Abstract
To investigate whether dietary α-linolenic acid (ALA) content alters the effect of β-carotene (βC) on mammary carcinogenesis, we conducted a chemically-induced mammary tumorigenesis experiment in rats randomly assigned to four nutritional groups (15 rats/group) varying in βC supplementation and ALA content. Two oil formulae-enriched diets (15%) were used: one with 4% ALA in the essential fatty acids (EFA) ratio linoleic acid to ALA = 5 (EFA 5 diet) and the other with 16% ALA in an EFA ratio = 1 (EFA 1 diet), both designed with a similar linoleic acid content βC was added (10 mg/kg diet/day) or not to these diets. βC led to decreased tumour incidence and tumour growth when supplemented to the EFA 5 diet, whereas it had no effect when supplemented to the EFA 1 diet. The decrease of tumour growth did not result from an involvement of lipoperoxidation (tumour malondialdehyde content similar between groups) or from an inhibition of tumour cell proliferation (unchanged S phase fraction in tumours). We concluded that an adequate content of ALA in diet is required for allowing protective effect of βC in mammary carcinogenesis. Whether such an interaction between ALA and βC influences the risk of breast cancer in women needs to be investigated.
Keywords: Adipose Tissue; chemistry; Animals; Antioxidants; administration & dosage; Cell Division; physiology; Diet; Dietary Supplements; Fatty Acids; analysis; Fatty Acids, Essential; administration & dosage; Female; Mammary Neoplasms, Experimental; prevention & control; Rats; Rats, Sprague-Dawley; S Phase; physiology; alpha-Linolenic Acid; administration & dosage; beta Carotene
Keywords: β-carotene, α-linolenic acid, ω-6/ω-3 fatty acid ratio, mammary tumours.
Introduction
Several epidemiological studies have consistently shown that individuals with high intake of vegetables and/or fruits had reduced risk of cancer, including breast cancer (Riboli & Norat, 2003). A potential explanation is that antioxidant nutrients, including carotenoids, prevent carcinogenesis by interfering with oxidative damage to DNA, lipids and proteins. However, results of epidemiological studies are inconclusive on the association between β-carotene (βC) and risk of breast cancer (IARC Working Group on the Evaluation of Cancer Preventive Agents., 1998a). Moreover, two intervention trials conducted in male smokers, the Alpha-Tocopherol, βC cancer prevention study (ATBC) and the βC and Retinol Efficacy Trial (CARET), which both used high-dose βC supplements, found an increased incidence of lung cancer in subjects who received supplements in comparison to non-recipients (The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group, 1994; Omenn et al., 1996).,- By contrast, in a trial conducted in healthy men, the Physicians’ Health Study (PHS), a high supplementation of βC on alternate days had no effect on cancer incidence (Hennekens et al., 1996) No clear mechanistic explanation has been provided yet to explain these conflicting findings. Nevertheless, some hypothesis have been advanced, involving the form of βC (synthetic/natural trans/cis), the amount of βC (physiological/pharmacological), the individuals exposed or not to high risk factors of cancer, genetic factors interfering with nutrition, and the possible interaction between βC and other nutrients.
Antioxidant vitamins supplements in mammary tumour-bearing rodents have generated contradictory results (IARC Working Group on the Evaluation of Cancer Preventive Agents., 1998b). It is still unclear whether contrasting results are due to differences in animal models, differences in supplement doses, or interference of antioxidants used with other dietary compounds or the combined effects of these confounding factors. In a model of chemically-induced mammary tumours in rats adding the antioxidant vitamin E to a diet rich in alpha-linolenic acid (ALA, 18:3n-3, the essential fatty acid of the omega-3 family) led to enhanced mammary tumour growth, whereas it had no effect when added to a diet devoid of ALA (Cognault et al., 2000). These data suggest that an interaction between antioxidant compounds and dietary omega-3 fatty acids is a determinant of mammary tumour growth.
To determine whether ALA content of diet alters the effect of βC on mammary carcinogenesis, we examined the effects of two oil formulae–enriched diets differing by their ALA content (4% and 16%) with ratios of linoleic acid (LA, 18:2n-6) to ALA of 4.66 and 1.05 respectively, in absence or presence of βC (10 mg/kg diet/day) on the characteristics of mammary carcinogenesis, and found that the omega-3 lipid environment of diet modifies the effect of βC.
Material and Methods
Animals and experimental carcinogenesis
Sixty 40-day-old female Sprague-Dawley rats were purchased from Harlan France (Gannat, France). The care of animals was in accordance with institution guidelines. Rats were housed 3 per cage and maintained at constant temperature (22°C) and humidity with a 12 hour light/dark cycle. Mammary tumours were induced by a single dose of N-Nitroso-N-methylurea (NMU) as previously described (Colas et al., 2004). Rats were randomly separated into four dietary groups (15 rats/group) Three weeks after carcinogenesis initiation and for 15 weeks, animals were palpated once weekly for the detection of mammary tumours. The largest length, width and depth of each tumour were measured with a calliper and the tumour area was calculated as the product of the two largest diameters. The tumour incidence (percentage of rats bearing at least one malignant mammary tumour) and the tumour growth (mean of tumour areas per tumour bearing rat each week) were determined. After 17 weeks of rat monitoring, animals were sacrificed.
Diets
Until NMU administration, rats were fed a recommended diet for the breeding and rearing of rodents (Harlan Teklad TRM Rat/Mouse Diet, France). Then, they were fed the experimental diets composed of a common basal diet (APAE, Jouy-en-Josas, France) as already described (Cognault et al., 2000) and 15% of an oils mixture (wt/wt). Diets, designed with a similar LA content, were as follows: EFA 5 diet containing a mixture of 60.2% African peanut oil and 39.8 European rapeseed oil (Bailly, Aulnay sous Bois, France) resulting in a 4%-ALA content in the recommended essential fatty acids ratio (EFA ratio: LA/ALA) for rats near 5 (actually 4.66) (Potier de Courcy et al., 1989); EFA I diet containing a mixture of 69% African peanut oil and 31 Linseed oil (ALA enriched oil Daudruy, Dunkerque, France) leading to a 16%-ALA content in an EFA ratio of 1.05 (Table 1) (βC (type I, Sigma, France) was added (10 mg/kg diet/day; + βC) or not (w/o βC, controls) to these diets. Animals received commercial and experimental diets and water ad libitum.
TABLE 1.
Fatty acid composition of commercial and experimental diets and triacylglycerides in rats’ adipose tissue.
| TRM diet1 | EFA 5 diet | EFA 1 diet | |||||
|---|---|---|---|---|---|---|---|
| Fatty acids (mole % of total fatty acids) | Diet2 | Diet3 | Adipose tissue4 | Diet3 | Adipose tissue4 | ||
| Mean | SD | Mean | SD | ||||
| Saturates | |||||||
| 16:0 | 16.3 | 8.5 | 15.4 | 0.4 | 9.3 | 17.5 | 0.5 |
| 18:0 | 2.7 | 2.4 | 2.6 | 0.07 | 3.4 | 2.9 | 0.09 |
| Total5 | 20.5 | 13.7 | 19.5 | 0.4 | 15.8 | 21.9 | 0.6 |
| Monounsaturates | |||||||
| 18:ln-9cis | 20.6 | 58.6 | 56.7 | 0.4 | 47.1 | 48.2 | 1.5 |
| Total6 | 24.7 | 63.0 | 62.4 | 0.3 | 49.8 | 54.6 | 1.4 |
| n-6 PUFA | |||||||
| 18:2n-6cis | 48.4 | 18.3 | 13.8 | 0.2 | 16.7 | 13.2 | 1.1 |
| Total7 | 48.4 | 18.4 | 14.3 | 0.2 | 17.3 | 13.5 | 1.1 |
| n-3 PUFA | |||||||
| 18:3n-3 | 4.7 | 3.9 | 1.3 | 0.05 | 16.3 | 7.0 | 0.4 |
| Total8 | 4.8 | 3.9 | 1.4 | 0.05 | 16.4 | 7.4 | 0.4 |
| Ratio | |||||||
| 18:2n-6cis/18:3n-3 | 10.3 | 4.66 | 10.63 | 0.4 | 1.05 | 1.9 | 0.1 |
Harlan Teklad TRM Rat/Mouse Diet, Harlan, France.
Fatty acid composition given by the supplier.
Fatty acid composition of one sample of each diet.
Ten rats were randomly selected in each dietary group to provide tissues for fatty acid analyses.
Including: 14:0, 15:0, 17:0, 20:0, 21:0, 22:0, 23:0 and 24:0.
Including: 14:1, 16:1, 17:1, I8:1 n-7cis, 20:1, 22:1 and 24:1.
Including: 18:3n-6, 20:2n-6, 20:3n-6, 20:4n-6, 22:2n-6 and 22:4n-6.
Including: 20:3n-3, 20:5n-3, 22:5n-3 and 22:6n-3.
The weight of rats was controlled weekly until the end of experiment.
Biochemical analyses
The fatty acid composition of adipose tissue was determined as previously described (Colas et al., 2004): after total lipids extraction, triglycerides were purified by preparative thin layer chromatography and fatty acids were methylated with boron trifluoride and analyzed by gas chromatography (Trace GC, Thermofinnigan, France) with a 60 m polar capillary column (BPX 70, SGE, France)
The βC absorption was controlled for each nutritional group by measuring the hepatic βC content. After extraction (Lyan et al., 2001) βC was analyzed by a HPLC system (Spectra System, Thermofinnigan, France) with two Adsorbosphere HS C18 3 μm cartridges (100 mm × 4.6 mm and 150 mm × 4.6 mm, Alltech, France) at 37°C and a photodiode array detector (UV6000LP, Thermofinnigan, France) as already described (Steghens et al., 1997).
Lipoperoxidation was evaluated by measuring total malondialdehyde (MDA) content in tumours. At the time of autopsy, necrotic tissues were carefully removed from tumours before freezing. Fifteen tumours (similarly distributed according to their age and their size) were used for the analysis. A fragment of tumour was cut and thawed at 4°C on Tris-HCl 100 mM, pH 7.4 (KCL 100 mM, EDTA 1 mM, butylhydroxytoluene 0.1 mM, Triton X-100 and 0.1% phenylmethanesulfonylfluoride 0.1 mM). The total lysat was centrifuged at 10000 g for 5 min and the supernatant extracted for analysis. The protein content in tumour was measured and standardized at 15–20 mg/mL for each sample. As previously described (Steghens et al., 2001), MDA was derivatizated with diaminonaphtalene in an acid medium at 37°C to form a MDA diazepinium. Analyses were carried out with a HPLC diode array system and an on line mass-spectrophotometer for confirmation. Results were expressed as nmol/g protein, instead of nmol/g tumours, to avoid MDA content variations due to weight of tumours.
Cell cycle
The distribution of cells within the cell cycle was assessed by flow cytometry after staining of DNA content with propidium iodide, as previously described (Cognault et al., 2000).
Statistical analyses
Effects of dietary conditions on carcinogenesis and biochemical parameters were evaluated, using the Statistica 6.0 software (StatSoft, Inc., France), by the following tests (p value < 0.10): 1) Pearson Chi2 test for tumour incidence; 2) repeated measures ANOVA with grouping factor (time) for tumour growth; 3) Mann-Whitney test for biochemical analyses and cell cycle. Data are expressed as mean ± standard error.
Results
During the experiment, no significant difference in weight gain among dietary groups was observed (data not shown).
Fatty acid composition of rat adipose tissue; (an indicator of dietary fatty acid intake); is presented in Table 1. Since βC supplementation did not change this composition whatever the ALA content, the Table 1 presents the results for the groups without βC. We showed that the EFA ratio was 5.6-fold greater in rats fed the EFA 5 diet than in rats fed the EFA 1 diet and that the LA content was similar among groups.
βC was detected only in livers of rats receiving the dietary βC supplementation. This content was not significantly different between rats fed EFA 5 (0.21 ± 0.04 μg/g of tissue) and EFA 1 diets (0.27 ± 0.04) (p = 0.35).
No significant difference in tumour incidence at the end of experiment was found between groups without βC: EFA 5 diet, 96% incidence, n = 14/15 rats with tumour; EFA 1 diet, 73.3%, 11/15 (p = 0.14) βC supplementation led to a reduced tumour incidence in rats fed the EFA 5 diet: 60.0%, n = 9/15 (p = 0.03), but not in rats fed the EFA 1 diet: 86.7%, n = 13/15 (p = 0.36) compared to their respective control (96%; 73.3%). No difference in tumour growth was observed between rats fed the EFA 5 and EFA 1 diets without βC (Figure 1). βC led to a decreased tumour growth in rats fed the EFA 5 diet (around a 50% decrease) but not in rats fed the EFA 1 diet (Figure 1).
FIGURE 1. Effects of β-carotene (βC) on mammary tumour growth according to EFA 5 and EFA 1 diets.
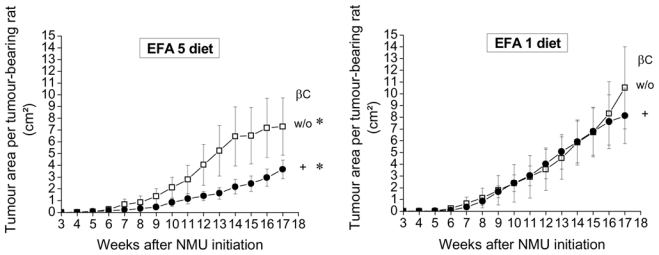
After chemical induction of mammary tumours, rats were randomly assigned to 4 nutritional groups (15 rats/group). Rats were fed either the EFA 5 diet (with an essential fatty acid (EFA) ratio LA to ALA = 5) or the EFA 1 diet (with an EFA ratio of 1), supplemented with βC (10 mg/kg diet/day, +) or not (w/o).
*: In rats fed the EFA 5 diet, tumour growths (means ± SEM) are significantly different between animals with βC and those w/o βC (p = 0.09, repeated measures ANOVA with time as grouping factor)
Tumor growths are not significantly different between rats fed the EFA 5 and EFA 1 diets w/o βC or between rats fed the EFA 1 diet with βC and w/o βC (p = 0.99, repeated measures ANOVA with time as grouping factor)
The measure of S phase fraction in tumours was not significantly different between groups: 3.8 ± 0.8 and 4.7 ± 0.9 w/o and + βC respectively in groups fed the EFA 5 diet; and 4.4 ± 0.7 and 3.7 ± 0.9 % w/o and + βC respectively in groups fed the EFA 1 diet (all p > 0.2).
The average MDA content in tumours was 220.6 pmol/mg proteins and not significantly different between dietary conditions (data not shown).
Discussion
The objective of this study was to determine whether dietary ALA content alters the effect of βC on mammary carcinogenesis. We provide evidence that βC had an inhibitory effect on tumour incidence and growth in rats fed the recommended EFA ratio of 5 (4% ALA), but failed to act as protective agent in rats fed an EFA ratio of 1 (16% ALA), which is pretty far above physiological levels in human diets. These data suggested that such a protective effect of βC on tumour growth may be dependent on ALA content in diets, although other nutritional factors associated with ALA in oils might interfere.
Since βC has been shown to display antioxidant (Sies & Stahl, 1995) or pro-oxidant properties (Palozza, 1998), we assessed the involvement of lipoperoxidation in the decrease of tumour growth. We found that the MDA content in tumours was not modified by βC supplementation whatever the dietary EFA ratio. In agreement with our data, Chew et al. did not find any significant difference in lipoperoxides products in transplanted mammary tumours of mice fed βC compared to controls without supplementation (Chew et al., 1999). In contrast βC was found to decrease the MDA content in colon adenocarcinoma cells supplemented with eicosapentaenoic acid (Palozza et al., 2000). These data suggest that βC could act as an antioxidant in presence of long chain omega-3 fatty acids which are more susceptible to peroxidation than ALA.
The S phase fraction was determined in tumours as an index of cell proliferation. We did not find any significant difference in S phase fractions between groups, suggesting that decreased tumour growth in rats fed the EFA diet with βC was a consequence of cell loss rather than an inhibition of cell proliferation.
Pathways implicated in the effects of βC in the present study are not known. However, several mechanisms have been proposed, including notably the modulation of apoptotic signalling (Palozza et al., 2004), of the immune response (Chew & Park, 2004), of the gap junction communications or the regulation of the detoxifying enzymes (Stahl et al., 2002).
We conclude that dietary ALA content alters the effect of βC on mammary carcinogenesis. Whether ALA content of diet modifies the protective effect of βC on breast cancer prevention in women needs to be determined and implies that more research be carried out in order to understand the effect of dietary βC supplementation along with omega-3 fatty acids in breast cancer.
Acknowledgments
This work was supported in part by grants from the French Ministry of Research (Nutrialis), La Ligue Nationale contre le Cancer (Comités d’Indre et Loire, Indre, Loire-et-Cher) and INSERM (ATC program). Carotenoids standards were kindly provided by Hoffman-La Roche (Basle, Switzerland). V. Maillard was a recipient of a fellowship from La Ligue Nationale contre le Cancer. We thank M. Pinault for her contribution to lipids chemistry, J. Montharu and his staff for animal care and V. Guérin for technical help.
References
- Chew BP, Park JS. Carotenoid action on the immune response. J Nutr. 2004;134:257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- Chew BP, Park JS, Wong MW, Wong TS. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999;19:1849–1853. [PubMed] [Google Scholar]
- Cognault S, Jourdan ML, Germain E, Pitavy R, Morel E, Durand G, Bougnoux P, Lhuillery C. Effect of an alpha-linolenic acid-rich diet on rat mammary tumor growth depends on the dietary oxidative status. Nutr Cancer. 2000;36:33–41. doi: 10.1207/S15327914NC3601_6. [DOI] [PubMed] [Google Scholar]
- Colas S, Paon L, Denis F, Prat M, Louisot P, Hoinard C, Le Floch O, Ogilvie G, Bougnoux P. Enhanced radiosensitivity of rat autochthonous mammary tumors by dietary docosahexaenoic acid. Int J Cancer. 2004;109:449–454. doi: 10.1002/ijc.11725. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Cancer Preventive Agents; IARC. IARC Handbooks of Cancer Prevention. Lyon: The International Agency for Research on Cancer; 1998a. Carotenoids; pp. 82pp. 87pp. 112–115. [Google Scholar]
- IARC Working Group on the Evaluation of Cancer Preventive Agents; IARC. IARC Handbooks of Cancer Prevention. Lyon: The International Agency for Research on Cancer; 1998b. Carotenoids; pp. 137–215. [Google Scholar]
- Lyan B, Azais-Braesco V, Cardinault N, Tyssandier V, Borel P, Alexandre-Gouabau MC, Grolier P. Simple method for clinical determination of 13 carotenoids in human plasma using an isocratic high-performance liquid chromatographic method. J Chromatogr B Biomed Sci Appl. 2001;751:297–303. doi: 10.1016/s0378-4347(00)00488-6. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Palozza P. Prooxidant actions of carotenoids in biologic systems. Nutr Rev. 1998;56:257–265. doi: 10.1111/j.1753-4887.1998.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Palozza P, Calviello G, Maggiano N, Lanza P, Ranelletti FO, Bartoli GM. Beta-carotene antagonizes the effects of eicosapentaenoic acid on cell growth and lipid peroxidation in WiDr adenocarcinoma cells. Free Radic Biol Med. 2000;28:228–234. doi: 10.1016/s0891-5849(99)00225-7. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Di Nicuolo F, Calviello G. Modulation of apoptotic signalling by carotenoids in cancer cells. Arch Biochem Biophys. 2004;430:104–109. doi: 10.1016/j.abb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Potier de Courcy G, Durand G, Abraham J, Gueguen L. Recommandations sur les conditions d’alimentation des animaux de laboratoire (rats et souris) Sciences des Aliments. 1989;9:209–217. [Google Scholar]
- Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62:1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- Stahl W, Ale-Agha N, Polidori MC. Non-antioxidant properties of carotenoids. Biol Chem. 2002;383:553–558. doi: 10.1515/BC.2002.056. [DOI] [PubMed] [Google Scholar]
- Steghens JP, van Kappel AL, Denis I, Collombel C. Diaminonaphtalene, a new highly specific reagent for HPLC-UV measurement of total and free malondialdehyde in human plasma or serum. Free Radic Biol Med. 2001;31:242–249. doi: 10.1016/s0891-5849(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Steghens JP, van Kappel AL, Riboli E, Collombel C. Simultaneous measurement of seven carotenoids, retinol and alpha-tocopherol in serum by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;694:71–81. doi: 10.1016/s0378-4347(97)00140-0. [DOI] [PubMed] [Google Scholar]
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]


