, 4H), 1.46 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 172.5, 159.4, 156.9, 149.5, 130.3, 125.5, 124.5, 121.4, 115.3, 113.3, 110.6, 52.4, 46.3, 37.7, 32.3, 30.8, 29.1, 25.0, 8.5; IR (KBR) νmax 3327, 2931, 2358, 1712, 1676, 1605, 1529, 1371, 1261, 1097, 1041, 771 cm−1; HRMS (FAB) m/z: [M+H+] for C44H57N4O8, calcd 769.4176; found, 769.4193.
(E)-N1,N6-bis(7-(3-(dimethylamino)propoxy)-8-methyl-2-oxo-2H-chromen-3-yl)hex-3-enediamide (36)
Colorless amorphous solid (24 mg, 34%); 1H NMR (500 MHz, CDCl3) δ 8.45 (s, 2H), 7.15 (dd, J = 3.9, 8.5, 2H), 6.72 (d, J = 8.5 Hz, 2H), 5.77 (t, J = 4.6 Hz, 4H), 3.94 (t, J = 5.4 Hz, 4H), 3.14 (m, 4H), 2.40 (m, 4H), 2.15 (s, 6H), 2.14 (s, 6H), 2.13 (s, 6H), 1.89 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 170.3, 159.1, 158.3, 149.1, 127.3, 125.6, 120.7, 113.6, 112.9, 108.6, 66.6, 56.1, 44.8, 44.5, 40.6, 26.9, 7.7; IR (KBR) νmax 3312, 2939, 2857, 1707, 1682, 1608, 1521, 1365, 1269, 1172, 1039, 903 cm−1; HRMS (FAB) m/z: [M+H+] for C36H45N4O8, calcd 661.3237; found, 661.3215.
Z)-N1,N8-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)oct-4-enediamide (38)
1H NMR (400 MHz, CDCl3) δ 8.58 (s, 2H), 7.30 (d, J = 8.6 Hz, 2H), 6.86 (d, J = 8.7 Hz, 2H), 5.43 (m, 2H), 4.63 (m, 2H), 2.94 (m, 8H), 2.57 (s, 6H), 2.45 (s, 6H), 2.31 (m, 12H), 2.07 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 178.3, 174.0, 160.9, 158.0, 151.3, 138.0, 132.2, 131.8, 130.3, 127.5, 125.7, 124.4, 123.6, 123.5, 116.6, 115.5, 112.3, 54.2, 52.6, 46.1, 39.2, 39.1, 36.3, 30.6, 30.0, 29.8, 26.7, 25.0, 24.9, 9.9; IR (KBR) νmax 3335, 3085, 3043, 2923, 2852, 1703, 1681, 1604, 1523, 1377, 1097, 771 cm−1; HRMS (FAB) m/z: [M+H+] for C40H49N4O8: calcd 713.3550; found, 713.3564.
Gemneral Procedure for peptide coupling of non-noviosylated saturated linker dimer
Pyridine (45 µL, 0.56 mmol) was added to a solution of amino coumarin 22 (80 mg, 0.28 mmol) in 4 mL of THF and stirred for 15 min at rt and adipoyl dichloride (16 µL, 0.11 mmol) was added drop wise. The resulting reaction mixture was stirred at rt for about 15 h and concentrated. The residue was purified by silica gel column chromotography (CH2Cl2/MeOH, 98/2) to get saturated linked dimer 42 (66 mg, 89%) as a colorless amorphous solid.
N1,N6-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)adipamide (42)
1H NMR (500 MHz, CD3OD) δ 8.35 (s, 2H), 7.06 (d, J = 8.7 Hz, 2H), 6.66 (d, J = 8.7 Hz, 2H), 4.31 (m, 2H), 2.42 (m, 4H), 2.28 (t, J = 6.5 Hz, 4H), 2.21 (m, 4H), 2.08 (s, 6H), 2.04 (s, 6H), 1.77 (m, 4H), 1.67 (m, 4H), 1.57 (m, 4H); 13C NMR (125 MHz, CD3OD) δ 172.8, 159.9, 157.3, 149.9, 125.6, 125.4, 120.9, 114.5, 112.9, 110.2, 51.6, 45.4, 36.3, 29.9, 24.5, 7.7; IR (KBR) νmax 3514, 3201, 2927, 2783, 1718, 1687, 1622, 1404, 1346, 1284, 1103, 992 cm−1; HRMS (FAB) m/z: [M+H+] for C38H47N4O8, calcd 687.3394; found, 687.3378.
N1,N8-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)octanediamide (43)
Colorless amorphous solid (59 mg, 81%); 1H NMR (500 MHz, CD3OD) δ 8.48 (s, 2H), 7.17 (d, I = 8.6 Hz, 2H), 6.75 (d, J = 8.8 Hz, 2H), 4.38 (m, 2H), 2.53 (t, J = 10.6 Hz, 4H), 2.32 (t, J = 7.5 Hz, 4H), 2.31 (m, 4H), 2.19 (s, 6H), 2.17 (s, 6H), 1.88 (m, 4H), 1.79 (m, 4H), 1.62 (m, 4H), 1.32 (m, 4H); 13C NMR (125 MHz, CD3OD) δ 173.0, 159.2, 156.7, 149.3, 125.5, 125.3, 121.0, 114.8, 113.1, 110.4, 52.0, 45.6, 36.9, 30.0, 28.6, 24.9, 8.0; IR (KBR) νmax 3378, 2928, 2783, 1716, 1685, 1612, 1422, 1354, 1289, 1111, 992 cm−1; HRMS (FAB) m/z: [M+H+] for C40H51N4O8, calcd 715.3707; found, 715.3700.
N1,N10-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)decanediamide (44)
Colorless amorphous solid (65 mg, 87%); 1H NMR (500 MHz, CDCl3) δ 8.60 (s, 2H), 7.25 (d, J = 8.6 Hz, 2H), 6.82 (d, J = 8.7 Hz, 2H), 4.46 (m, 2H), 2.65 (m, 4H), 2.41 (m, 4H), 2.38 (t, J = 7.6 Hz, 4H), 2.31 (s, 6H), 2.28 (s, 6H), 1.99 (m, 4H), 1.89 (m, 4H), 1.69 (m, 4H), 1.28 (m, 8H); 13C NMR (125 MHz, CDCl3) δ 172.7, 159.4, 156.7, 149.3, 125.6, 124.8, 121.2, 115.1, 113.3, 110.5, 72.0, 51.9, 46.0, 37.5, 30.3, 29.2, 29.1, 25.3, 8.3; IR (KBR) νmax 3323, 2931, 2852, 2470, 1713, 1674, 1623, 1604, 1527, 1408, 1267, 1043, 729 cm−1; HRMS (FAB) m/z: [M+H+] C42H55N4O8, calcd 743.4020; found, 743.4009.
methyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate (50)
Bis(pinacolate)diboron (7.24 g, 28.49 mmol) and potassium acetate (6.45 g, 65.75 mmol) followed by Pd(dppf)2Cl2 (894 mg, 1.1 mmol) were added simultaneously to a solution of methyl 3-(trifluoromethylsulfonyloxy)benzoate 47 (6.22 g, 21.92 mmol) in 1, 4-dioxane (80 mL) at rt. The resulting reaction mixture was stirred at 90 °C for 16 h and diluted with 1N hydrogen chloride (100 mL). The aqueous layer was extracted with ethyl acetate (3 × 100 mL) and the combined extracts were washed with saturated NaCl, dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (hexane/EtOAc, 7/3) to give methyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate 50 as a amorphous brown solid, (4.59 g, 80%). 1H NMR (500 MHz, CDCl3) δ 8.47 (s, 1H), 8.13 (dt, J = 1.5, 7.8 Hz, 1H), 7.99 (dt, J = 1.3, 7.4, 1H), 7.45 (t, J = 7.6, 1H), 3.92 (s, 3H), 1.39 (m, 12H); 13C NMR (125 MHz, CDCl3) δ 167.3, 139.3, 135.9, 132.4, 127.9, 84.2, 52.2, 25.0; HRMS (FAB) m/z: [M+Na+] for C14H19BNaO4, calcd 285.1274; found, 285.1272.
methyl 4-methoxy-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate (51)
amorphous brown solid (4.35 g, 68%). 1H NMR (400 MHz, DMSO-d6) δ 8.18 (d, J = 2.3 Hz, 1H), 8.05 (dd, J = 2.4, 8.7 Hz, 1H), 7.10 (d, J = 8.8 Hz, 1H), 3.83 (s, 3H), 3.82 (s, 3H), 1.29 (s, 12H); 13C NMR (100 MHz, DMSO-d6) δ 167.9, 165.7, 137.8, 134.3, 121.3, 110.9, 83.6, 53.8, 51.4, 24.3; HRMS (FAB) m/z: [M+Na+] for C15H21BNaO5, calcd 315.1380; found, 315.1377.
methyl 3-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate (52)
Colorless amorphous solid, (4.26 g, 71%); 1H NMR (400 MHz, CDCl3) δ 8.01 (t, J = 1.1 Hz, 1H), 7.65 (dd, J = 1.7, 2.8 Hz, 1H), 7.52 (dd, J = 1.7, 1.0 Hz, 1H), 3.93 (s, 3H), 3.85 (s, 3H), 1.35 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 167.1, 159.5, 131.0, 128.1, 124.5, 117.6, 84.1, 55.5, 52.1, 25.0; HRMS (FAB) m/z: [M+Na+] for C15H21BNaO5, calcd 315.1380; found, 315.1379.
dimethyl biphenyl-3,3’-dicarboxylate (53)
Pd(dppf)2Cl2 (475 mg, 0.52 mmol) and K2CO3 (4.83 g, 34.93 mmol) were added to the miture of methyl 3-(trifluoromethylsulfonyloxy)benzoate 47 (3.3 g, 11.64 mmol) and methyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate 50 (3.05 g, 11.64 mmol) in dioxane (50 mL) at rt. The resulting reaction mixture was stirred at 90 °C for 14 h then filtered through a pad of silica gel, eluted with EtOAc and the eluents were concentrated. The residue was purified by silica gel column chromatography (hexane/EtOAc, 4/1) to give dimethyl biphenyl-3,3’-dicarboxylate 53 (2.13 g, 68%) as a amorphous white solid. 1H NMR (400 MHz, CDCl3) δ 8.31 (s, 2H), 8.06 (d, J = 7.7 Hz, 2H), 7.83 (d, J = 7.7 Hz, 2H), 7.55 (t, J = 7.8 Hz, 2H), 3.97 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 167.1, 140.5, 131.7, 131.0, 129.2, 129.0, 128.4, 52.4, 25.0; HRMS (FAB) m/z: [M+Na+] for C16H14NaO4, calcd 293.0790; found, 293.0793.
dimethyl 6,6’-dimethoxybiphenyl-3,3’-dicarboxylate: General procedure for Suzuki-Coupling reaction (54)
(2.73 g, 71%) as a colorless amorphous solid. 1H NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 2.2, 8.7 Hz, 2H), 7.95 (d, J = 2.2 Hz, 2H), 7.01 (d, J = 8.7 Hz, 2H), 3.91 (s, 6H), 3.84 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 166.9, 160.8, 133.0, 131.3, 126.8, 122.3, 110.4, 55.9, 51.9; HRMS (FAB) m/z: [M+Na+] for C18H18NaO6, calcd 353.1001; found, 353.0999.
dimethyl 5,6’-dimethoxybiphenyl-3,3’-dicarboxylate (55)
Colorless amorphous solid (1.89 g, 76%). 1H NMR (500 MHz, CDCl3) δ 8.05 (dd, J = 2.2, 8.6 Hz, 1H), 8.02 (d, J = 2.2 Hz, 1H), 7.79 (t, J = 1.5 Hz, 1H), 7.56 (dd, J = 1.4, 2.6 Hz, 1H), 7.28 (dd, J = 1.6, 2.6 Hz, 1H), 7.01 (d, J = 8.7 Hz, 1H), 3.93 (s, 3H), 3.91 (s, 3H), 3.89 (s, 3H), 3.88 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 167.1, 166.9, 160.2, 159.4, 139.2, 132.2, 131.4, 129.6, 123.5, 122.9, 121.0, 113.0, 110.8, 56.0, 55.7, 52.1; HRMS (FAB) m/z: [M+Na+] for C18H18NaO6, calcd 353.1001; found, 353.0999.
dimethyl 5,5’-dimethoxybiphenyl-3,3’-dicarboxylate (56)
Colorless amorphous solid (0.81 g, 58%). 1H NMR (500 MHz, CDCl3) δ 7.42 (d, J = 1.1 Hz, 2H), 7.10 (d, J = 1.1 Hz, 2H), 6.86 (d, J = 1.4 Hz, 2H), 3.49 (s, 6H), 3.44 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 166.9, 160.1, 141.7, 132.1, 121.0, 118.3, 113.4, 55.8, 52.4; HRMS (FAB) m/z: [M+Na+] for C18H18NaO6, calcd 353.1001; found, 353.0999.
Biphenyl-3,3’-dicarboxylic acid (57)
LiOH (3.4 g, 80.9 mmol) was added to the solution of dimethyl biphenyl-3,3’-dicarboxylate 53 (2.19 g, 8.09 mmol) in 40 mL of THF:MeOH:H2O (3:2:2) at room teperature and stirred for 6 h. The resulting reaction mixture was acidfied to pH ~ 4 with 2N HCl, the solid product was precpitated out and filtered off the solid product, re-suspended in CH3CN and concentrated to get biphenyl-3,3’-dicarboxylic acid 57 (1.88 g, 96%) as a colorless amorphous solid. 1H NMR (400 MHz, DMSO-d6) δ 13.18 (s, 2H), 8.21 (s, 2H), 7.98 (m, 4H), 7.64 (t, J = 8.8 Hz, 2H); 13C NMR (100 MHz, DMSO) δ 167.3, 139.7, 131.8, 131.3, 129.7, 128.8, 127.5; (FAB) m/z: [M-H+] for C14H9O4, calcd 241.0501; found, 241.0506.
6,6’-dimethoxybiphenyl-3,3’-dicarboxylic acid (58)
Colorless amorphous solid, (2.19 g, 90%) 1H NMR (500 MHz, DMSO-d6) δ 7.97 (dd, J = 2.1, 8.6 Hz, 2H), 7.71 (d, J = 2.1 Hz, 2H), 7.20 (t, J = 12.1 Hz, 2H), 3.79 (s, 6H); 13C NMR (125 MHz, DMSO-d6) δ 167.0, 160.3, 132.2, 130.9, 126.3, 122.8; HRMS (FAB) m/z: [M+Cl−] for C16H14ClO6, calcd 337.0479; found, 337.0482.
5,6’-dimethoxybiphenyl-3,3’-dicarboxylic acid (59)
Colorless amorphous solid (1.71 g, 92%). 1H NMR (500 MHz, DMSO-d6) δ 12.93 (s, 2H), 7.98 (dd, J = 2.1, 8.6 Hz, 1H), 7.86 (d, J = 2.1 Hz, 1H), 7.63 (s, 1H), 7.45 (s, 1H), 7.30 (s, 1H), 7.23 (d, J = 8.7 Hz, 1H), 3.86 (s, 3H), 3.85 (s, 3H); 13C NMR (125 MHz, DMSO) δ 167.1, 166.9, 159.7, 159.1, 138.9, 132.1, 131.5, 131.2, 128.6, 123.2, 122.5, 119.8, 112.9, 111.7, 56.1, 55.5; HRMS (FAB) m/z: [M-H+] for C16H13O6, calcd 301.0712; found, 301.0707.
5,5’-dimethoxybiphenyl-3,3’-dicarboxylic acid (60)
Colorless amorphous solid (0.64 g, 93%). 1H NMR (500 MHz, DMSO-d6) δ 13.18 (s, 2H), 7.78 (t, J = 1.5 Hz, 2H), 7.90 (m, 4H), 3.90 (s, 6H); 13C NMR (125 MHz, DMSO-d6) δ 167.0, 159.9, 141.0, 132.7, 120.0, 117.2, 113.7, 55.6; HRMS (FAB) m/z: [M-H+] for C16H13O6, calcd 301.0712; found, 301.0707.
General Procedure for peptide coupling of biaryl linkers
Thionyl chloride (0.12 mL, 1.6 mmol) was added to a solution of diacid acid 57 (39 mg, 0.16 mmol) in 3 mL of THF. The resulting reaction mixture was refluxed for 3 h, solvent was evapourated under reduced pressure and kept under high vaccume for 1–2 h to get biphenyl-3,3’-dicarbonyl dichloride 61 as a colorless soild, used immediately for next coupling reaction without any further purification.
Pyridine (67 µL, 0.83 mmol) was added to a solution of amino coumarin 13 (120 mg, 0.41 mmol) in 4 mL of THF, stirred for 15 min at rt and above freshly prepared diacid chloride 61 was added drop wise in 2 mL of THF. The resulting reaction mixture was stirred at rt for about 15 h and concentrated to get crude product. The residue was purified by silica gel column chromotography to get tilte biaryl dimer as colorless amorphous solid.
General Procedure for noviosylated biaryl dimers cyclic carbonate cleavage
Et3N (10% total volume) was added drop wise to a solution of above cyclic carbonate di amides in methanol. The resulting mixture was stirred for 14 h, and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH, 19/:1) to yield olefin linked noviosylated dimer 65 (89 mg, 61% yield, over all in two steps) as a colorless amorphous solid.
N3-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N3’-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)biphenyl-3,3’-dicarboxamide (65)
1H NMR (500 MHz, CDCl3) δ 8.84 (s, 2H), 8.82 (s, 2H), 8.19 (s, 2H), 7.92 (d, J = 8.0 Hz, , 2H), 7.86 (d, J = 8.3 Hz, 2H), 7.64 (t, J = 7.7 Hz, 2H), 7.37 (d, J = 8.7 Hz, 2H), 7.21 (d, J = 8.8 Hz, 2H), 5.63 (d, J = 1.7 Hz, 2H), 4.27 (m, 4H), 3.62 (s, 6H), 3.39 (d, J = 8.9 Hz, 2H), 2.72 (br s, 4H), 2.30 (s, 6H), 1.40 (s, 6H), 1.16 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 165.9, 159.5, 156.2, 149.3, 141.0, 134.7, 131.3, 129.7, 126.4, 126.0, 124.9, 121.8, 114.4, 114.1, 111.3, 97.8, 84.4, 78.7, 71.3, 68.7, 62.1, 29.4, 22.6, 8.6; ; IR (KBR) νmax 3392, 3315, 2926, 2869, 1710, 1168, 1665, 1607, 1520, 1367, 1253, 1211, 1140, 1085, 964 cm−1; HRMS (FAB) m/z: [M+Na+] for C50H52N2NaO16, calcd 959.3215; found, 959.3209. This material was determined to be 95.6% pure (Retention time = 28.147) by HPLC (Phenomenex Luna C-18, 5 micron, 10 ×250 mm column eluting with 50% CH3CN/50% H2O, flow rate 5.0 mL/min.
N3-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N3’-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-6,6’-dimethoxybiphenyl-3,3’-dicarboxamide (66)
Colorless amorphous solid, (37 mg, 58% yield, over all in two steps). 1H NMR (500 MHz, CDCl3) δ 8.81 (s, 2H), 8.72 (s, 2H), 8.00 (d, J = 8.7 Hz, 2H), 7.86 (s, 2H), 7.36 (d, J = 8.6 Hz, 2H), 7.21 (d, J = 8.8 Hz, 2H), 7.11 (d, J = 8.8 Hz, 2H), 5.63 (s, 2H), 4.26 (m, 4H), 3.89 (s, 6H), 3.62 (s, 6H), 3.40 (d, J = 9.6 Hz, 2H), 2.57 (br s, 4OH), 2.30 (s, 6H), 1.40 (s, 6H), 1.16 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 166.9, 161.3, 160.4, 157.1, 149.9, 127.7, 126.7, 126.3, 122.4, 114.9, 114.7, 112.1, 111.8, 99.3, 85.0, 79.4, 72.1, 69.2, 62.5, 56.7, 29.6, 23.2, 8.9; IR (KBR) νmax 3402, 3312, 2927, 2867, 1712, 1169, 1667, 1604, 1521, 1498, 1367, 1251, 1207, 1142, 1080, 964 cm−1; HRMS (FAB) m/z: [M+Na+] for C52H56N2NaO18, calcd 1019.3426; found, 1019.3413. This material was determined to be 99.2% pure (Retention time = 2.3123) by HPLC (Phenomenex Luna C-18, 5 micron, 10 ×250 mm column eluting with 49% CHCl3\49% MeOH and 2% H2O, flow rate 5.0 mL/min.
N3’-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N3-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-5,6’-dimethoxybiphenyl-3,3’-dicarboxamide (67)
Isolated using 5% of methanol in dichlorometane, colorless amorphous solid (59 mg, 75% yield, over all in two steps); 1H NMR (500 MHz, CDCl3) δ 8.81 (s, 1H), 8.79 (s, 1H), 8.78 (s, 1H), 8.72 (s, 1H), 7.93 (m, 2H), 7.65 (s, 1H), 7.47 (s, 1H), 7.34 (dd, J = 2.0, 8.6 Hz, 2H), 7.26 (m, 1H), 7.19 (d, J = 8.6 Hz, 2H), 7.10 (d, J = 8.6 Hz, 1H), 5.61 (s, 2H), 4.25 (s, 4H), 3.95 (s, 3H), 3.94 (s, 3H), 3.61 (s, 6H), 3.39 (d, J = 8.9 Hz, 2H), 2.74 (br s, 2H), 2.65 (br s, 2H), 2.29 (s, 6H), 1.39 (s, 6H), 1.15 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 170.3, 169.8, 163.8, 163.7, 163.5, 163.4, 160.3, 160.2, 153.1, 153.1, 143.3, 138.8, 133.8, 133.5, 132.8, 129.9, 129.8, 129.8, 129.5, 129.4, 125.4, 125.3, 124.3, 123.5, 118.0, 118.0, 117.7, 117.6, 115.5, 115.2, 102.4, 88.0, 82.5, 75.1, 72.3, 65.5, 64.5, 59.7, 59.4, 32.6, 26.3, 11.9; IR (KBR) νmax 3371, 3301, 2927, 2852, 1714, 1700, 1670, 1604, 1521, 1500, 1367, 1251, 1205, 1138, 1082, 964 cm−1; HRMS (FAB) m/z: [M+H+] for C52H57N2O18, calcd 997.3606; found, 997.3618.
N3-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N3’-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-5,5’-dimethoxybiphenyl-3,3’-dicarboxamide (68)
Isolated using 5% of methanol in dichlorometane, colorless amorphous solid (12 mg, 54% yield, over all in two steps); 1H NMR (500 MHz, acetone-d6) δ 8.98 (m, 2H), 8.62 (s, 2H), 7.77 (s, 2H), 7.41 (m, 6H), 7.13 (d, J = 8.7 Hz, 2H), 5.47 (s, 2H), 4.33 (m, 2OH), 3.99 (m, 4H), 3.87 (s, 6H), 3.25 (d, J = 8.8 Hz, 2H), 2.14 (s, 6H), 1.19 (s, 6H), 0.98 (s, 6H); 13C NMR (125 MHz, acetone-d6) δ 166.3, 161.4, 159.2, 157.4, 150.2, 142.9, 137.2, 126.8, 125.9, 119.2, 117.6, 114.6, 114.5 113.0, 112.0, 99.6, 84.7, 79.0, 72.3, 69.5, 61.8, 56.1, 23.3, 8.5; IR (KBR) νmax 3401, 3387, 2927, 2877, 1712, 1700, 1668, 1604, 1525, 1501, 1367, 1248, 1205, 1136, 1080, 962 cm−1; HRMS (FAB) m/z: [M+Na+] for C52H56N2NaO18, calcd 1019.3426; found, 1019.3401.
N3-(7-((2R,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-6-methoxy-8-methyl-2-oxo-2H-chromen-3-yl)-N3’-(7-((2S,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-6-methoxy-8-methyl-2-oxo-2H-chromen-3-yl)-6,6’-dimethoxybiphenyl-3,3’-dicarboxamide (69)
Isolated using 5% of methanol in dichlorometane, colorless amorphous solid (94 mg, 77% yield, over all in two steps); 1H NMR (500 MHz, DMSO) δ 9.59 (s, 2H), 8.53 (s, 2H), 8.04 (dd, J = 1.9, 8.6 Hz, 2H), 7.87 (d, J = 1.4 Hz, 2H), 7.32 (s, 2H), 7.26 (d, J = 8.9 Hz, 2H), 5.23 (d, J = 3.0 Hz, 2H), 5.05 (d, J = 4.8 Hz, 2OH), 4.95 (d, J = 5.9 Hz, 2OH), 4.03 (m, 2H), 3.86 (m, 2H), 3.84 (s, 6H), 3.82 (s, 6H), 3.48 (s, 6H), 3.19 (d, J = 8.6 Hz, 2H), 2.30 (s, 6H), 1.27 (s, 6H), 1.25 (s, 6H); 13C NMR (125 MHz, DMSO-d6) δ 165.1, 160.0, 158.1, 149.2, 146.0, 143.5, 130.6, 129.3, 127.7, 126.5, 125.3, 122.9, 119.1, 114.8, 117.2, 107.9, 103.8, 83.3, 77.9, 70.6, 67.6, 56.3, 56.0, 28.0, 24.1, 9.7; IR (KBR) νmax 3458, 3400, 2976, 2937, 1714, 1672, 1604, 1523, 1462, 1365, 1250, 1110, 950, 760 cm−1; HRMS (FAB) m/z: [M+Na+] for C54H60N2NaO20, calcd 1079.3637; found, 1079.3622. This material was determined to be 95.6% pure (Retention time = 11.138) by HPLC (Phenomenex Luna C-18, 5 micron, 10 ×250 mm column eluting with 450% CH3CN3 50% H2O, flow rate 5.0 mL/min.
N3-(7-((2R,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methoxy-2-oxo-2H-chromen-3-yl)-N3’-(7-((2S,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methoxy-2-oxo-2H-chromen-3-yl)-6,6’-dimethoxybiphenyl-3,3’-dicarboxamide (70)
Isolated using 5% of methanol in dichlorometane, colorless amorphous solid, (67 mg, 82% yield, over all in two steps); 1H NMR (500 MHz, CDCl3) δ 8.78 (s, 2H), 8.70 (s, 2H), 7.98 (dd, J = 2.4, 8.7 Hz, 2H), 7.83 (d, J = 2.4 Hz, 2H), 7.23 (d, J = 8.9 Hz, 2H), 7.20 (d, J = 8.9 Hz, 2H), 7.09 (d, J = 8.8 Hz, 2H), 5.56 (d, J = 2.4 Hz, 2H), 4.27 (m, 4H), 3.95 (s, 6H), 3.87 (s, 6H), 3.60 (s, 6H), 3.36 (d, J = 8.7 Hz, 2H), 2.76 (br s, 2H), 2.18 (br s, 2H), 1.40 (s, 6H), 1.22 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 165.6, 160.5, 158.9, 151.2, 144.0, 136.7, 130.7, 129.1, 126.8, 125.6, 123.8, 122.8, 122.6, 115.4, 113.3, 111.1, 98.8, 84.2, 78.8, 71.1, 68.7, 61.9, 61.9, 56.1, 28.9, 23.0; IR (KBR) νmax 3458, 3400, 2976, 2937, 1714, 1672, 1604, 1523, 1462, 1365, 1250, 1110, 950, 760 cm−1; HRMS (FAB) m/z: [M+Na+] for C52H56N2NaO20, calcd 1051.3324; found, 1051.3339; This material was determined to be 95.1% pure (Retention time = 2.314) by HPLC (Phenomenex Luna C-18, 5 micron, 10 ×250 mm column eluting with 49% CHCl3/49% MeOH and 2% H2O, flow rate 5.0 mL/min.
6,6’-dimethoxy-N3,N3’-bis(8-methyl-7-(1-methylpiperidin-4-yloxy)-2-oxo-2H-chromen-3-yl)biphenyl-3,3’-dicarboxamide (71)
Isolated using 10% of methanol in dichlorometane, colorless amorphous solid, (46 mg, 87%); 1H NMR (500 MHz, CDCl3) δ 8.79 (s, 2H), 8.70 (s, 2H), 7.99 (dd, J = 2.4, 8.7 Hz, 2H), 7.85 (d, J = 2.4 Hz, 2H), 7.31 (d, J = 8.6 Hz, 2H), 7.09 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 8.8 Hz, 2H), 4.46 (m, 2H), 3.88 (s, 6H), 2.65 (m, 4H), 2.36 (m, 4H), 2.34 (s, 6H), 2.32 (s, 6H), 2.02 (m, 4H), 1.91 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 165.5, 160.4, 159.6, 157.0, 149.5, 130.7, 129.0, 126.8, 125.8, 125.6, 124.4, 121.7, 115.3, 113.5, 111.0, 110.6, 72.5, 56.1, 52.4, 46.4, 30.9, 8.5; IR (KBR) νmax 3406, 2937, 2843, 1707, 1664, 1603, 1521, 1491, 1367, 1238, 1103, 1041, 762 cm−1; HRMS (FAB) m/z: [M+H+] for C48H51N4O10, calcd 843.3605; found, 843.3570.
N3,N3’-bis(7-(3-(dimethylamino)propoxy)-8-methyl-2-oxo-2H-chromen-3-yl)-6,6’-dimethoxybiphenyl-3,3’-dicarboxamide (72)
Isolated using 10%-15% of methanol in dichlorometane, colorless amorphous solid, (27 mg, 69%); 1H NMR (400 MHz, DMSO-d6) δ 9.61 (s, 2H), 8.46 (s, 2H), 8.04 (d, J = 8.7 Hz, 2H), 7.87 (d, J = 2.2 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.25 (d, J = 8.9 Hz, 2H), 7.08 (d, J = 8.8 Hz, 2H), 4.19 (t, J = 5.8 Hz, 4H), 3.82 (s, 6H), 3.21 (t, J = 6.9 Hz, 4H), 2.76 (s, 12H), 2.24 (s, 6H), 2.20 (m, 4H); 13C NMR (100 MHz, DMSO-d6) δ 165.1, 159.9, 158.2, 157.9, 149.5, 130.6, 129.6, 129.3, 126.5, 126.3, 125.3, 121.3, 112.9, 112.5, 111.1, 109.2, 65.9, 55.9, 54.2, 54.1, 42.4, 24.2, 8.0; IR (KBR) νmax 3413, 2958, 2941, 1699, 1668, 1606, 1529, 1502, 1371, 1265, 1159, 1020, 762 cm−1; HRMS (FAB) m/z: [M+H+] for C46H51N4O10, calcd 819.3605; found, 819.3602.
3-(2’,6-dimethoxy-5’-(7-acetyloxy-8-methyl-2-oxo-2H-chromen-3-ylcarbamoyl)biphenyl-3-ylcarboxamido)-8-methyl-2-oxo-2H-chromen-7-yl acetate (73)
Isolated using 4% of methanol in dichlorometane, colorless amorphous solid, (19 g, 47%); 1H NMR (500 MHz, DMSO-d6) δ 9.67 (s, 2H), 8.58 (s, 2H), 8.04 (s, 2H), 7.88 (s, 2H), 7.65 (d, J = 8.3 Hz, 2H), 7.27 (d, J = 8.3 Hz, 2H), 7.15 (d, J = 8.4 Hz, 2H), 3.83 (s, 6H), 2.36 (s, 6H), 2.19 (s, 6H). 13C NMR (125 MHz, DMSO-d6) δ 168.9, 165.2, 160.0, 157.7, 150.0, 149.0, 130.7, 129.5, 127.3, 126.5, 125.8, 125.2, 123.6, 119.3, 118.0, 117.2, 111.2, 56.0, 20.6, 8.8; IR (KBR) νmax 3270, 2977, 2942, 1717, 1702, 1680, 1618, 1529, 14675, 1367, 1124, 1114, 950, 769 cm−1; HRMS (FAB) m/z: [M+Na+] for C40H32N2NaO12, calcd 755.1853; found, 755.1853.
methyl 3-bromo-4-(2-(methoxycarbonyl)phenoxy)benzoate (75)
Sodium carbonate (2.54 g, 23.94 mmol) was to a solution of methyl 3-bromo-4-fluorobenzoate 74 (1.86 g, 7.98 mmol) and methyl salicylate (1.21 g, 7.98 mmol) in 10 mL of dimethyl acetamde (DMA) at rt. The resulting reaction mixture was stirred at 120 °C for 16 h and quenched with water and aqueous layer was extracted with EtOAc (3 × 50 mL); the combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (hexanes/EtOAc, 5/1) to afford methyl 3-bromo-4-(2-(methoxycarbonyl)phenoxy)benzoate 75 (2.27 g, 78%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.34 (d, J = 2.0 Hz, 1H), 8.03 (dd, J = 1.7, 7.8 Hz, 1H), 7.85 (dd, J = 2.0, 8.6 Hz, 1H), 7.59 (td, J = 1.6, 7.7 Hz, 1H), 7.34 (t, J = 7.6 Hz, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.60 (d, J = 8.6 Hz, 1H), 3.91 (s, 3H), 3.76 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 165.5, 165.3, 158.9, 154.0, 135.3, 134.2, 132.6, 131.7, 130.3, 125.5, 125.6, 122.5, 120.2, 115.8, 112.1, 52.3; IR (KBr) νmax 2951, 2843, 1721, 1597, 1481, 1433, 1300, 1256, 963, 760 cm−1; HRMS (FAB) m/z: [M+H+] for C16H14BrO5, calcd 365.0025; found, 365.0018.
dimethyl dibenzo[b,d]furan-2,6-dicarboxylate (76)
Potassium carbonate (1.61 g, 16.4 mmol) followed by Pd(dppf)2Cl2 (313 mg, 0.38 mmol, 7 mol%) were added simultaneously to a solution of methyl 3-bromo-4-(2-(methoxycarbonyl)phenoxy)benzoate 75 (2.0 g, 5.48 mmol) in 15 mL of N,N-dimethyl acetamde (DMA) at rt. The reaction mixture was stirred at 120 °C for 3 h, quenched with water, aqueous layer was extracted with EtOAc (3 × 40 mL) and combined organic layers were washed with saturated aqueous NaCl, dried with anhydrous Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel column chromatography (hexanes/EtOAc, 4/1) to provide dimethyl dibenzo[b,d]furan-2,6-dicarboxylate 76 (1.34 g, 86%) as a colorless oil. 1H NMR (500 MHz, DMSO-d6) δ 8.68 (d, J = 1.6 Hz, 1H), 8.23 (dd, J = 1.7, 8.7 Hz, 1H), 8.18 (dd, J = 1.2, 7.6 Hz, 1H), 8.15 (dd, J = 1.2, 7.7 Hz, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.46 (t, J = 7.7 Hz, 1H), 4.06 (s, 3H), 3.99 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 166.9, 165.1, 159.2, 155.6, 130.1, 129.7, 125.7, 125.6, 125.4, 123.5, 123.2, 123.0, 115.8, 112.2, 52.6, 52.4. IR (KBr) νmax 2951, 2843, 1721, 1597, 1481, 1433, 1300, 1256, 963, 760 cm−1; HRMS (FAB) m/z: [M+Na+] for C16H12NaO5, calcd 307.0582; found, 307.0571.
(methoxycarbonyl)phenoxy)methyl)benzoate (78)
Potassium carbonate (4.33 g, 31.34 mmol) was added to a solution of methyl 4-(bromomethyl)-3-iodobenzoate 77 (3.7 g, 10.42 mmol) and methyl salicylate (1.59 g, 10.45 mmol) in 45 mL of DMF at rt. The resulting reaction mixture was stirred at 70 °C for 16 h, diluted with water and aqueous layer was extracted with EtOAc (2 × 60 mL); combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by silica gel column chromatography (hexanes-/EtOAc, 5/2) to afforded (methoxycarbonyl)phenoxy)methyl)benzoate 78 (3.01 g, 68%) as a colorless amorphous solid. 1H NMR (400 MHz, CDCl3) δ 8.51 (d, J = 1.6 Hz, 1H), 8.09 (dd, J = 1.6, 8.1 Hz, 1H), 7.90 (dd, J = 1.5, 7.8 Hz, 2H), 7.50 (td, J = 1.8, 8.4 Hz, 1H), 7.05 (dd, J = 8.1, 16.3 Hz, 2H), 5.13 (s, 2H), 3.94 (s, 3H), 3.93 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 166.6, 165.6, 157.6, 143.9, 140.0, 133.9, 132.2, 131.0, 129.8, 128.0, 121.2, 120.5, 113.7, 95.1, 74.4, 52.5, 52.2; HRMS (FAB) m/z: [M+Na+] for C17H15INaO5, calcd 448.9862; found, 448.9863.
methyl 3-iodo-4-((3-(methoxycarbonyl)phenoxy)methyl)benzoate (79)
Colorless amorphous solid (2.68 g, 91%). 1H NMR (500 MHz, CDCl3) δ 8.55 (d, J = 1.6 Hz, 1H), 8.05 (dd, J = 1.7, 8.0 Hz, 1H), 7.71 (dt, J = 1.4, 9.0 Hz, 1H), 7.68 (m, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.40 (t, J = 8.0 Hz, 1H), 7.20 (m, 1H), 5.12 (s, 2H), 3.95 (s, 3H), 3.94 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 166.9, 165.5, 158.2, 143.8, 140.4, 131.8, 131.3, 129.8, 129.6, 128.2, 122.9, 120.1, 115.5, 96.2, 73.8, 52.6, 52.2; IR (KBR) νmax 2951, 2921, 1722, 1595, 1435, 1286, 1256, 1218, 1113, 1031, 756 cm−1; HRMS (FAB) m/z: [M+Na+] for C17H15INaO5, calcd 448.9862; found, 448.9863.
methyl 3-iodo-4-((4-(methoxycarbonyl)phenoxy)methyl)benzoate (80)
Colorless amorphous solid (1.84 g, 87%). 1H NMR (500 MHz, CDCl3) δ 8.53 (d, J = 1.5 Hz, 1H), 8.03 (d, J = 9.3 Hz, 2H), 8.02 (m, 1H), 7.58 (d, J = 8.1 Hz, 1H), 7.00 (d, J = 9.3 Hz, 2H), 5.12 (s, 2H), 3.93 (s, 3H), 3.90 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 166.8, 165.5, 161.8, 143.4, 140.4, 131.9, 131.4, 129.6, 128.1, 123.6, 114.6, 96.0, 73.7, 52.6, 52.1; IR (KBR) νmax 2949, 2849, 1720, 1718, 1607, 1508, 1435, 1277, 1252, 1172, 1111, 1031, 767; HRMS (FAB) m/z: [M+Na+] for C17H15INaO5, calcd 448.9862; found, 448.9863.
dimethyl 6H-benzo[c]chromene-4,9-dicarboxylate (81)
Potassium acetate (1.87 g, 19.07 mmol) followed by Pd(dppf)2Cl2 (363 mg, 0.45 mmol) were added simultaniously to a solution of (methoxycarbonyl)phenoxy)methyl)benzoate 78 (2.71 g, 6.36 mmol) in 25 mL of dimethyl acetamde (DMA) at rt. The reaction mixture was stirred at 140 °C for 3 h and diluted with water. The aqueous layer was extracted with EtOAc (3×10mL); combined organic layers were washed with saturated aqueous NaCl, dried with anhydrous Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (hexanes/EtOAc, 4/1) to provide dimethyl 6H-benzo[c]chromene-4,9-dicarboxylate 81 (1.56 g, 82%) as a colorless amorphous solid. 1H NMR (500 MHz, CDCl3) δ 8.37 (d, J = 1.4 Hz, 1H), 7.99 (ddd, J = 1.6, 4.3, 7.8 Hz, 2H), 7.80 (dd, J = 1.6, 7.8 Hz, 1H), 7.27 (d, J = 7.8 Hz, 1H), 7.13 (t, J = 7.8 Hz, 1H), 5.25 (s, 2H), 3.97 (s, 3H), 3.93 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 166.8, 166.3, 154.6, 135.8, 132.0, 130.7, 129.9, 129.4, 127.7, 125.0, 123.7, 123.6, 121.7, 120.8, 68.6, 52.5, 52.4; IR (KBr) νmax 2951, 2865, 1723, 1721, 1595, 1577, 1433, 1406, 1267, 1196, 1151, 1111, 1060, 1018, 964, 758 cm−1; HRMS (FAB) m/z: [M+Na+] for C17H14NaO5, calcd 321.0739; found, 321.0738.
dimethyl 6H-benzo[c]chromene-3,9-dicarboxylate (82)
Colorless amorphous solid (1.07 g, 84%). 1H NMR (500 MHz, CDCl3) δ 8.42 (s, 1H), 8.02 (d, J = 7.8 Hz, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.76 (dd, J = 1.7, 8.1 Hz, 1H), 7.66 (d, J = 1.6 Hz, 1H), 7.25 (d, J = 8.1 Hz, 1H), 5.20 (s, 2H), 3.97 (s, 3H), 3.94 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 166.7, 166.6, 154.6, 136.6, 131.6, 130.7, 129.9, 129.7, 126.4, 125.2, 124.0, 123.7, 123.6, 118.9, 68.4, 52.5, 52.4; IR (KBR) νmax 2952, 2920, 1718, 1585, 1430, 1408, 1292, 1255, 1196, 1093, 887, 756 cm−1; HRMS (FAB) m/z: [M+Na+] for C17H14NaO5, calcd 321.0739; found, 321.0738.
dimethyl 6H-benzo[c]chromene-2,9-dicarboxylate (83)
Colorless amorphous solid (1.17 g, 86%). 1H NMR (500 MHz, CDCl3) δ 8.49 (d, J = 1.6 Hz, 1H), 8.42 (s, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.93 (dd, J = 2.0, 8.5 Hz, 1H), 7.21 (d, J = 7.8 Hz, 1H), 7.00 (d, J = 6.8 Hz, 1H), 5.20 (s, 2H), 3.97 (s, 3H), 3.94 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 166.7, 166.7, 158.5, 135.4, 131.6, 130.8, 129.6, 129.4, 125.6, 124.9, 124.4, 123.5, 121.8, 117.6, 68.4, 52.4, 52.2; IR (KBR) νmax 2952, 2920, 1718, 1585, 1430, 1408, 1292, 1255, 1196, 1093, 887, 756 cm−1; HRMS (FAB) m/z: [M+Na+] for C17H14NaO5, calcd 321.0739; found, 321.0738.
2-methoxy-3-(methoxycarbonyl)phenylboronic acid (85)
Bis(pinacolate)diboron (1.71g, 6.73 mmol), potassium acetate (1.32 g, 13.46 mmol), and followed by bis(diphenylphosphinoferrocene)palladium dichloride (183 g, 0.224 mmol, 5 mol%) were added simultaneously to a solution of methyl-5-bromo-2-methylbenzoate 84 (1.1 g, 4.49 mmol) in 30 mL of 1,4-dioxane at rt. The resulting mixture was heated to 110 °C and stirred for 2 h before adding 10 mL of 1N hydrogen chloride. The aqueous layer was extracted with EtOAc (3 × 15 mL) and combined extracts were washed with saturated aqueous NaCl, dried with anhydrous Na2SO4, filtered, and concentrated to give the corresponding crude boronic ester.
Ammonium acetate (1.04 g, 13.46 mmol) and sodium periodate (2.88 g, 13.46 mmol) were added sequentially to a solution of above crude boronic ester in mixed solution of acetone (10 mL) and water (10 mL). The resulting mixture was stirred at rt for 17 h. The precipitate was filtered off, and the filtrate was concentrated under reduced pressure. The residue was extracted with EtOAc (3 × 15 mL) and combined organic extracts were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered, and concentrated. The product was purified by silica gel column chromatography (hexane/EtOAc, 1/1) to give 2-methoxy-3-(methoxycarbonyl)phenylboronic acid 85 (556 mg, 59%) as a pale brown amorphous solid. 1H NMR (500 MHz, DMSO-d6) δ 8.18 (br s, 2H), 7.65 (dd, J = 1.8, 7.6 Hz, 1H), 7.61 (dd, J = 1.8, 7.3 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 3.83 (s, 3H), 3.77 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 166.7, 162.1, 137.9, 131.4, 123.8, 122.9, 62.1, 52.1; HRMS (FAB) m/z: [M+Na+] for C9H11BNaO5, calcd 233.0597; found, 233.0599.
dimethyl 2-methoxy-6’-(2-methoxy-2-oxoethyl)biphenyl-3,3’-dicarboxylate (87)
Potassium carbonate (987 mg, 7.14 mmol) and Pd(dppf)2Cl2 (98 mg, 0.12 mmol) were simultaneously added to the solution of 2-methoxy-3-(methoxycarbonyl)phenylboronic acid 80 (500 mg, 2.38 mmol) and methyl 3-iodo-4-(2-methoxy-2-oxoethyl)benzoate 86 (0.96 mg, 2.86 mmol) in 1,4-dioxane (8 mL) at rt. The resulting reaction mixture was stirred at 90 °C for 14 h, filtrated by celite, and the mother layer was evaporated. The residue was purified by silica gel column chromatography (hexane/EtOAc, 3/1) to give dimethyl 2-methoxy-6’-(2-methoxy-2-oxoethyl)biphenyl-3,3’-dicarboxylate 87 (656 mg, 74%) as a viscous liquid. 1H NMR (500 MHz, CDCl3) δ 8.04 (dd, J = 1.9, 8.0 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H), 7.83 (dd, J = 1.8, 7.8 Hz, 1H), 7.46 (d, J = 8.1 Hz, 1H), 7.37 (dd, J = 1.8, 7.5 Hz, 1H), 7.21 (dd, J = 1.8, 7.5 Hz, 1H), 3.92 (s, 3H), 3.90 (s, 3H), 3.58 (d, J = 2.2 Hz, 2H), 3.55 (s, 3H), 3.40 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 166.8, 166.6, 157.1, 138.3, 138.2, 135.6, 135.1, 131.6, 131.5, 130.6, 129.2, 129.1, 125.4, 123.9, 61.9, 52.4, 52.3, 52.0, 38.9; IR (KBr) νmax 2997, 2951, 1724, 1608, 1591, 1465, 1419, 1435, 1288, 1256, 1161, 1111, 1004, 964, 764 HRMS (FAB) m/z: [M+Na+] for C20H20NaO7, calcd 395.1107; found, 395.1110.
dimethyl 6’-(2-hydroxyethyl)-2-methoxybiphenyl-3,3’-dicarboxylate (88)
1M DIBAL-H in dichloromethane (2.7 mL, 2.7 mmol) was added drop wise to a solution of dimethyl 2-methoxy-6’-(2-methoxy-2-oxoethyl)biphenyl-3,3’-dicarboxylate 87 (0.63 g, 1.69 mmol) in dichloromethane (17 mL) at −78 °C over 10 min under argon atmosphere. The resulting reaction mixture was stirred at same temperature for 2 h, quenched with 1:1 mixture of MeOH and H2O (3 mL), followed by saturated sodium potassium tartarate (20 mL) and stirred for 1 h at rt. The aqueous layer was extracted with CH2Cl2 (2 × 15 mL) and combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered and concentrated to get crude dimethyl 2-methoxy-6’-(2-oxoethyl)biphenyl-3,3’-dicarboxylate as a viscous liquid.
The crude product of above dimethyl 2-methoxy-6’-(2-oxoethyl)biphenyl-3,3’-dicarboxylate was dissolved in MeOH (10 mL) and sodium borohydride (161 mg, 4.23 mmol) was added portions wise at 0 °C. The resulting reaction mixture was stirred at rt for 2 h, MeOH was removed under reduced pressure and re-suspended in water and extracted with EtOAc (3 × 15 mL), and the combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by silica gel column chromatography (hexane/EtOAc, 3/2) to give dimethyl 6’-(2-hydroxyethyl)-2-methoxybiphenyl-3,3’-dicarboxylate 88 (297 mg, 51%) as colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.01 (dd, J = 1.7, 8.1 Hz, 1H), 7.92 (d, J = 1.8 Hz, 1H), 7.83 (dd, J = 1.7, 7.8 Hz, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.35 (dt, J = 2.3, 7.6 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 3.92 (s, 3H), 3.90 (s, 3H), 3.72 (td, J = 3.6, 6.6 Hz, 2H), 3.45 (s, 3H), 2.78 (m, 2H), 1.90 (br s, OH); 13C NMR (125 MHz, CDCl3) δ 166.9, 166.7, 157.0, 143.1, 138.2, 135.9, 135.4, 131.5, 131.3, 129.6, 129.2, 128.3, 125.5, 124.0, 62.6, 62.1, 52.4, 52.2, 36.6; HRMS (FAB) m/z: [M+Na+] for C19H20NaO6, calcd 367.1158; found, 367.1151.
dimethyl 2-hydroxy-6’-(2-hydroxyethyl)biphenyl-3,3’-dicarboxylate
1M BCl3 in hexanes (2.45 mL, 2.45 mmol) was added drop wise to a solution of dimethyl 6’-(2-hydroxyethyl)-2-methoxybiphenyl-3,3’-dicarboxylate 88 (0.28 g, 0.81 mmol) in dichloromethane (6 mL) at −78 °C over 4 min under argon atmosphere. The resulting reaction mixture was stirred over 15 min at the same temperature, quenched with 3 mL of cold water followed by saturated NaHCO3 (10 mL). The aqueous layer was extracted with EtOAc (3 × 15 mL), combined organic layers were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (hexane/EtOAc, 3/7) to give dimethyl 2-hydroxy-6’-(2-hydroxyethyl)biphenyl-3,3’-dicarboxylate (232 mg, 94%) as a pale yellow amorphous solid. 1H NMR (500 MHz, CDCl3) δ 11.14 (s, 1H), 8.02 (dd, J = 1.8, 8.0 Hz, 1H), 7.92 (dd, J = 1.7, 8.0 Hz, 1H), 7.89 (d, J = 1.8 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.37 (dd, J = 1.6, 7.4 Hz, 1H), 6.98 (t, J = 5.8, 9.6 Hz, 1H), 3.98 (s, 3H), 3.90 (s, 3H), 3.73 (d, J = 4.3 Hz, 2H), 2.83 (dd, J = 6.6, 10.8 Hz, 2H), 1.58 (s, 1H); 13C NMR (125 MHz, CDCl3) δ 170.9, 167.0, 158.7, 143.0, 137.8, 137.1, 131.9, 130.0, 129.6, 129.3, 128.5, 119.2, 112.7, 62.8, 52.6, 52.2, 36.8; HRMS (FAB) m/z: [M+Na+] for C18H18NaO6, calcd 353.1001; found, 353.1010.
dimethyl 2-hydroxy-6’-(2-(tosyloxy)ethyl)biphenyl-3,3’-dicarboxylate (89)
Pyridine (0.28 mL, 3.42 mmol), and tosyl chloride (169 mg, 0.89 mmol) were added sequentially to a solution of 2-hydroxy-6’-(2-hydroxyethyl)biphenyl-3,3’-dicarboxylate (226 mg, 0.68 mmol) in dichloromethane (5 mL) under argon atmosphere at 0 °C. The resulting reaction mixture was stirred at rt for 2 h, and then concentrated. The residue was purified by silica gel column chromatography (hexane/ethyl acetate 4:1) to give dimethyl 2-hydroxy-6’-(2-(tosyloxy)ethyl)biphenyl-3,3’-dicarboxylate 89 (305 mg, 92%) as a viscous liquid. 1H NMR (500 MHz, CDCl3) δ 11.04 (s, 1H), 7.94 (dd, J = 1.8, 8.0 Hz, 1H), 7.91 (dd, J = 1.6, 8.0 Hz, 1H), 7.83 (d, J = 1.8 Hz, 1H), 7.61 (s, 1H), 7.60 (s, 1H), 7.25 (m, 5H), 6.94 (t, J = 7.7 Hz, 1H), 4.08 (dd, J = 7.2, 14.5 Hz, 2H), 3.98 (s, 3H), 3.90 (s, 3H), 2.92 (dt, J = 5.1, 6.9 Hz, 2H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.8, 166.8, 158.6, 144.8, 140.7, 137.8, 137.1, 131.9, 130.2, 129.9, 129.8, 129.3, 129.1, 129.0, 127.9, 119.3, 112.8, 69.8, 52.7, 52.2, 33.1, 21.7; HRMS (FAB) m/z: [M+H+] for C25H25O8S, calcd 485.1270; found, 485.1265.
dimethyl 6,7-dihydrodibenzo[b,d]oxepine-4,10-dicarboxylate (90)
Potassium carbonate (256 mg, 1.86 mmol) was added to a solution of dimethyl 2-hydroxy-6’-(2-(tosyloxy)ethyl)biphenyl-3,3’-dicarboxylate 89 (0.3 g, 0.62 mmol) in 5 mL of DMF at rt under argon atmosphere. The resulting reaction mixture was stirred at 90 °C for 3 h and quenched with water. The aqueous layer was extracted with EtOAc (3 × 10 mL); the combined organic layers were washed with saturated aqoueous NaCl, dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (hexanes/EtOAc, 3/1) to offerd dimethyl 6,7-dihydrodibenzo[b,d]oxepine-4,10-dicarboxylate 90 (149 mg, 77%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 1.5 Hz, 1H), 7.81 (dd, J = 1.5, 7.6 Hz, 1H), 7.63 (dd, J = Hz, 1.6, 7.8 Hz, 1H), 7.38 (d, J = Hz, 1.6, 7.8 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H), 7.30 (t, J = 8.2 Hz, 1H), 4.73 (t, J = 6.5 Hz, 2H), 3.94 (s, 3H), 3.93 (s, 3H), 2.87 (t, J = 6.5 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 167.0, 166.9, 153.7, 142.8, 138.6, 136.4, 133.1, 131.0, 129.5, 129.5, 129.4, 128.6, 126.5, 124.4, 78.7, 52.4, 52.3, 33.5; HRMS (FAB) m/z: [M+H+] for C18H17O5, calcd 313.1076; found, 313.1064.
dibenzo[b,d]furan-2,6-dicarboxylic acid (91)
LiOH (1.77 g, 42.0 mmol) was added to solution of 76(1.2 g, 4.22 mmol) in 18 ml of THF:MeOH:H2O (3:2:2) at rt. The resulting reaction mixture was stirred for 4 h, acidified to pH ~4 with 2N HCl. The acidified aqueous layer was extracted with EtOAc (3 × 15 mL); combined organic layers were washed with saturated aqueous NaCl, dried with anhydrous Na2SO4, filtered, and concentrated. The crude solid product was recrystalized with EtOAc to get dibenzo[b,d]furan-2,6-dicarboxylic acid 91 (0.96 g, 89%) as a colorless amorphous solid. 1H NMR (500 MHz, DMSO-d6) δ 13.24 (br s 2H), 8.85 (d, J = 1.6 Hz, 1H), 8.57 (dd, J = 1.2, 7.7 Hz, 1H), 8.15 (dd, J = 1.8, 8.6 Hz, 1H), 8.07 (dd, J = 1.2, 7.7 Hz, 1H), 7.89 (d, J = 8.6 Hz, 1H), 7.55 (t, J = 7.7 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 167.1, 165.2, 158.3, 154.8, 130.1, 129.6, 126.5, 124.9, 123.5, 123.4, 123.1, 116.3, 112.0; HRMS (FAB) m/z: [M-H+] for C14H7O5, calcd 255.0293; found, 255.0296.
6H-benzo[c]chromene-4,9-dicarboxylic acid (92)
Colorless amorphous solid, (1.17 g, 94%) as a colorless amorphous solid. 1H NMR (500 MHz, DMSO-d6) δ 12.99 (s, 2H), 8.34 (d, J = 1.5 Hz, 1H), 8.11 (dd, J = 1.5, 7.8 Hz, 1H), 7.94 (dd, J = 5.7, 13.5 Hz, 1H), 7.66 (dd, J = 1.5, 7.7 Hz, 1H), 7.45 (d, J = 7.9 Hz, 1H), 7.16 (t, J = 7.7 Hz, 1H), 5.25 (s, 2H). 13C NMR (125 MHz, DMSO-d6) δ 167.0, 166.8, 153.5, 135.8, 131.3, 131.2, 129.3, 129.1, 127.3, 125.5, 123.1, 122.9, 122.1, 121.9, 67.6; HRMS (FAB) m/z: [M-H+] for C15H9O5, calcd 269.0450; found, 269.0444.
6H-benzo[c]chromene-3,9-dicarboxylic acid (93)
Colorless amorphous solid (0.77g, 95%). 1H NMR (500 MHz, DMSO-d6) δ 13.21 (br s, 2H), 8.37 (s, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.95 (dd, J = 1.4, 7.8 Hz, 1H), 7.66 (dd, J = 1.6, 8.1 Hz, 1H), 7.47 (d, J = 1.6 Hz, 1H), 7.45 (d, J = 7.9 Hz, 1H), 5.27 (s, 2H). 13C NMR (125 MHz, DMSO-d6) δ 166.9, 166.7, 154.2, 136.3, 132.3, 131.5, 129.8, 128.9, 125.8, 125.7, 124.0, 123.4, 123.4, 117.8, 67.6; HRMS (FAB) m/z: [M-H+] for C15H9O5, calcd 269.0450; found, 269.0444.
6H-benzo[c]chromene-2,9-dicarboxylic acid (94)
Colorless amorphous solid (0.89 g, 92%). 1H NMR (500 MHz, DMSO-d6) δ 13.06 (s, 2H), 8.39 (s, 1H), 8.30 (s, 1H), 7.93 (d, J = 7.9 Hz, 1H), 7.88 (d, J = 8.5 Hz, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 5.32 (s, 2H). 13C NMR (125 MHz, DMSO-d6) δ 167.0, 166.8, 158.0, 135.5, 131.5, 131.4, 129.3, 128.8, 125.7, 125.0, 124.8, 122.5, 121.4, 117.6, 67.7; HRMS (FAB) m/z: [M-H+] for C15H9O5, calcd 269.0450; found, 269.0452.
6,7-dihydrodibenzo[b,d]oxepine-4,10-dicarboxylic acid (95)
Cololess amorphous solid, (117 g, 90%). 1H NMR (500 MHz, DMSO-d6) δ 12.95 (br s, 2H), 7.98 (d, J = 1.5 Hz, 1H), 7.94 (dd, J = 1.5, 7.7 Hz, 1H), 7.73 (dd, J = 1.6, 7.8 Hz, 1H), 7.68 (dd, J = 1.6, 7.8 Hz, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.36 (t, J = 8.1 Hz, 1H), 4.61 (t, J = 6.4 Hz, 2H), 2.85 (t, J = 6.4 Hz, 2H). 13C NMR (125 MHz, CDCl3) δ 167.5, 167.2, 152.5, 142.6, 138.1, 135.6, 132.2, 130.3, 130.0, 129.0, 128.9, 128.7, 127.8, 124.6, 78.3, 32.5; HRMS (FAB) m/z: [M-H+] for C16H11O5, calcd 283.0606; found, 283.0608.
General Procedure for peptide coupling of tricyclic tether linkers
Thionyl chloride (0.12 mL, 1.6 mmol) was added to a solution of dibenzo[b,d]furan-2,6-dicarboxylic acid 91 (30 mg, 0.117 mmol) in 2 mL of THF. The resulting reaction mixture was refluxed for 3 h, solvent was evapourated under reduced pressure and kept under high vaccume for 1–2 h to get dibenzo[b,d]furan-2,6-dicarbonyl dichloride 96 as colorless soild, used immediately for next coupling reaction without any further purification.
Pyridine (67 µL, 0.83 mmol) was added to a solution of amino coumarin 13 (114 mg, 0.29 mmol) in 3 mL of THF and stirred for 15 min at rt and then above freshly prepared diacid chloride 96 was added drop wise in 1 mL of THF. The resulting reaction mixture was stirred at rt for about 15 h, tand concentrated. Th residue was purified by silica gel column chromotography (CH2Cl2/acetone; 3/97) to get tilte biaryl dimer as colorless amorphous solid.
General Procedure for noviosylated tricyclic dimers cyclic carbonate hydrolysis
Et3N (10% total volume) was added dropwise to a solution of above cyclic carbonate di amides in methanol. The resulting mixture was stirred for 14 h, and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH, 19/1) to yield tricyclic tether dimer 101 (53% yield, over all in two steps) as a colorless amorphous solid.
N6-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N2-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)dibenzo[b,d]furan-2,6-dicarboxamide (101)
1H NMR (400 MHz, DMSO-d6) δ 9.68 (s, 1H), 8.29 (s, 2H), 8.19 (d, J = 3.1 Hz, 1H), 7.90 (s, 1H), 7.71 (d, J = 6.7 Hz, 1H), 7.53 (d, J = 8.5 Hz, 1H), 7.27 (d, J = 8.6 H, 1H z), 7.27 (d, J = 8.6 Hz, 1H), 7.01 (t, J = 7.7 Hz, 1H), 6.78 (d, J = 8.8 Hz, 2H), 6.62 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 8.8 Hz, 1H), 5.01 (d, J = 2.4 Hz, 1H), 4.95 (d, J = 2.4 Hz, 1H), 3.67 (m, 2H), 3.62 (m, 2H), 3.41 (s, 6H), 3.32 (d, J = 8.8 Hz, 2H), 2.25 (s, 6H), 1.36 (s, 3H), 1.35 (s, 3H), 1.11 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 165.0, 161.8, 159.3, 159.2, 157.4, 156.1, 156.0, 153.1, 149.1, 149.0, 129.5, 129.2, 127.3, 125.9, 125.8, 125.4, 124.9, 124.8, 124.2, 124.1, 123.4, 121.9, 121.4, 120.3, 117.3, 114.2, 113.8, 113.7, 112.4, 111.1, 111.0, 70.9, 68.5, 61.9, 61.8, 61.7, 31.6, 28.7, 28.6, 22.7, 22.6, 22.6, 14.1, 8.3, 8.2; IR (KBR) νmax 3437, 3400, 2967, 2922, 1712, 1707, 1664, 1604, 1529, 1367, 1249, 1080, 992, 761 cm−1; HRMS (FAB) m/z: [M+Na+] for C50H50N2NaO17, calcd 973.3007; found, 973.3010.
N4-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N9-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-6H-benzo[c]chromene-4,9-dicarboxamide (102)
Colorless amorphous solid, (79% yield, over all in two steps); 1H NMR (500 MHz, DMSO-d6) δ 10.70 (s, 1H), 9.94 (s, 1H), 8.78 (s, 1H), 8.51 (s, 1H), 8.51 (s, 1H), 8.31 (d, J = 6.5 Hz, 1H), 8.08 (d, J = 6.5 Hz, 1H), 7.97 (d, J = 8.7 Hz, 1H), 7.60 (m, 3H), 7.37 (t, J = 7.8 Hz, 1H), 7.16 (t, J = 8.3 Hz, 2H), 5.52 (m, 3H), 5.35 (s, 2H), 5.05 (s, 2H), 4.01 (m, 2H), 3.91 (m, 2H), 3.50 (s, 6H), 3.28 (dd, J = 1.4, 9.2 Hz, 2H), 3.17 (d, J = 4.1 Hz, 1H), 2.23 (s, 6H), 1.25 (s, 6H), 1.03 (s, 6H). 1H NMR (125 MHz, DMSO-d6) δ 165.5, 162.6, 158.4, 158.1, 156.3, 155.6, 152.5, 149.7, 148.7, 134.3, 134.2, 131.6, 130.3, 128.7, 128.0, 126.3, 126.1, 125.6, 124.1, 123.3, 122.9, 122.0, 121.3, 121.1, 113.4, 113.0, 112.9, 112.9, 110.9, 110.8, 98.5, 83.4, 77.9, 77.9, 70.9, 68.4, 67.6, 61.2, 55.0, 28.6, 23.0, 23.0, 8.2; IR (KBR) νmax 3446, 3402, 3035, 2975, 2935, 1716, 1704, 1664, 1607, 1527, 1367, 1246, 1083, 994, 762 cm−1; HRMS (FAB) m/z: [M+Na+] for C51H52N2NaO17, calcd 987.3164; found, 987.3164.
N3-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N8-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-6H-benzo[c]chromene-3,8-dicarboxamide (103)
Colorless amorphous solid, (75% yield, over all in two steps); 1H NMR (500 MHz, DMSO-d6) δ 9.92 (s, 1H), 9.70 (s, 1H), 8.50 (dd, J = 4.3, 7.8 Hz, 3H), 8.18 (d, J = 8.2 Hz, 1H), 7.96 (d, J = 7.8 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.59 (m, 3H), 7.49 (d, J = 8.1 Hz, 1H), 7.16 (dd, J = 4.4, 8.8 Hz, 2H), 5.52 (t, J = 2.3 Hz, 2H), 5.30 (s, 2H), 5.30 (d, J = 6.1 Hz, 1H), 5.02 (d, J = 6.1 Hz, 1H), 4.00 (m, 2H), 3.90 (m, 2H), 3.50 (s, 6H), 3.30 (br s, 2H), 3.28 (d, J = 9.2 Hz, 2H), 2.22 (s, 3H), 2.22 (s, 3H), 1.25 (s, 6H), 1.03 (s, 6H); 1H NMR (125 MHz, DMSO-d6) δ 165.5, 164.9, 158.1, 156.3, 156.2, 154.3, 149.7, 149.6, 135.5, 134.9, 134.1, 130.3, 129.6, 128.7, 128.4, 126.3, 125.6, 125.3, 124.3, 121.9, 121.6, 121.3, 121.2, 116.5, 113.0, 112.9, 110.8, 98.5, 83.4, 83.4, 77.9, 70.9, 70.8, 67.6, 67.6, 61.2, 28.6, 23.0, 8.2, 8.2; IR (KBR) νmax 3442, 3406, 2978, 2935, 1712, 1664, 1630, 1606, 1529, 1369, 1246, 1136, 1083, 1060, 993, 750 cm−1; HRMS (FAB) m/z: [M+Na+] for C51H52N2NaO17, calcd 987.3164; found, 987.3135.
N2-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N8-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-6H-benzo[c]chromene-2,8-dicarboxamide (104)
Colorless amorphous solid, (57% yield, over all in two steps); 1H NMR (500 MHz, DMSO) δ 9.99 (s, 1H), 9.86 (s, 1H), 8.65 (s, 1H), 8.55 (s, 1H), 8.49 (d, J = 8.8 Hz, 2H), 7.94 (m, 2H), 7.60 (dd, J = 4.3, 8.7 Hz, 2H), 7.49 (d, J = 8.0 Hz, 1H), 7.17 (m, 3H), 5.52 (s, 2H), 5.34 (s, 2H), 5.34 (d, J = 5.5 Hz, 2H), 5.03 (d, J = 6.2 Hz, 2H), 4.01 (m, 2H), 3.91 (m, 2H), 3.50 (s, 6H), 3.28 (d, J = 9.2 Hz, 1H), 3.17 (d, J = 5.2 Hz, 1H), 2.22 (s, 6H), 1.25 (s, 6H), 1.02 (s, 6H); 13C NMR (125 MHz, DMSO) δ 165.7, 165.3, 158.2, 158.1, 157.4, 156.3, 156.2, 149.8, 149.7, 134.7, 134.2, 130.4, 130.1, 128.9, 127.4, 126.3, 126.3, 125.4, 123.9, 121.8, 121.6, 121.5, 121.3, 117.4, 113.1, 113.0, 112.9, 110.8, 98.4, 83.4, 77.9, 70.9, 67.7, 67.6, 61.1, 55.0, 28.6, 22.9, 8.2. IR (KBR) νmax 3433, 3404, 2978, 2933, 1716, 1707, 1664, 1607, 1527, 1367, 1246, 1111, 1084, 993, 762 cm−1; HRMS (FAB) m/z: [M+Na+] for C51H52N2NaO17, calcd 987.3164; found, 987.3157.
N4-(7-((2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-N10-(7-((2S,3S,4R,5S)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yloxy)-8-methyl-2-oxo-2H-chromen-3-yl)-6,7-dihydrodibenzo[b,d]oxepine-4,10-dicarboxamide (105)
Colorless amorphous solid, (60% yield, over all in two steps); 1H NMR (500 MHz, CDCl3) δ 11.05 (s, 1H), 8.81 (s, 2H), 8.79 (s, 2H), 8.76 (s, 1H), 8.29 (dd, J = 1.6, 8.0 Hz, 1H), 7.96 (d, J = 1.6 Hz, 1H), 7.90 (dd, J = 1.9, 8.0 Hz, 1H), 7.68 (dd, J = 1.8, 7.6 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.32 (d, J = 8.7 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 7.18 (d, J = 8.8 Hz, 1H), 7.14 (d, J = 8.8 Hz, 1H), 5.59 (s, 2H), 4.85 (t, J = 6.7 Hz, 2H), 4.25 (m, 4H), 3.62 (s, 6H), 3.39 (dd, J = 2.5, 8.9 Hz, 2H), 2.90 (t, J = 6.7 Hz, 2H), 2.29 (s, 3H), 2.24 (s, 3H), 1.77 (br s, 4OH), 1.40 (s, 3H), 1.39 (s, 3H), 1.15 (s, 3H), 1.14 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 165.4, 163.8, 159.5, 159.4, 156.1, 156.0, 152.1, 149.2, 141.5, 139.0, 135.3, 133.9, 133.1, 132.1, 128.9, 127.4, 126.9, 126.0, 125.8, 125.8, 125.5, 125.0, 124.7, 122.5, 121.8, 114.4, 114.3, 114.2, 114.0, 112.3, 111.1, 97.9, 97.8, 84.4, 78.7, 78.7, 71.3, 71.2, 68.8, 62.1, 62.0, 45.4, 32.8, 29.3, 29.1, 22.7, 22.6, 8.5, 8.4; IR (KBR) νmax 3446, 3384, 2978, 2921, 1772, 1701, 1627, 1605, 1521, 1491, 1367, 1254, 1080, 1053, 962 cm−1; HRMS (FAB) m/z: [M+Na+] for C52H54N2NaO17, calcd 1001.3320; found, 1001.3334.
Biological Evaluation
Anti-Proliferation Assays
MCF-7 and SKBr3 cells were maintained in Advanced DMEM/F12 (1:1; Gibco) supplemented with non-essential amino acids, L-glutamine (2 mM), streptomycin (500 µg/mL), penicillin (100 units/mL), and 10% FBS. Cells were grown to confluence in a humidified atmosphere (37 °C, 5% CO2), seeded (2000/well, 100 µL) in 96-well plates, and allowed to attach overnight. Compound or geldanamycin at varying concentrations in DMSO (1% DMSO final concentration) was added, and cells were returned to the incubator for 72 h. After 72 h, the number of viable cells was determined using an MTS/PMS cell proliferation kit (Promega) per the manufacturer’s instructions. Cells incubated in 1% DMSO were used as 100% proliferation, and values were adjusted accordingly. IC50 values were calculated from separate experiments performed in triplicate using GraphPad Prism.
Western Blot Analyses
MCF-7 cells were cultured as described above and treated with various concentrations of drug, GDA in DMSO (1% DMSO final concentration), or vehicle (DMSO) for 24 h. Cells were harvested in cold PBS and lysed in RIPA lysis buffer containing 1 mM PMSF, 2 mM sodium orthovanadate, and protease inhibitors on ice for 1 h. Lysates were clarified at 14,000g for 15 min at 4° C. Protein concentrations were determined using the Pierce BCA protein assay kit per the manufacturer’s instructions. Equal amounts of protein (20 µg) were electrophoresed under reducing conditions, transferred to a PVDF, and immunoblotted with the corresponding specific antibodies. Membranes were incubated with an appropriate horseradish peroxidase-labeled secondary antibody, developed with a chemiluminescent substrate, and visualized.
Supplementary Material
Figure 1.
Hsp90 C-terminal inhibitors
Acknowledgements
The authors gratefully acknowledge the support of this project by NIH CA120458, NIH Training Grant (T32 GM008545) on Dynamic Aspects in Chemical Biology (L.B.P.), and the ACS Division of Medicinal Chemistry Pre-doctoral Fellowship (L.B.P.)
Abbreviations
- Hsp90
90 kilodalton heat shock protein
- ATP
Adenosine triphosphate
- DNA
Deoxyribonucleic acid
- SAR
Structure–activity relationships
- Akt
Protein kinase B
- Her2
Human epidermal growth factor receptor 2
Footnotes
Supporting Information Available: Experimental procedures and characterization for all compounds.
References
- 1.Duerfeldt AS, Blagg BSJ. Hsp90 inhibition: Elimination of shock and stress. Bioorg. Med. Chem. Lett. 2010;20:4983–4987. doi: 10.1016/j.bmcl.2010.06.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L, Trepel JB. Update on Hsp90 inhibitors in clinical trial. Curr. Top Med. Chem. 2009;9:1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. Heat shock protein 90: inhibitors in clinical trials. J. Med. Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 4.Marcu MG, Chadli A, Bouhouche I, Catelli MG, Neckers L. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 2000;2000:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 5.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signalling proteins. J. Natl. Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 6.Bishop SC, Burlison JA, Blagg BSJ. Hsp90: a novel target for the disruption of multiple signaling cascades. Curr. Cancer Drug Tar. 2007;7:369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly A, Blagg BSJ. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide binding pocket. Curr. Med. Chem. 2008;15:2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solit DB, Chiosis G. Development and application of Hsp90 inhibitors. Drug Discov. Today. 2008;13:38–43. doi: 10.1016/j.drudis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Peterson LB, Blagg BSJ. To fold or not to fold: modulation and consequences of Hsp90 inhibition. Future Med. Chem. 2009;1:267–283. doi: 10.4155/fmc.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W, Neckers L. Targeting the Molecular Chaperone Heat Shock Protein 90 Provides a Multifaceted Effect on Diverse Cell Signaling Pathways of Cancer Cells. Clin. Cancer Res. 2007;13:1625–1629. doi: 10.1158/1078-0432.CCR-06-2966. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J. Mol. Med. 2004;82:488–499. doi: 10.1007/s00109-004-0549-9. [DOI] [PubMed] [Google Scholar]
- 12.Hooper DC, Wolfson JS, McHugh GL, Winters MB, Swartz MN. Effects of novobiocin, coumermycin A1, clorobiocin, and their analogs on Escherichia coli DNA gyrase and bacterial growth. Antimicrob. Agents Chemother. 1982;22:662–671. doi: 10.1128/aac.22.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 14.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl. Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 15.Allan RK, Mok D, Ward BK, Ratajczak T. Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J. Biol. Chem. 2006;281:7161–7171. doi: 10.1074/jbc.M512406200. [DOI] [PubMed] [Google Scholar]
- 16.Yu XM, Shen G, Neckers L, Blake H, Holzbeierlein J, Cronk B, Blagg BSJ. Hsp90 Inhibitors Identified from a Library of Novobiocin Analogues. J. Am. Chem. Soc. 2005;127:12778–12779. doi: 10.1021/ja0535864. [DOI] [PubMed] [Google Scholar]
- 17.Burlison JA, Neckers L, Smith AB, Maxwell A, Blagg BSJ. Novobiocin:? Redesigning a DNA Gyrase Inhibitor for Selective Inhibition of Hsp90. J. Am. Chem. Soc. 2006;128:15529–15536. doi: 10.1021/ja065793p. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly A, Mays JR, Burlison JA, Nelson JT, Vielhauer G, Holzbeierlein J, Blagg BSJ. The design, synthesis, and evaluation of coumarin ring derivatives of the novobiocin scaffold that exhibit antiproliferative activity. J. Org. Chem. 2008;73:8901–8920. doi: 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnelly AC, Zhao H, Kusuma BR, Blagg BSJ. Cytotoxic sugar analogues of an optimized novobiocin scaffold. Med. Chem. Comm. 2010;1:165–170. [Google Scholar]
- 20.Zhao H, Kusuma BR, Blagg BSJ. Synthesis and Evaluation of Noviose Replacements on Novobiocin That Manifest Antiproliferative Activity. ACS Med. Chem. Lett. 2010;1:311–315. doi: 10.1021/ml100070r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burlison JA, Avila C, Vielhauer G, Lubbers DJ, Holzbeierlein J, Blagg BS. Development of novobiocin analogues that manifest anti-proliferative activity against several cancer cell lines. J. Org. Chem. 2008;73:2130–2137. doi: 10.1021/jo702191a. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y-T, Blagg BSJ. A Library of Noviosylated Coumarin Analogues. J. Org. Chem. 2007;72:3609–3613. doi: 10.1021/jo062083t. [DOI] [PubMed] [Google Scholar]
- 23.Burlison JA, Blagg BS. Synthesis and Evaluation of Coumermycin A1 Analogues that Inhibit the Hsp90 Protein Folding Machinery. Org. Lett. 2006;8:4855–4858. doi: 10.1021/ol061918j. [DOI] [PubMed] [Google Scholar]
- 24.Yu XM, Han H, Blagg BSJ. Synthesis of Mono- and Dihydroxylated Furanoses, Pyranoses, and an Oxepanose for the Preparation of Natural Product Analogue Libraries. J. Org. Chem. 2005;70:5599–5605. doi: 10.1021/jo050558v. [DOI] [PubMed] [Google Scholar]
- 25.Yu XM, Shen G, Blagg BSJ. Synthesis of (−)-Noviose from 2,3-O-Isopropylidene-d-erythronolactol. J. Org. Chem. 2004;69:7375–7378. doi: 10.1021/jo048953t. [DOI] [PubMed] [Google Scholar]
- 26.Shen G, Yu Xm, Blagg BSJ. Syntheses of photolabile novobiocin analogues. Bioorg. Med. Chem. Lett. 2004;14:5903–5906. doi: 10.1016/j.bmcl.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Donnelly AC, Kusuma BR, Brandt GEL, Brown D, Rajewski RA, Vielhauer G, Holzbeierlein J, Blagg BSJ. Engineering an antibiotic to fight cancer: Optimization of the novobiocin scaffold to produce anti- proliferative agents. J. Med. Chem. 2011;54:3839–3853. doi: 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen G, Blagg BSJ. Radester, a novel inhibitor of the Hsp90 protein folding machinery. Org. Lett. 2005;7:2157–2160. doi: 10.1021/ol050580a. [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty TK, Reddy GV. Studies directed toward the synthesis of glycopeptide antibiotic teicoplanin: first synthesis of the N- terminal 14-membered ring. J. Org. Chem. 1992;57:5462–5469. [Google Scholar]
- 30.Chausset-Boissarie L, Arvai R, Cumming GR, Besnard C, Kundig EP. Total synthesis of (+/−)-Vertine with Z-selective RCM as a key step. Chemical Communications. 2010;46:6264–6266. doi: 10.1039/c0cc01885f. [DOI] [PubMed] [Google Scholar]
- 31.Morgan BJ, Mulrooney CA, O’Brien EM, Kozlowski MC. Perylenequinone Natural Products: Total Syntheses of the Diastereomers (+)-Phleichrome and (+)-Calphostin D by Assembly of Centrochiral and Axial Chiral Fragments. J. Org. Chem. 2009;75:30–43. doi: 10.1021/jo901384h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner H, Goldbrunner J. Asymmetrische Katalysen, IL: Optisch aktive Binaphthylderivate — Synthese und Einsatz in Übergangsmetallkatalysatoren. Chemische Berichte. 1989;122:2005–2009. [Google Scholar]
- 33.Drège E, Tominiaux C, Morgant G, Desmaële D. Synthetic Studies on Cyathin Terpenoids: Enantioselective Synthesis of the Tricyclic Core of Cyathin through Intramolecular Heck Cyclisation. Eur. J. Org. Chem. 2006;2006:4825–4840. [Google Scholar]
- 34.Scribner A, Dennis R, Hong J, Lee S, McIntyre D, Perrey D, Feng D, Fisher M, Wyvratt M, Leavitt P, Liberator P, Gurnett A, Brown C, Mathew J, Thompson D, Schmatz D, Biftu T. Synthesis and biological activity of imidazopyridine anticoccidial agents: Part I. Eur. J. Med. Chem. 42:1334–1357. doi: 10.1016/j.ejmech.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Link JT. Organic Reactions. Vol. 60. John Wiley & Sons, Inc.; 2004. The Intramolecular Heck Reaction; pp. 157–561. [Google Scholar]
- 36.Álvarez S, Khanwalkar H, Álvarez R, Erb C, Martínez C, Rodríguez-Barrios F, Germain P, Gronemeyer H, de Lera AR. C3 Halogen and C8′′ Substituents on Stilbene Arotinoids Modulate Retinoic Acid Receptor Subtype Function. ChemMedChem. 2009;4:1630–1640. doi: 10.1002/cmdc.200900214. [DOI] [PubMed] [Google Scholar]
- 37.Dong Y, Shi Q, Pai H-C, Peng C-Y, Pan S-L, Teng C-M, Nakagawa-Goto K, Yu D, Liu Y-N, Wu P-C, Bastow KF, Morris-Natschke SL, Brossi A, Lang J-Y, Hsu JL, Hung M-C, Lee EYHP, Lee K-H. Antitumor Agents. 272. Structure-Activity Relationships and In Vivo Selective Anti-Breast Cancer Activity of Novel Neo-tanshinlactone Analogues. J. Med. Chem. 2010;53:2299–2308. doi: 10.1021/jm1000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kline T, Bowman J, Iglewski BH, de Kievit T, Kakai Y, Passador L. Novel synthetic analogs of the Pseudomonas autoinducer. Bioorg. Med. Chem. Lett. 1999;9:3447–3452. doi: 10.1016/s0960-894x(99)00626-5. [DOI] [PubMed] [Google Scholar]
- 39.Pechlivanidis Z, Hopf H, Ernst L. Paracyclophanes: Extending the Bridges. Synthesis. Eur. J. Org. Chem. 2009;2009:223–237. [Google Scholar]
- 40.Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E. A Critical Role for HSP90 in Cancer Cell Invasion Involves Interaction with the Extracellular Domain of HER -2. J. Biol. Chem. 2008;283:2031–2041. doi: 10.1074/jbc.M701803200. [DOI] [PubMed] [Google Scholar]
- 41.Sidera K, Patsavoudi E. Extracellular HSP90: conquering the cell surface. Cell Cylce. 2008;7:1564–1568. doi: 10.4161/cc.7.11.6054. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsumi S, Scroggins B, Koga F, Lee MJ, Trepel J, Felts S, Carreras C, Neckers L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2007;27:2478–2487. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.