Abstract
Orthotopic liver transplantation is frequently associated with hyperfibrinolysis, the origin and clinical relevance of which is largely unknown. In 20 orthotopic liver transplantations, we studied the occurrence and systemic effects of hyperfibrinolysis. Severe fibrinolysis was defined to be present when the euglobulin-clot lysis time and the whole-blood-clot lysis time, as measured by thrombelastography, were shorter than 60 and 90 min, respectively, at some time during the operation. Based on these criteria, 7 patients had minimal fibrinolysis (group I), and 13 patients had severe fibrinolysis (group II). In group II a gradual increase of tissue-type plasminogen activator (t-PA) activity was seen during the anhepatic stage, followed by an “explosive” increase immediately after graft reperfusion (P=0.0004, compared with group I), and a reduction of plasminogen activator inhibitor (PAI) activity. Plasma degradation products of fibrinogen and fibrin increased parallel to t-PA activity, and levels were significantly higher at 45 min after graft reperfusion in group II compared with group I (P<0.04). Thrombin-antithrombin III complexes showed an identical steady increase in both groups, indicating that increased t-PA activity was not related to thrombin formation. A combination of increased endothelial release and reduced hepatic clearance may have caused the increased t-PA activity. The t-PA—associated destruction of fibrinogen and fibrin after graft reperfusion is consistent with the clinical signs of severe oozing often seen in this period. These observations may have important clinical implications for the treatment of bleeding in patients undergoing orthotopic liver transplantation.
Orthotopic liver transplantation has become an accepted and clinically useful treatment for patients with a variety of irreversible liver disease (1). The gradual improvements of the surgical technique, anesthesiologic management, and immunosuppressive therapy have contributed to an increase in the success rate and long-term survival (1, 2). The surgical operation however is an extensive procedure, which may be frequently associated with serious bleeding, requiring massive blood transfusions (3). Maintenance of surgical hemostasis may be seriously complicated by disturbances in the hemostatic system. Previous studies have suggested an important role of hyperfibrinolysis in the origin of bleeding complications during orthotopic liver transplantation (4, 5).
Recently, studies involving only a few subjects have shown that the hyperfibrinolysis during orthotopic liver transplantation may be related to increased plasma levels of tissue-type plasminogen activator (t-PA)* (6, 7), a key enzyme of the fibrinolytic system (8). Under normal physiologic conditions, t-PA activity in the circulation is low, due to its rapid inactivation by formation of complexes with PA-inhibitors (PAI), but t-PA activity may increase several fold after specific stimuli (8). Both t-PA and its major inhibitor (PAI-1) are produced and secreted by endothelial cells, whereas hepatocytes and blood platelets are additional sources of PAI-1 (8, 9). Elimination of t-PA from the blood is mainly regulated by the liver with a t1/2 of 3–5 minutes (10). Increased levels of t-PA during orthotopic liver transplantation probably result from a combination of increased endothelial release and decreased hepatic clearance during the anhepatic stage (6). The mechanisms underlying the increased release of t-PA and the role of PAI in the regulation of t-PA activity during liver transplantation however are still unknown.
Some investigators have suggested that hvperfibrinolysis in orthotopic liver transplantation may be secondary to disseminated intravascular coagulation (DIC) (7, 11, 12). Differentiation between secondary and primary fibrinolytic activity however has been difficult, mainly due to lack of appropriate laboratory tests. Lack of specific parameters has also hampered the assessment of the role of increased fibrinolytic activity in the actual breakdown of coagulation factors and the development of a bleeding diathesis. Especially under strongly hyper-fibrinolytic conditions, fibrinogenolysis may also occur (13). Although increased serum levels of fibrin(ogen) degradation products have been found during liver transplantation (4, 11), whether these were the result of fibrin breakdown or plasmin destruction of fibrinogen could not be determined.
In the present study, we examined the origin and clinical relevance of hyperfibrinolysis in orthotopic liver transplantation. Measurement of t-PA and PAI activity in combination with the separate quantitation of plasma degradation products of fibrinogen and fibrin enabled us to study the origin of hyperfibrinolysis and its role in the development of a systemic lytic state. Thrombin-antithrombin III (TAT) complexes were measured to study the role of clotting activation and thrombin formation in the origin of hyperfibrinolysis.
MATERIALS AND METHODS
Patients
Twenty-three adult patients who underwent their first liver transplantation at Presbyterian University Hospital between June 1 and 27, 1988 were observed prospectively. In an otherwise consecutive series, 3 patients, of whom more than 2 blood samples were missing due to technical errors, were omitted. The remaining 20 patients were categorized by pathological diagnosis as described previously (14). Five different diagnostic groups could be distinguished, as shown in Table 1. Orthotopic liver transplantation was performed by a standard technique, using a venovenous bypass in all patients (15). The surgical procedure can be divided into 3 stages. During the preanhepatic stage (stage I), the host liver is isolated. The anhepatic stage (stage II) begins with the clamping of the vessels of the native liver and ends with the completion of the vascular anastomosis of the graft liver. The postanhepatic stage (stage III) lasts from graft reperfusion to the end of surgery. Intraoperative blood loss was compensated by the transfusion of modified whole blood (from which platelets have been removed) or packed RBC and fresh frozen plasma in an approximate ratio of 1:1. In case of massive blood loss, a rapid infusion system was used, by which a mixture of packed RBC, fresh-frozen plasma, and Plasmalyte A was infused in a ratio of 1 U:1 U:250 ml. Platelets and cryoprecipitate were usually not given before stage III. All patients gave their informed consent for blood sampling during the operation, as part of the intraoperative patient care.
Table 1.
Diagnosis and characteristics in 20 patients undergoing orthothopic liver transplantation
| Diagnosis | No. | F | M | Age range |
|---|---|---|---|---|
| Postnecrotic cirrhosis | 9 | 3 | 6 | 27–54 |
| Primary biliary cirrhosis | 5 | 5 | - | 27–60 |
| Sclerosing cholangitis | 3 | 1 | 2 | 29–41 |
| Carcinoma | 1 | - | 1 | 63 |
| Miscellaneousa | 2 | 1 | 1 | 22, 37 |
| Total | 20 | 10 | 10 | 22–63 |
Two patients with Wilson’s disease.
Intraoperative blood samples for hemostasis monitoring were collected from an arterial line. Blood (9 ml) was collected in 1 ml 0.13 mol/L trisodium citrate and immediately centrifuged at 2800×g for 10 min. Plasma was either directly used for testing or frozen at −70°C. Whole blood (0.36 ml) was used for thrombelastographic monitoring within 2 nun after sampling. Blood samples were taken according to the following schedule: immediately after induction of anesthesia (BASE); 30 min before removing the liver (II−30); 5 min in the anhepatic stage (II+5); 5 min before graft reperfusion (III−5); 5 min after graft reperfusion (III+5); 45 min after reperfusion (III+45); 150 min after reperfusion (III+150), and at the end of the operation (END).
Assays
Standard hemostasis tests were performed using previously described methods (16, 17). Thrombelastographic monitoring of whole blood coagulation and fibrinolysis was performed using a Thromb Elastograph-D (Haemoscope Corporation, Morton Grove, IL). The whole blood clot lysis time (WBLT) was defined as the time between the maximum amplitude and the registration of complete lysis on the thrombelastographic recording (normal >150 min) (18).
Levels of t-PA activity (normal range, 0–1 IU/ml) and PAI activity were measured using chromogenic substrate methods (Coasets t-PA and PAI, Kabi Vitrum Hematology, Stockholm, Sweden). For the measurement of t-PA activity, 100 µl of plasma was acidified (pH 4.0–4.1) with 100 µl of acetate buffer and 20 µl of 20% acetic acid, both supplied in the assay kit. T-PA activity was determined by measuring the amidolytic activity of plasmin onto the chromogenic substrate S-2251, after incubation in the presence of plasminogen and human fibrin(ogen) fragments (19, 20). The fibrinolytic activity of t-PA was expressed in International Units assessed by calibration against the international standard of t-PA from human melanoma cells (lot 83/517, National Institute for Biological Standards and Control, London, UK). PAI activity was measured by adding 40 IU/ml t-PA to an equal volume of plasma. After incubation for 10 min at room temperature, samples were diluted with sterile water (1:80), and residual t-PA activity was determined as described above. PAI activity was expressed in arbitrary units (AU), defined as the amount that inhibits 1 IU of t-PA in 10 min (21) (normal, 0–40 AU/ml).
Two different sandwich-type enzyme-linked immunosorbent assays (Fibrinostika, Organon Teknika, Turnhout, Belgium) were used for the quantitation of plasma levels of fibrinogen degradation products (FgDP; normal <0.5 µg/ml) and fibrin degradation products (FbDP; normal <0.5 µg/ml). In both ELISAs, a monoclonal antibody, which reacts exclusively with FgDP and FbDP and not with intact fibrinogen or fibrin, is used as catching antibody. The FgDP ELISA contains a monoclonal tagging antibody that is specific for covalently bound fibrinopeptide A. Since fibrinopeptide A is split off during the activation of fibrinogen by thrombin, this ELISA tags only FgDPs that result from the plasmin-mediated destruction of fibrinogen (22). The FbDP ELISA gets its specificity for FbDP by using a monoclonal antibody that is elicited with D-dimer as immunogen (23).
TAT complexes (normal range, 1.0–4.1 µg/L) were measured by an ELISA (Behringwerke, Marburg, FRG), based on rabbit antibodies to human thrombin and antithrombin III, respectively (24). Samples with TAT levels exceeding the highest standard contained in the assay kit (60 µ/L), were diluted (1:2 or 1:4) in normal pooled plasma, which had been shown to have a TAT concentration of 1.2 µg/ml.
Statistical analysis
Statistical analysis was performed using the NPAR1WAY computer program of the Statistical Analysis System (SAS Institute Inc., Cary, NC). The significance of differences within and between groups were tested using the Wilcoxon rank-sum test and two-sample test, respectively. Values for P<0.05 were considered to be significant.
RESULTS
In all but 1 patient, slightly to severely increased fibrinolytic activity, as measured by shortening of the euglobulin clot lysis time (ELT; normal >120 min) or WBLT (normal >150 min), was found in at least 1 blood sample during the operation. Signs of hyperfibrinolysis were most frequent at the end of the anhepatic stage and early after graft reperfusion of the donor liver. Fibrinolysis was defined as minimal if the ELT was longer than 60 min or when the WBLT was longer than 90 min in all blood samples. Severe fibrinolysis was defined as being present when the ELT and WBLT were shorter than 60 and 90 min, respectively, in at least one of the intraoperative blood samples. According to these criteria, the patients were divided into 2 groups. Group I was formed by 7 patients with minimal fibrinolysis. Group II consisted of 13 patients with severe fibrinolysis. Comparison of the preoperative hemostasis parameters showed no significant differences between the 2 groups (Table 2). Both groups included patients with different diagnoses, without an accumulation of any diagnostic group in either of the 2 groups.
Table 2.
Comparison of preoperative hemostasis profile in patients with minimal (group I) and severe (group II) intraoperative fibrinolysis
| Variables | Reference values | Median (range) | |
|---|---|---|---|
| Group I | Group II | ||
| Coagulation: | |||
| PT (sec) | 10.8–13.0 | 11.3 (10.4–21.2) | 12.2 (9.7–21.2) |
| aPTT (sec) | 26–34 | 36.0 (28.9–51.6) | 42.9 (29.1–127) |
| ThT (sec) | 13–18 | 17.1 (15.3–32.9) | 22.0 (14.3–47.7) |
| Fibrinogen (mg/dl) | 150–450 | 285 (159–460) | 140 (85–350) |
| Factor II (%)a | 50–150 | 66 (32–130) | 38 (15–135) |
| Factor VIII (%)a | 50–150 | 185 (130–300) | 120 (82–280) |
| TAT complex (µg/ml) | 1.0–4.1 | 8.0 (2.0–16.0) | 4.3 (1.6–60.0) |
| Platelets (109/L) | 150–450 | 81 (56–510) | 118 (39–336) |
| Fibrinolysis: | |||
| ELT (min) | >120 | >120 (105–>120) | 60 (15–>120) |
| WBLT (min) | >150 | >150b | >150b |
| t-PA act. (IU/ml) | 0–1.0 | 2.4 (0–6.0) | 1.4 (0–8.0) |
| PAI act. (IU/ml) | 0–40.0 | 18.5 (14.0–36.0) | 18.8 (3.0–37.5) |
| FgDP (µg/ml) | <0.5 | 0.28 (0.20–3.0) | 0.50 (0.20–2.6) |
| FbDP (µg/ml) | <0.5 | 0.30 (0.26–4.5) | 0.84 (0.22–6.0) |
Percentage of pooled normal plasma.
For all patients.
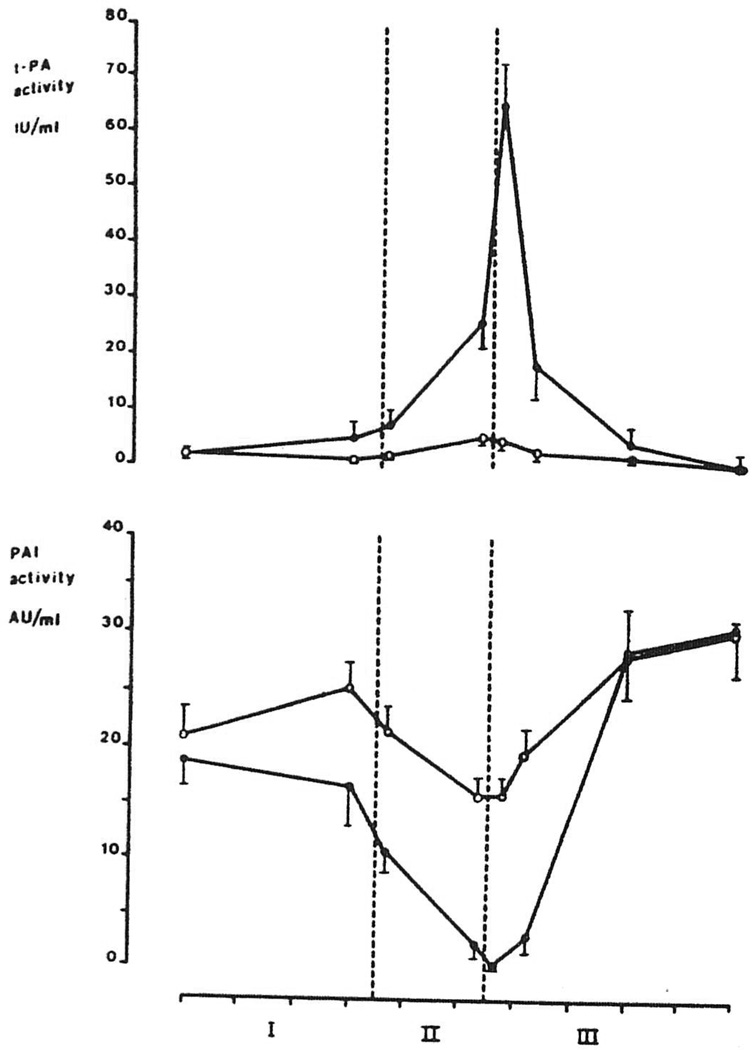
Mean intraoperative levels of t-PA activity and PAI activity of group I and II are depicted in Figure 1. There were no significant changes in t-PA and PAI activity during the preanhepatic stage in both groups. In group I t-PA levels remained below 12 IU/ml during the rest of the operation in all patients. In group II t-PA activity increased after clamping of the vessels of the native liver, and levels were significantly higher at the end of the anhepatic stage (III−5), compared with group I (P=0.002). During reperfusion of the graft, t-PA levels increased sharply, resulting in a more than doubling at 5 min after reperfusion, compared with the values at 5 min before reperfusion (P<0.007). At this time t-PA activity in group II (65.1±8.5 IU/ml, mean ±SEM) was about 30 times higher than the preoperative value and more than 10 times higher than t-PA activity in group I (P=0.0004). Later in the postanhepatic stage, a rapid disappearance of t-PA activity was seen, and levels fell into the normal range (0–1 IU/ml) at the end of the operation in all but 2 patients. In these 2 patients t-PA levels were still moderately increased (3.4 and 10 IU/ml). In group II free PAI activity showed a pattern inverse that of t-PA activity, and only minimal PAI activity (1.1 ±0.7 AU/ml) was left at the peak of fibrinolytic activity, but levels increased during the later postanhepatic period in both groups.
Figure 1.
Intraoperative levels of t-PA activity and PAI activity (mean ±SEM) in patients with minimal ( , group I, n=7) and severe fibrinolysis (
, group I, n=7) and severe fibrinolysis ( , group II, n=13). Each tic on the abscissa indicates 1 hr. The area between the dotted lines represents the anhepatic stage (stage II).
, group II, n=13). Each tic on the abscissa indicates 1 hr. The area between the dotted lines represents the anhepatic stage (stage II).
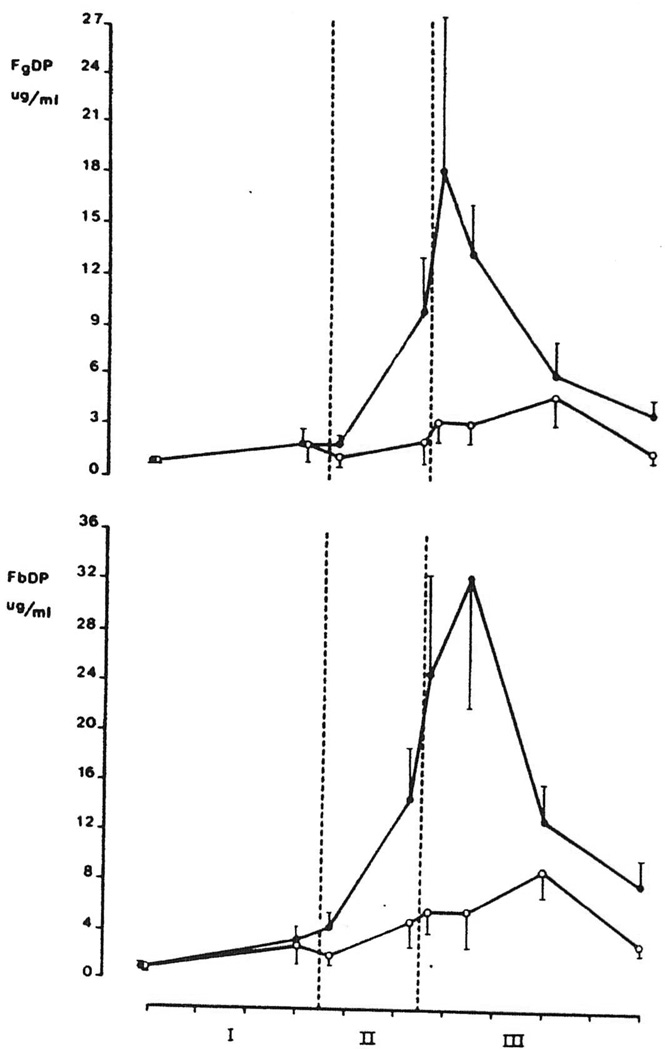
Mean plasma levels of FbDP and FgDP in groups I and II are shown in Figure 2. Although an increase of FbDP and FgDP was seen in both groups, levels in group II were significantly higher in the postanhepatic period at 45 min after graft reperfusion when compared with group I (P<0.04). In group II the highest FgDP level (18.4±7.9 µg/ml) coincided with the peak in t-PA activity (II+5), whereas the maximum in FbDP (32.5±11.2 µg/ml) occurred somewhat later in the postanhepatic stage (III+45).
Figure 2.
Intraoperative levels (mean ±SEM) of fibrinogen degradation products (FgDP) and fibrin degradation products (FbDP) in orthotopic liver transplantation.  , group I: patients with minimal fibrinolysis (n=7);
, group I: patients with minimal fibrinolysis (n=7);  : Group II: patients with severe fibrinolysis (n=13). Each tic on the abscissa indicates 1 hr. The area between the dotted lines represents the anhepatic stage (stage II).
: Group II: patients with severe fibrinolysis (n=13). Each tic on the abscissa indicates 1 hr. The area between the dotted lines represents the anhepatic stage (stage II).
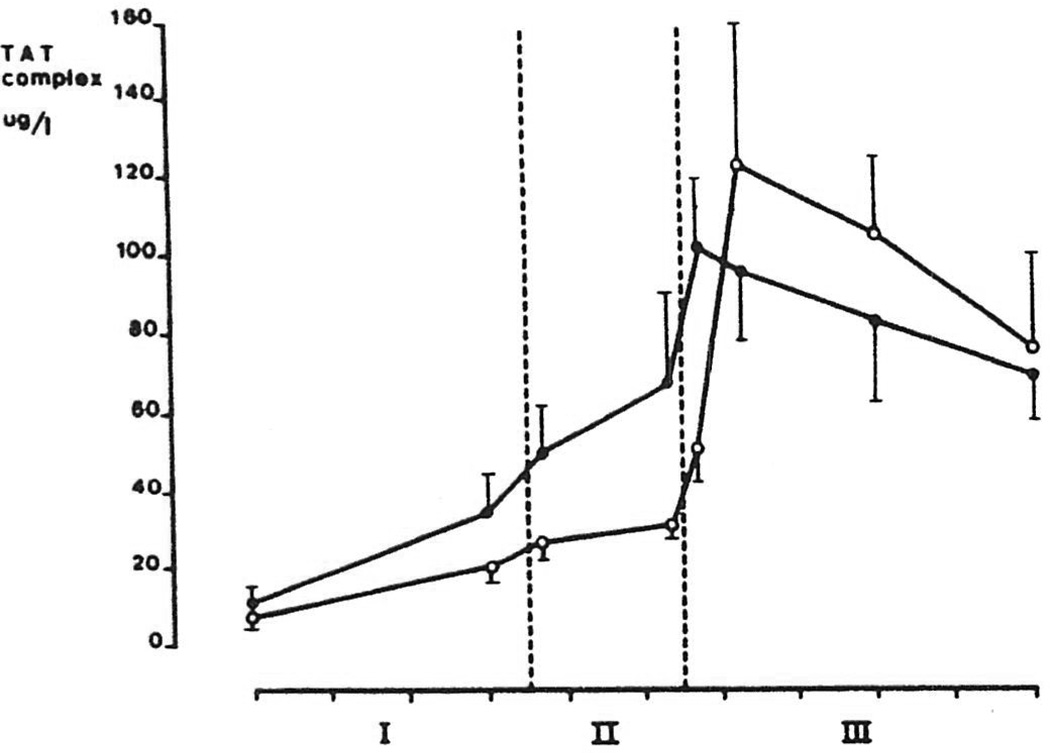
Mean levels of TAT complexes in group I and II are shown in Figure 3. An identical increasing pattern was seen in both groups, and at none of the time points were TAT levels significantly different between the 2 groups. Highest TAT levels were found during the postanhepatic stage, and levels were still above the normal upper limit (>4.1 µg/L) at the end of the operation in all patients.
Figure 3.
Intraoperative levels (mean ±SEM) of thrombin-antithrombin III (TAT) complexes in orthotopic liver transplantation.  , group I: patients with minimal fibrinolysis (n=7);
, group I: patients with minimal fibrinolysis (n=7);  , group II: patients with severe fibrinolysis (n=13). Each tic on the abscissa indicates 1 hr. The area between the dotted lines represents the anhepatic stage (stage II).
, group II: patients with severe fibrinolysis (n=13). Each tic on the abscissa indicates 1 hr. The area between the dotted lines represents the anhepatic stage (stage II).
Comparison of changes in arterial pH, pO2, and blood pressure in group I and II are shown in Table 3. There was no evidence for a relationship between intraoperative changes in hemodynamics and the increase in t-PA activity. Periods of shock, as determined by a drop in blood pressure and pH, were found among both patients with high and low t-PA activities.
Table 3.
Comparison of intraoperative hemodynamic changes in patients with low (group I) and high (group II) t-PA activity
| Hemodynamics | Group I (n=7) | Group II (n=13) |
|---|---|---|
| At the end of stage II: | ||
| Arterial pHa | 7.37 (7.33–7.45) | 7.40 (7.34–7.46) |
| Arterial pO2 (mm Hg) | 218 (113–311) | 279 (122–397) |
| No. patients with a hypotensive period | 1/7 (14%) | 3/13 (23%) |
| Early in stage III: | ||
| Arterial pH | 7.31 (7.22–7.42) | 7.32 (7.15–7.42) |
| Arterial pO2 (mm Hg) | 272 (150–486) | 275 (114–531) |
| No. patients with a hypotensive period | 1/7 (14%) | 5/13 (38%) |
The pH and pO2 are expressed in median (range).
The intraoperative use of blood products is shown in Table 4. There were no differences in the use of cryoprecipitate and platelets between the 2 groups. However, intraoperative blood loss, as reflected by the total use of modified whole blood or packed RBC and fresh-frozen plasma, was significantly higher in group II than in group I (P<0.02).
Table 4.
Intraoperative blood use in patients with low (group I) and high (group II) t-PA activity
| Group I (n=7) | Group II (n=13) | ||
|---|---|---|---|
| RBCa (U) | Mean | 5 | 19 |
| Median (range) | 4 (1–8) | 10 (3–101) | |
| FFP (U) | Mean | 1 | 11 |
| Median (range) | 0 (0–8) | 4 (0–68) | |
| RBC + FFP (U) | Mean | 6 | 30 |
| Median (range) | 4 (2–16) | 10 (3–169) | |
| Cryoprecipitate (U) | Mean | 0 | 3 |
| Median (range) | 0b | 0 (0–12) | |
| Platelets (U) | Mean | 3 | 5 |
| Median (range) | 0 (0–10) | 8 (0–28) |
RBC includes packed red blood cells and modified whole blood.
For all patients.
DISCUSSION
In earlier studies we have found that increased fibrinolytic activity, as measured by the ELT or thrombelastography (TEG), occurs in about 80% of the patients undergoing orthotopic liver transplantation (18, 25). Fibrinolytic activity may increase during the anhepatic stage and is most often severe early after graft recirculation. A simultaneous decrease of plasminogen and α2-antiplasmin, the main inhibitor of plasmin, has been found during this period, which supports the view of an active fibrinolytic process (5). However, the use of ELT and TEG in these studies did not allow an exact characterization of the fibrinolytic defect, and the origin and clinical relevance of the increased fibrinolytic activity have remained largely unclear.
In this study we found an extreme increase of t-PA activity, and concomitant decrease of PAI activity during the anhepatic and early postanhepatic period in patients with severe fibrinolysis, as measured by the ELT and TEG. Reduction in PAI activity can be explained by the formation of complexes with t-PA. After saturation of free PAI, a further increase of t-PA will result in the increase of free t-PA activity in the circulation (9, 21). Recently, Dzik et al. (6) and Palareti et al. (7) have reported a similar increase of t-PA during the anhepatic stage in a limited number of patients undergoing OLT. However, these studies did not show the explosive t-PA increase, occurring directly after graft reperfusion, as seen in our patients. Since we observed a rapid normalization of t-PA activity after its maximum, this peak could have been easily missed in the other studies if no blood samples were taken within 10 min after reperfusion.
The intraoperative course of t-PA activity suggests that there are 2 different mechanisms responsible for the increase of t-PA during orthotopic liver transplantation. The initial rise of t-PA during the anhepatic stage has been explained by a combination of increased release of t-PA and reduced hepatic clearance (6). This view is supported by the lack of fibrinolytic activation during auxiliary, heterotopic liver transplantation in which the native liver is not removed (26, 27). Dzik et al. (6) found that increase of t-PA during orthotopic liver transplantation may be associated with signs of shock. They suggested a mechanism of increased t-PA release due to hypotension and acidosis. We could not find any differences in blood pressure, arterial pH, or pO2 in patients with high or low t-PA levels. Probably mechanisms other than changes in the arterial circulation may also attribute to an increased release of t-PA. Patients with liver disease are known to be prone to activation of their fibrinolytic system upon specific stimuli such as physical stress and exercise (28, 29). The surgical stress, with the manipulations and extensive trauma to the vascular bed and abdominal circulation therefore might have contributed to an increased release of t-PA.
The rapid increase during graft reperfusion suggests a second mechanism that is associated with the restoration of blood flow through the donor liver. In studies with pigs, we recently demonstrated that fibrinolytic activity in the hepatic venous outflow immediately after graft reperfusion is significantly higher than the fibrinolvtic activity in the systemic circulation (Porte RY, Blauw E, Knot EAR, 1988, unpublished data). These data, in combination with the “explosive” t-PA increase after reperfusion in this study, strongly suggest an increased release of t-PA from the reperfused donor liver. Several factors, including vasoreactive agents, venous occlusion, anoxia, and thrombin, have been found to stimulate t-PA release from endothelial cells in in vitro and in vivo studies (8, 30, 31). It can be theorized that, during graft reperfusion and the subsequent restoration of normal portal blood flow, one or more of these factors, or leakage from ischemic damaged endothelial cells, contribute to an increase of t-PA. The normalization of t-PA activity during the late postanhepatic stage can be explained by the restoration of the normal hepatic clearance of t-PA after the implantation of a viable donor liver. Reduction of t-PA activity might have been enhanced by an increase of PAI toward the end of the operation, which is generally seen after major surgery and which is consistent with the behavior of PAI as an acute phase reactant (32).
Some investigators have suggested that hyperfibrinolysis in orthotopic liver transplantation may be secondary to thrombin formation during DIC (7, 11). For several reasons we do not believe that DIC is the main cause of t-PA increase during orthotopic liver transplantation.
First of all, in large series of patients, we previously found no evidence for a combined decrease of coagulation factors and inhibitors, as occurs during DIC (24, 33). Repeatedly negative findings regarding thromboembolic processes in histopathologic and clinical examination support the view that DIC does not play a clinically important role in OLT (34, 35).
Second, this study provided evidence for a thrombin-independent increase in t-PA during liver transplantation. Although TAT complexes, which are formed early during clotting activation by complex formation between thrombin and antithrombin III (24), increased steadily from the beginning of the operation and were still elevated at the end of the operation, t-PA increase was clearly limited to the anhepatic and early postanhepatic stage. Additionally, there was no difference in TAT increase in patients with high or low t-PA levels, indicating that the t-PA increase was independent of thrombin formation. The rise in TAT levels in our patients was most probably a sign of local clotting activation at the wound surface. Further studies, preferably with patients undergoing other major abdominal surgical procedures as control group, however, are necessary to establish the role of increased TAT levels in orthotopic liver transplantation.
Third, secondary fibrinolysis during DIC is probably a more local process that does not result in detectable increased fibrinolytic activity in the systemic circulation. In an analysis of 346 patients with DIC, Spero et al. (36) found evidence of systemic fibrinolytic activation, as demonstrated by shortened ELT or recalcified clot lysis time, in only 10% of the patients. Francis and Seyfert (37) recently demonstrated that although t-PA antigen is elevated in patients with DIC, detectable free t-PA activity is less frequently present than in hospitalized controls. These investigators concluded that PAI levels are also increased in DIC, leading to masking of the increased endothelial secretion of t-PA. This view is supported by experiments with cultured endothelial cells in which thrombin was shown to stimulate the release of both t-PA and PAI (30), resulting in no detectable net free t-PA activity despite increased levels of t-PA antigen. We observed a 40–50-fold systemic increase of t-PA activity and a concomitant saturation of PAI, resulting in a decrease of free PAI activity during the period of hyperfibrinolysis. Another process than DIC therefore seems responsible for the increased t-PA activity in orthotopic liver transplantation. Why some patients develop a high increase of t-PA activity, whereas others do not, remains unclear. We could not find any difference in diagnosis, preoperative hemostasis profile, or intraoperative hemodynamics in patients with high or low intraoperative t-PA activity levels.
Irrespective of its origin, the extremely high t-PA levels during the early postanhepatic stage may be clinically important. Especially in this period, formation of fibrin and stable hemostatic clots is necessary to prevent or stop bleeding from the vascular anastomosis and the extensive wound surface. Although the systemic increase of t-PA activity had a transient character, the clinical effect may extend over a considerably longer period, as t-PA may bind to fibrin and become incorporated in newly formed hemostatic clots, resulting in early lysis and delayed bleeding from fresh wounds (38). The role of t-PA in the development of a systemic lytic state was demonstrated in this study by the increase of plasma levels of both FbDP and FgDP in patients with high t-PA levels. This confirms earlier studies, which suggested a plasmin-mediated destruction of coagulation factors during orthotopic liver transplantation (5, 33). FgDPs are known to have an anticoagulant effect by inhibiting the polymerization of fibrin monomers into cross-linked fibrin (39). The occurrence of FgDPs in the circulation therefore may well be attributed to a further deterioration of the hemostatic function in patients undergoing orthotopic liver transplantation, as has been suggested before by Blecher et al. (40). The peak of FbDPs in the early postanhepatic stage is consistent with the clinical picture of delayed oozing and increased blood loss in this period (18, 25). We indeed found an significantly higher blood loss in patients with severe fibrinolysis (group II). However, these data should be interpreted with some reserve, as blood loss is influenced by multiple factors and larger series of patients are necessary to confirm this observation.
Since the primary increase in t-PA activity was associated with an active proteolytic destruction of fibrinogen, and fibrin and a high blood loss, the use of antifibrinolytic drugs seems justified in patients with life-threatening hemorrhages where active fibrinolysis is likely. To identify these patients, it is advisable to include at least 1 test method for the assessment of fibrinolytic activity in the intraoperative hemostasis monitoring in orthotopic liver transplantation.
Footnotes
This study was sponsored by the Netherlands Digestive Diseases Foundation and the A. A. Van Beek Fund. Assay kits were gifts from Behringwerke (Marburg, FRG), Kabi Vitrum Haematology (Stockholm, Sweden), and Organon Teknika (Turnhout, Belgium).
Abbreviations: AU, arbitrary units; DIC, disseminated intravascular coagulation; ELT, euglobin clot lysis time; FbDP, fibrin degradation products; FgDP, fibrinogen degradation products; PAI, plasminogen activator inhibitor; TAT, thrombin-antithrombin III; TEG, thrombelastography; t-PA, tissue-type plasminogen activator; WBLT, whole blood clot lysis time.
REFERENCES
- 1.Maddrey WC, Van Thiel DH. Liver transplantation, an overview. Hepatology. 1988;8:948. doi: 10.1002/hep.1840080440. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JH, Bontempo FA, Cornell FW, et al. Blood use in liver transplantation. Transfusion. 1987;27:222. doi: 10.1046/j.1537-2995.1987.27387235624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groth CG. Changes in coagulation. In: Starzl TE, Putman CW, editors. Experience in hepatic transplantation. Philadelphia: Saunders; 1969. p. 159. [Google Scholar]
- 5.Lewis JH, Bontempo FA, Kang YG, Spero JA, Ragni MV, Starzl TE. Intraoperative coagulation changes in liver transplantation. In: Winter PM, Kang YG, editors. Hepatic transplantation. New York: Praeger; 1986. p. 142. [Google Scholar]
- 6.Dzik WH, Arkin CF, Jenkins RL, Stump DC. Fibrinolysis during liver transplantation in humans: role of tissue-type plasminogen activator. Blood. 1988;71:1090. [PubMed] [Google Scholar]
- 7.Palareti, De Rosa V, Fortunato G, et al. Control of hemostasis during orthotopic liver transplantation. Fibrinolysis. 1988;2 suppl 3:61. [Google Scholar]
- 8.Collen D. On the regulation and control of fibrinolysis. Thromb Haemost. 1982;43:77. [PubMed] [Google Scholar]
- 9.Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69:381. [PubMed] [Google Scholar]
- 10.Brommer EJP, Derkx FHM, Schalenkamp MADH, Dooijewaard Gvd, Klaauw MM. Renal and hepatic handling of endogenous tissue-type plasminogen activator (t-PA) and its inhibitor in man. Thromb Haemost. 1988;59:404. [PubMed] [Google Scholar]
- 11.Bohmig HJ. The coagulation disorder of orthotopic hepatic transplantation. Semin Thromb Hemostas. 1977;4:57. doi: 10.1055/s-0028-1087128. [DOI] [PubMed] [Google Scholar]
- 12.Von Kaulla KN, Kayne H, Von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation before and after hepatectomy or transplantation in dogs and man. Arch Surg. 1966;92:71. doi: 10.1001/archsurg.1966.01320190073016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CY, Sobel JH, Weitz JI, Kaplan KL, Nossel HL. Immunologic identification of the cleavage products from Aα- and Bβ-chains in the early stages of plasmin digestion of fibrinogen. Thromb Haemost. 1986;56:100. [PubMed] [Google Scholar]
- 14.Bontempo FA, Lewis JH, Van Thiel DH, et al. The relation of preoperative coagulation findings to diagnosis, blood usage, and survival in adult liver transplantation. Transplantation. 1985;39:532. doi: 10.1097/00007890-198505000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwatsuki S, Shaw BW, Jr, Starzl TE. Current status of hepatic transplantation. Semin Liv Dis. 1983;3:173. doi: 10.1055/s-2008-1040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis JH. Hemostasis and hemorrhage. Sci Clin. 1971;1:1. [Google Scholar]
- 17.Lewis JH, Spero JA, Hasiba U. Diagnostic methods: laboratory tests: bleeding disorders. Garden City: Medical Exam Publishing Co.; 1978. p. 22. [Google Scholar]
- 18.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888. [PMC free article] [PubMed] [Google Scholar]
- 19.Wiman B, Mellbring G, Ranby M. Plasminogen activator release during venous stasis and exercise as determined by a new specific assay. Clin Chim Acta. 1983;48:266. doi: 10.1016/s0009-8981(83)80012-6. [DOI] [PubMed] [Google Scholar]
- 20.Verheijen JH, Mullaert E, Chang GTG, Kluft C, Wijngaards G. A simple sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurement in plasma. Thromb Haemost. 1982;48:266. [PubMed] [Google Scholar]
- 21.Chmielewska J, Ranby M, Wiman B. Evidence for a rapid inhibitor of tissue plasminogen activator in plasma. Thromb Res. 1983;31:427. doi: 10.1016/0049-3848(83)90407-3. [DOI] [PubMed] [Google Scholar]
- 22.Koppert PW, Kuipers W, Hoegee-de Nobel E, Brommer EJP, Koopman J, Nieuwenhuizen W. A quantitative enzyme immunoassay for primary fibrinogenolysis products in plasma. Thromb Haemost. 1987;57:25. [PubMed] [Google Scholar]
- 23.Koppert PW, Hoegee-de Nobel E, Nieuwenhuizen W. A monoclonal antibody-based enzyme immunoassay for fibrin degradation products in plasma. Thromb Haemost. 1988;59:310. [PubMed] [Google Scholar]
- 24.Pelzer H, Schwarz A, Heimburger N. Determination of human thrombin-antithrombin III complex in plasma with an enzyme-linked immunosorbent assay. Thromb Haemost. 1988;59:101. [PubMed] [Google Scholar]
- 25.Kang YG, Lewis JH, Navalgund A, et al. Epsilon-aminocaproic acid for treatment of fibrinolysis during liver transplantation. Anesthesiology. 1987;66:766. [PMC free article] [PubMed] [Google Scholar]
- 26.Knot EAR, Porte RJ, Terpstra OT, et al. Coagulation and fibrinolysis in the first human auxiliary partial liver transplantation in Rotterdam. Fibrinolysis. 1988;2:111. [Google Scholar]
- 27.Porte RJ, Knot EAR, de Maat MPM, et al. Fibrinolysis detected by TEG in heterotopic, auxiliary liver transplantation: effect of tissue type plasminogen activator. Fibrinolysis. 1988;2 suppl 3:67. [Google Scholar]
- 28.Das PC, Cash JD. Fibrinolysis at rest and after exercise in hepatic cirrhosis. Br J Haematol. 1969;17:431. doi: 10.1111/j.1365-2141.1969.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 29.Tytgat G, Collen D, De Vreker R, Verstraete M. Investigations on the fibrinolytic system in liver cirrhosis. Acta Haematol. 1968;50:265. doi: 10.1159/000208914. [DOI] [PubMed] [Google Scholar]
- 30.Gelehrter TD, Sznycer-Laszuk R. Thrombin induction of plasminogen activator inhibitor in cultured human endothelial cells. J Clin Invest. 1986;77:165. doi: 10.1172/JCI112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith D, Gilbert M, Owen WG. Tissue plasminogen activator release in vivo in response to vasoactive agents. Blood. 1985;66:835. [PubMed] [Google Scholar]
- 32.Kluft C, Verheijen JH, Jie AFH, et al. The postoperative fibrinolytic shutdown: a rapid reverting acute phase pattern for the fast acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest. 1985;45:605. doi: 10.3109/00365518509155267. [DOI] [PubMed] [Google Scholar]
- 33.Lewis JH, Bontempo FA, Awad S, et al. Liver transplantation: intraoperative changes in coagulation factors in 100 first transplants. Hepatology. doi: 10.1002/hep.1840090509. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchison DE, Genton E, Porter KA, et al. Platelet changes following clinical and experimental hepatic homotransplantation. Arch Surg. 1968;97:27. doi: 10.1001/archsurg.1968.01340010057003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, Putman CW, editors. Experience in hepatic transplantation. Philadelphia: Saunders; 1969. p. 422. [Google Scholar]
- 36.Spero JA, Lewis JH, Hasiba U. Disseminated intravascular coagulation: findings in 346 patients. Thromb Haeraost. 1980;43:28. [PubMed] [Google Scholar]
- 37.Francis RB, Seyfert U. Tissue plasminogen activator antigen and activity in disseminated intravascular coagulation: clincopathologic correlations. J Lab Clin Med. 1987;110:541. [PubMed] [Google Scholar]
- 38.Brommer EJP. The level of extrinsic plasminogen activator (t-PA) during clotting as a determinant of the rate of fibrinolysis: inefficiency of t-PA added afterwards. Thromb Res. 1984;34:109. doi: 10.1016/0049-3848(84)90067-7. [DOI] [PubMed] [Google Scholar]
- 39.Kowalski E. Fibrinogen derived inhibitors of coagulation. Thromb Diath Haemorrh. 1960;4 suppl:211. [PubMed] [Google Scholar]
- 40.Blecher TE, Terblanche J, Peacock JH. Orthotopic liver tranplantation: coagulation and hematologic changes in the pig. Arch Surg. 1968;96:331. doi: 10.1001/archsurg.1968.01330210009003. [DOI] [PubMed] [Google Scholar]