Abstract
The critical contribution of the Notch signaling pathway to vascular morphogenesis has been underscored by loss-of-function studies in mouse and zebrafish. Nonetheless, a comprehensive understanding as to how this signaling system influences the formation of blood vessels at the cellular and molecular level is far from reached. Here, we provide a detailed analysis of the distribution of active Notch1 in relation to its DSL (Delta, Serrate, Lag2) ligands, Jagged1, Delta-like1, and Delta-like4, during progressive stages of vascular morphogenesis and maturation. Important differences in the cellular distribution of Notch ligands were found. Jagged1 (Jag1) was detected in “stalk cells” of the leading vasculature and at arterial branch points, a site where Delta-like4 (Dll4) was clearly absent. Dll4 was the only ligand expressed in “tip cells” at the end of the growing vascular sprouts. It was also present in stalk cells, capillaries, arterial endothelium, and in mural cells of mature arteries in a homogenous manner. Delta-like1 (Dll1) was observed in both arteries and veins of the developing network, but was also excluded from mature arterial branch points. These findings support alternative and distinct roles for Notch ligands during the angiogenic process.
Keywords: arteries, blood vessels, capillaries, delta-like1, delta-like4, endothelial, jagged1, vascular remodeling, vasculature, veins
1. Results and Discussion
The Notch signaling pathway is widely known for its role in a myriad of processes during development. Nonetheless, the essential contribution of the Notch pathway to vascular morphogenesis has been revealed only recently. Deletion of many genes involved in Notch signal transduction, including: receptors, ligands, transcription factors, downstream targets, and molecules that participate in Notch processing, has resulted in severe vascular defects and embryonic lethality in mice (for reviews see Alva and Iruela-Arispe, 2004; Karsan, 2005; Shawber and Kitajewski, 2004). Of the Notch receptors, the loss of Notch1 proved to be the most deleterious to vascular development (Conlon et al., 1995; Krebs et al., 2000; Swiatek et al., 1994). Furthermore, several studies have demonstrated expression of Notch receptors and ligands in blood vessels and mural cells (Alva and Iruela-Arispe, 2004; Benedito and Duarte, 2005; Claxton and Fruttiger, 2004; Duarte et al., 2004; Gale et al., 2004; Shutter et al., 2000; Villa et al., 2001). These studies have also generally agreed that in addition to capillaries, Notch ligands and receptors are mostly confined to arteries, a finding that was subsequently supported by the requirement of Notch in establishing arterial identity through expression of EphrinB2 (Lawson et al., 2001). However, despite these findings, a comprehensive analysis of the relationship between Notch signaling molecules throughout the stages of angiogenesis is lacking. Notch 2 and Notch 3 have been implicated in vascular regression and arterial homeostasis respectively, but do not appear to participate in vascular morphogenesis (Joutel et al., 1996; McCright et al., 2001). In terms of the ligands, only the loss of either Jag1 or Dll4 results in vascular defects, indicating that the other three Notch DSL ligands may not be as involved in vessel development. Notably, analysis of the phenotypes exhibited by Jag1 (Xue et al., 1999) and Dll4 (Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004) knockout mice, suggested that these two ligands are not functionally redundant. Indeed, the onset of lethality would indicate that each one of these molecules might provide a different array of signals through activation of Notch receptors. Expression studies can offer critical information that could subsequently be used as a platform for mechanistic exploration. In the present study, our goal was to map the expression patterns of Notch1 and its ligands during the morphogenesis and maturation of the vasculature to gain insight as to which ligands contribute to specific events during vascular morphogenesis.
The embryonic vasculature expands in a three-dimensional manner and the distinction between immature and mature vessels is often difficult. Therefore, a two-dimensional model of angiogenesis, such as that of the retina, provides an ideal system for studying gene expression patterns (for review see Dorrell and Friedlander, 2006). In addition, the retinal vasculature displays all of the morphological hallmarks of angiogenesis, (sprouting, branching, fusion, remodeling, and maturation), making it an excellent platform in which to examine the expression of signaling molecules in relation to each stage of the angiogenic process (Fruttiger, 2002).
The vessels of the mouse retina develop post-natally during the first three weeks of life, although much of the maturation and remodeling takes place during the first two weeks. The primary vascular plexus migrates from the central retinal artery and expands in a planar manner with radially alternating veins and arteries from the optic nerve head, allowing the simultaneous observation of different stages of angiogenic expansion, as well as progressive maturation of arteries and veins (Fig.1A, B)(Claxton and Fruttiger, 2004; Fruttiger, 2002; Fruttiger et al., 2000; Stone and Dreher, 1987). We selected the time points of post-natal day (P) 3, 5, 7, and 15 for analysis based on the significant morphological events occurring at those times. Using a repertoire of antibodies to Notch receptor and ligand domains, including active Notch1, Jagged1, Delta-like1, and Delta-like4, we systematically examined their temporal and spatial distribution. PECAM-1 (also known as CD31) was used as a marker for vessel identification. Since the mere presence of Notch1 is not equivalent to activity, we used an antibody that recognizes the cytosolic domain of Notch1 only after it has been cleaved by γ-secretase, the last step in the activation of this receptor (Weinmaster, 2000).
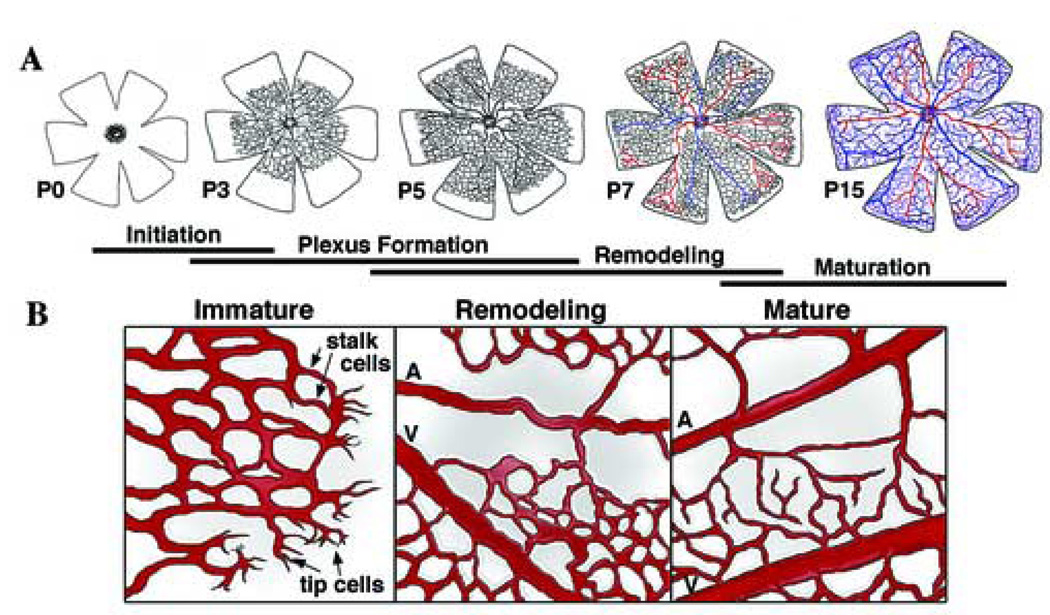
Figure 1. Vascular morphogenesis in the mouse retina.
(A) The schemes depict the stages development of the mouse retinal vasculature during the first 15 post-natal days (P). Initiation of the primary vascular network begins at P0 when vessels sprout from the optic nerve head, and begin extending and fusing to form a capillary plexus (Dorrell et al., 2002). The more mature vessels near the optic nerve head begin to remodel after P3 and develop into an alternating radial pattern of arteries and veins, connected by capillary beds. As the edge of the plexus reaches the perimeter of the retina (P7 and 8), vascular sprouts grow downward and form a secondary vascular plexus. By P15, the primary and secondary networks have matured and an intermediate plexus develops between the two established vascular layers (Dorrell et al., 2002). (B) Progression and maturation of the vascular plexus. Non-proliferating “tip cells” containing long filopodia guide the vascular sprout, consisting of dividing “stalk cells” (Gerhardt et al., 2003), as the immature network extends and forms by branching and fusion. Older vessels behind the leading edge remodel into arteries (which lack proximity of capillaries), veins, and capillary beds. Arteries, arterioles, veins, venuoles, and capillary beds are present in the mature vasculature. A, artery; V, vein.
1.1. Active Notch1 signaling in endothelial cells during vascular morphogenesis
Early in vessel initiation, at P3, active Notch1 was seen in the outer half of the vascular plexus, in immature capillaries and in tip and stalk cells (Fig. 2A–C). By P5 and P7, active Notch1 signaling appeared more sporadic and remained in the arterioles and capillaries at the peripheral two-thirds of the retina (Fig. 2D–I). At these immature stages, we also noted the presence of active Notch1 in vessels that appear to be destined for a venous fate (Fig. 2H, arrowhead). It is possible to distinguish arteries from veins by P5, as the more mature vessels near the optic nerve head have begun to remodel and capillary clearance is observed in the vicinity of the arteries (Fig. 1B). This arterial-venous distinction is even more obvious by P7, as the radially alternating major arteries and veins from the center of the retina are thicker in girth than other vessels. The identification of vessels based on these characteristics was confirmed by EphB4 antibody staining (data not shown), a marker for venous endothelial cells (Gerety et al., 1999). By P15, Notch1 activity was found in mature arteries and arterioles, although infrequent activity in venous endothelial cells was also detected at this time (Fig. 2J, arrowheads). Despite some activity in major vessels, the distribution of Notch1 during this late time point was mainly in the lower vascular plexus, particularly at branch points (Fig. 2J–L).
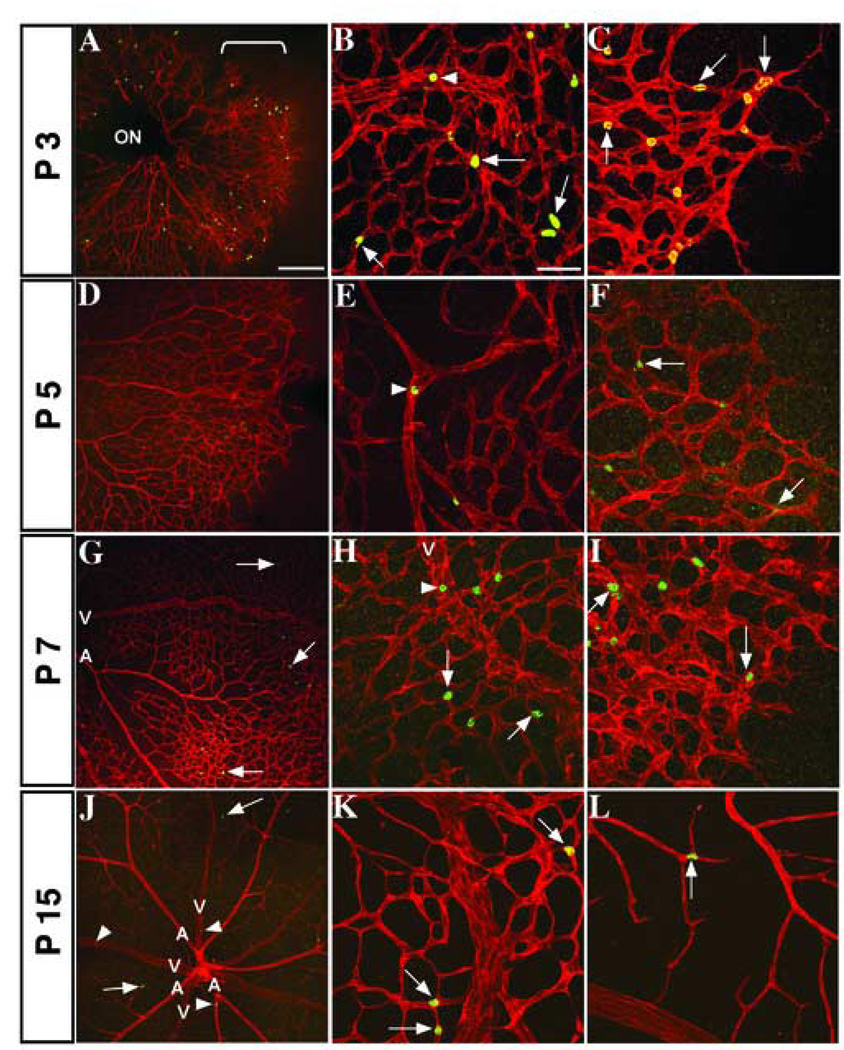
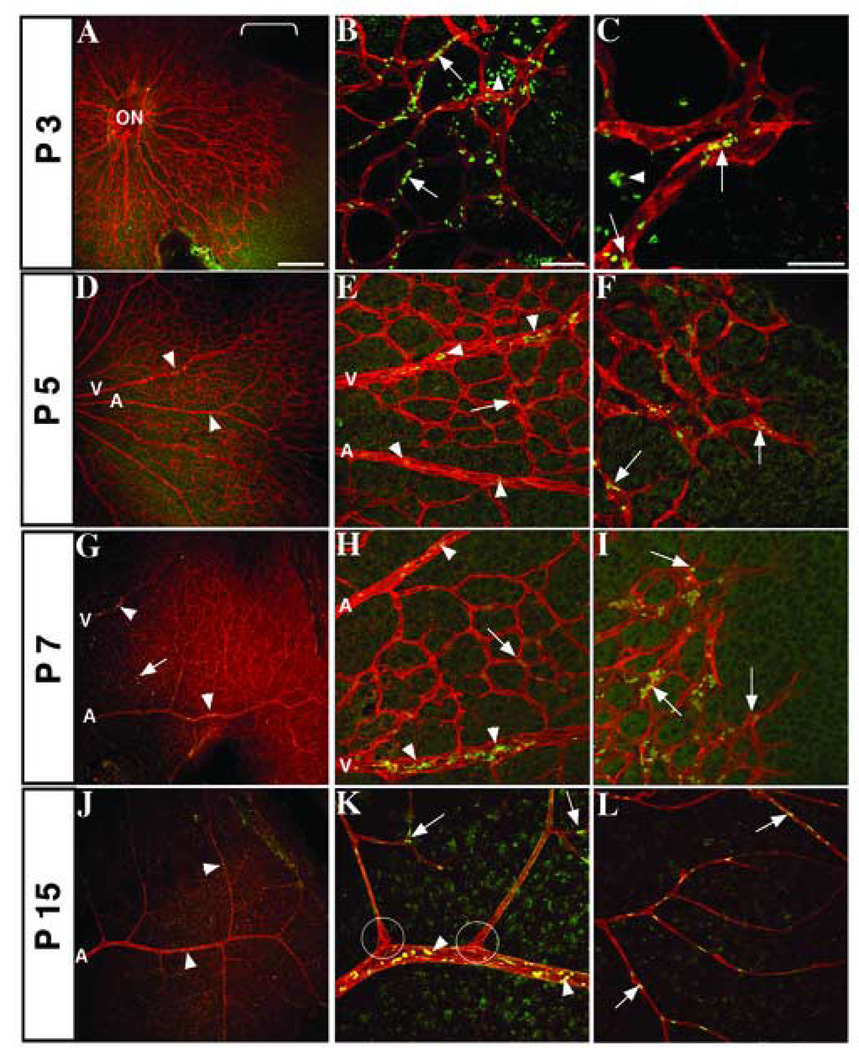
Figure 2. Pattern of active Notch1 expression in the developing retinal vasculature.
Fluorescence immunohistochemistry of post-natal mouse retinas with PECAM-1 (red) positive vessels and active Notch1 (green). Developmental time points are noted along the vertical axis. Low magnification images of retinal vasculature (A, D, G, J) show distribution of active Notch1 throughout angiogenesis. (A) Bracket shows Notch1 activation along the leading edge of the growing capillary plexus at P3, with expression in maturing vessels (B, arrowhead), capillaries, and stalk cells (B and C, arrows). Notch1 is sporadically found in mature vessels at P5 and 7 (E, H, arrowheads) and in capillaries (E–I, arrows), particularly at branching points. By P15, active Notch1 is further diminished in the mature vessels, although it is still found in arteries, arterioles, and some veins (J, arrowheads), branch points of capillaries (K), and the lower plexus (J, L arrows). ON, optic nerve; A, artery; V, vein. Scale bars: 200 µm (A, D, G, J) and 50 µm for the rest.
1.2. Jagged1 expression in developing retinal vasculature
In comparison to Notch1, Jag1 expression was reduced at P3, but when present, was also scattered along the periphery of the retinal vessels. Cells expressing Jag1 were observed near branching points, as well as in the stalks at the leading edge of the vasculature, but we did not detect Jag1 in tip cells (Fig. 3B, C). As the vascular network begins to mature and remodel, the pattern of Jag1 staining expanded to cover the outer two-thirds of the growing plexus, major arteries and smaller arterioles, as well as neighboring non-endothelial cells by P5 and 7 (Fig. 3D–I). Many of the Jag1 positive cells appeared to be along the edge of vessels and were likely pericytes or smooth muscle cells. The Jag1 positive cells outside the vascular template were probably of a neuronal nature (Fig. 3H, I), as Jag1 is also expressed in the central nervous system (Wang et al., 1998). Importantly, at P15, we found a concentration of Jag1 positive cells in branch points of arteries (Fig. 3J arrows, K circles and Fig. 6A, C). At these sites, cells expressing Jag1 appeared to be both endothelial and mural. This observation was particularly worth noting, considering Jag1 null mice die by embryonic day 11.5 as a result of a lack of vascular remodeling (Xue et al., 1999). We quantified the distribution of Jag1 at these arterial branch points at P15. From three independent samples we determined the percentage of Jag1 positive cells at branches to be 67%, 51%, and 60%, (average of 58%). In addition, increased Jag1 expression was also seen in mature arteries and arterioles, as well as in the lower vascular plexus during this time point (Fig. 3L).
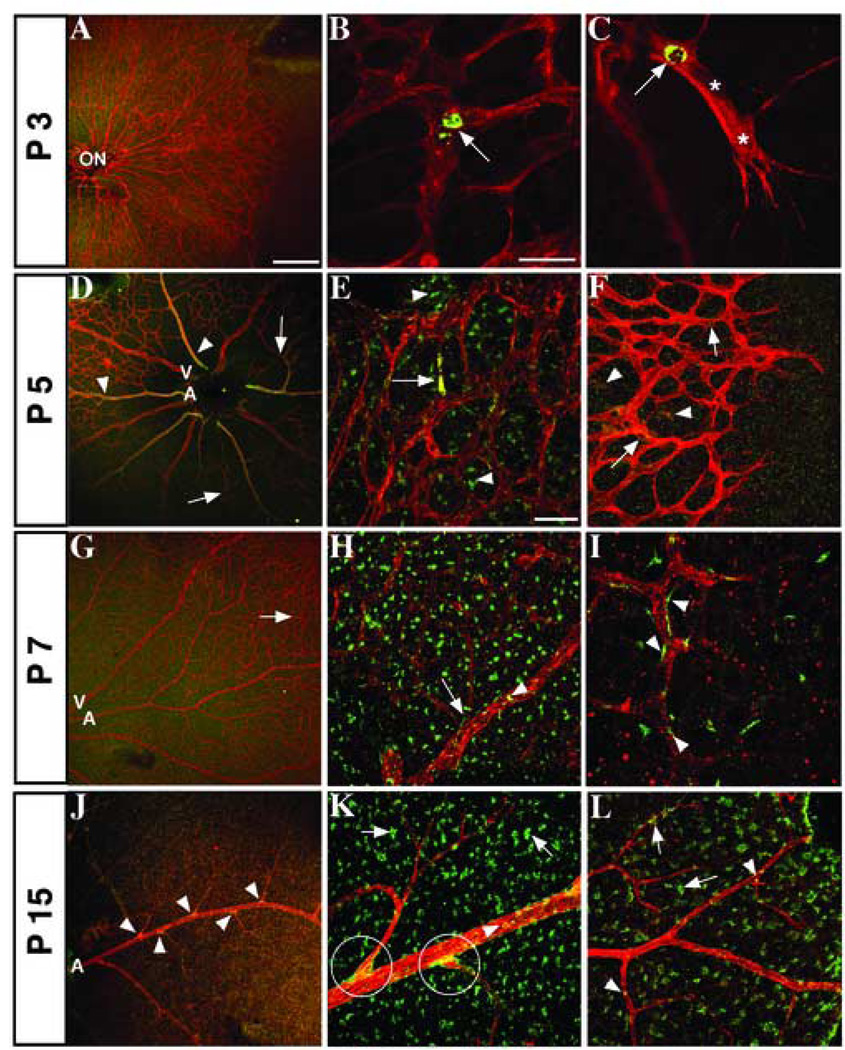
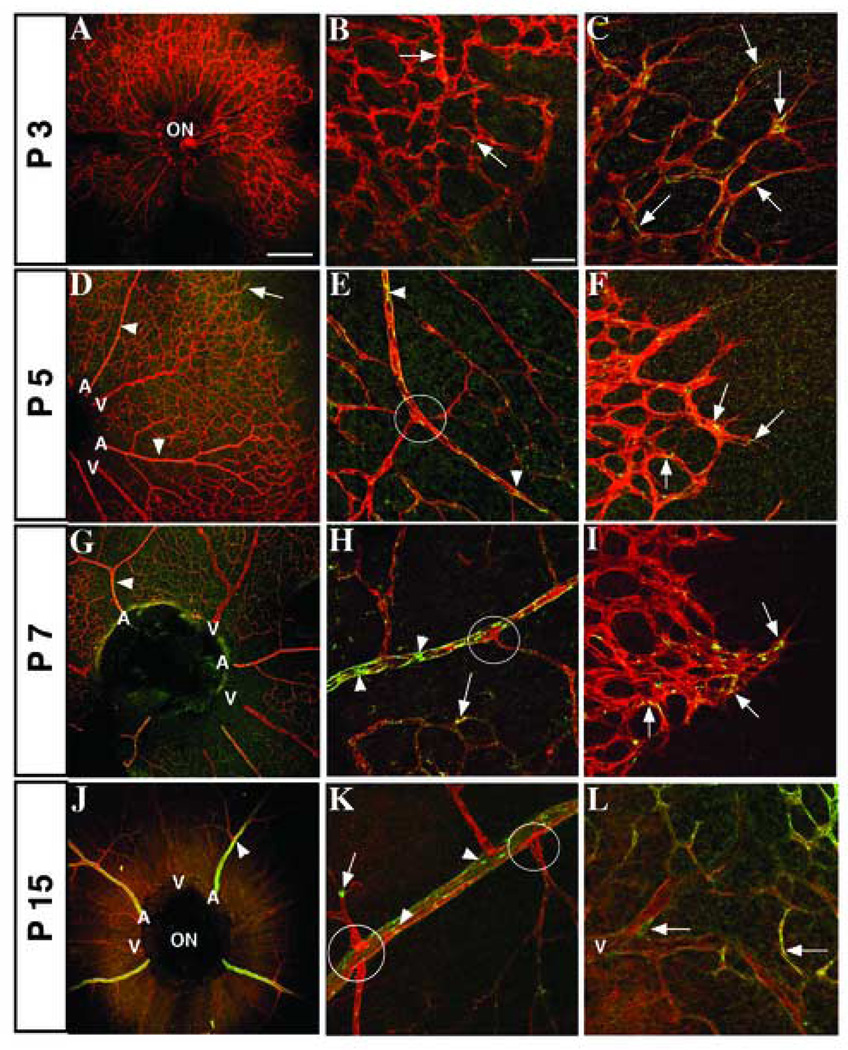
Figure 3. Distribution of Jagged1 in developing vessels, surrounding cells, and arterial branch points.
Confocal images of mouse retinas stained with antibodies for endothelial marker PECAM-1 (red) and Jagged1 (green). (A–C) P3 expression of Jag1 is sparse, although some staining is seen in the immature plexus (B) and stalk cells (C) near points of vessel branching. Nuclei, verified by staining with nuclear dye TO-PRO-3 (data not shown), are marked by asterisks. Jag1 expression is visible in arteries (D, arrowhead) and is still found in stalk cells (F, arrows) at P5. Concurrently, Jag1 expression increases in the remodeling plexus (D, E, arrows) and in surrounding cells outside the vasculature (E, F, arrowheads). By P7, many Jag1 positive cells are seen adjacent to the vessels (H, arrow and I), although Jag1 can still be found inside capillaries (G, arrow) and mature vessels (H, arrowhead). At P15, cells expressing Jag1 are observed wrapping around arterial branch points (J and K, circles). Jag1 is also found in arteries and arterioles (K, L, arrowheads), and non-vascular cells (K, L, arrows). ON, optic nerve; A, artery; V, vein. Scale bars: 200 µm (A, D, G, J), 50 µm (E, F, H, I, K, L), and 25 µm (B, C).
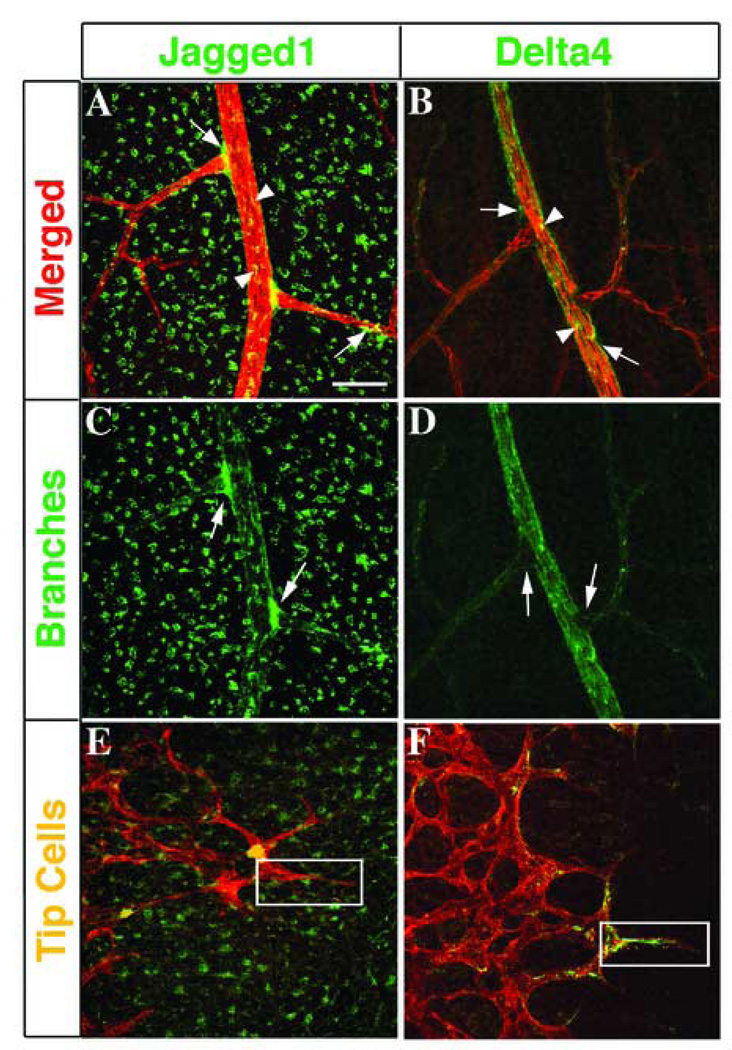
Figure 6. Differential expression of Jagged1 and Delta-like4 in arterial branches and tip cells.
Comparison of immunostaining for PECAM-1 (red), Jag1 (A, C, E, green), and Dll4 (B, D, F, green). Jag1 and Dll4 are both expressed in mature arteries (A, B, arrowheads) and in cells immediately adjacent to arteries in P15 mouse retinas (A, B, arrows), while expression of Jag1 is also seen in non-vascular associated cells (A). When the expression of Jag1 and Dll4 are viewed separately, the localization of Jag1 around arterial branch points (C, arrows) and the exclusion of Dll4 from these branches (D, arrows) are more clearly defined. Similarly in the leading edge of the early primary plexus, the patterns of expression of Jag1 and Dll4 appear to be associated with separate cell types, with Jag1 excluded from tip cells (E, boxed area) and Dll4 highly expressed in tip cells (F, boxed area). Scale bar: 50 µm.
1.3. Delta-like1 expression patterns during vascular morphogenesis
Expression of Dll1 at P3 was high at the leading edge of the developing vasculature (outer third of the retinal plexus), but it was also observed along vessels that appear to be arterial (Fig. 4A–C). As the vessels remodeled and matured at P5 and 7, Dll1 remained in the capillaries, although expression was weak. At the leading edge, Dll1 was found in stalk cells, but it was absent in tip cells (Fig. 4C). During this developmental time point, expression in arteries was also noted (Fig. 4D–I). Additionally, Dll1 was observed in veins at levels higher than that of arteries and capillaries (Fig. 4E, H). Although Dll1 has not been fully evaluated in the vasculature, its pattern constitutes an exception, as up to this point Notch receptors and ligands have only been detected in arterial and capillary vessels (Benedito and Duarte, 2005; Shutter et al., 2000; Villa et al., 2001)(Fig. 4E, H). At P15, Dll1 was found throughout the retinal vasculature, in arteries, arterioles, capillaries, venuoles and veins. Expression was not present in the vicinity of branching points of either arteries or veins. In addition, Dll1 was also found in cells outside of the vasculature, similar to the pattern of Jag1-stained retinas (Fig. 4J–L).
Figure 4. Expression of Delta-like1 during vascular morphogenesis.
Co-immunolocalization of PECAM-1 (red) and Dll1 (green) antibodies in post-natal mouse retinas. (A–C) Dll1 is expressed in the periphery of P3 retinas (A, bracket), in capillaries (B, arrows), stalk cells (C, arrows), and in cells not associated with the vasculature (B, C, arrowheads). Remodeling vessels of both arterial and venous nature have increased Dll1 expression at P5 (D, E, arrowheads). Staining patterns also show Dll1 in the capillaries (E, arrow) and stalk cells (F, arrows). By P7, arteries and veins are more distinct, Dll1 expression is clearly seen in both types of vessels and appears increased in veins when compared to arteries (G, H, arrowheads). Distribution of Dll1 in capillary and stalk cells is also increased and encompasses the entire vascular network (G–I, arrows). (J–L) This coverage is also visible at P15, as Dll1 is found in arteries, arterioles, capillaries, veins, venuoles, and the lower vascular plexus, but not at arterial branch points (K, circles). Non-vascular cells are also positive (K, L). ON, optic nerve; A, artery; V, vein. Scale bars: 200 µm (A, D, G, J), 50 µm (B, E, F, H, I, K, L), and 25 µm (C).
1.4. Expression of Delta-like4 during vascular development
Much like the other DSL ligands, the expression pattern of Dll4 was also confined to the edges of the growing plexus and in early stalk and tip cells at P3 (Fig. 5A–C). However, by P5 and 7, Dll4 was found in arteries and it was completely excluded from veins (Fig. 5D, G). Protein distribution of Dll4 displayed a banded pattern reminiscent of the previously reported transverse striping of Dll4 mRNA expression (Fig. 5E, H)(Claxton and Fruttiger, 2004). Dll4 also remained in the capillary plexus and smaller vessels, all of which was consistent with earlier in situ hybridization studies (Claxton and Fruttiger, 2004). By P7, Dll4 staining was conspicuously absent from arterial branching points (Fig. 5H). The arterial staining of Dll4 appeared to be increased by P15, in comparison to earlier stages and despite the reported decrease in Dll4 mRNA at this point (Fig. 5J–K)(Claxton and Fruttiger, 2004). A lack of Dll4 was also noted at branch points of mature arteries, based on the lack of co-localization of PECAM1 (Fig. 5K, circles). This Notch ligand was also detected throughout the capillary plexus, the lower vascular layer, and at the termini of veins by this time (Fig. 5K, L).
Figure 5. Localization of Delta-like4 in tip cells, capillaries, and arterial vessels throughout retinal vascular development.
Examination of the post-natal mouse retinal vasculature using fluorescence immunohistochemistry for PECAM-1 (red) and Dll4 (green). (A, B) Expression of Dll4 is not well defined in the vascular plexus P3 retinas, but is present in the center of the plexus (B) and in stalk and tip cells of the leading vascular sprouts (C). Dll4 is also found in early maturing arteries by P5 (D, arrowheads), as well as in capillaries, arterioles (E, arrowheads), tip cells and stalk cells (D, F, arrows). However, arterial branch points lack Dll4 expression at P5, 7 and 15 (E, H, K, circles), despite increased arterial staining (G, H, J, K, arrowheads). Dll4 is still seen in capillaries, stalk cells, and tip cells at P7 (H, I, arrows), but as the primary plexus reaches the edge of the retina and the lower vascular layers begin to form, Dll4 expression can be found near the termini of veins (L). ON, optic nerve; A, artery; V, vein. Scale bars: 200 µm (A, D, G, J), and 50 µm (B, C, E, F, H, I, K, L).
1.5. Distinct Jagged1 and Delta-like4 expression patterns in the mature vasculature
As the vasculature matures, we observed expression of Jag1 and Dll4 in mural cells directly associated with the vessels (Fig. 6A,B, arrows), as well as in the endothelial cells of the mature arteries (Fig. 6A,B, arrowheads). Jag1 was located at arterial branch points during vessel maturation, while Dll4 was excluded from this site (Fig. 6C, D). In addition, Jag1 expression was detected in stalk cells, immediately behind the Dll4 positive tip cells, during the expansion of the vascular plexus. This pattern indicates alternative modes for activation of Notch signaling in these two non-overlapping cellular compartments (Fig. 6E, F).
1.6. Jagged1 and Delta-like4 expression in vascular smooth muscle cells
The finding of Jag1 and Dll4 positive cells that did not co-localize with PECAM1, led us to consider whether these cells were vascular smooth muscle cells (VSMC). Several cells expressing αSMA were also positive for Jag1 staining at both P7 and P15 (Fig. 7A, arrows), and in particular, αSMA staining at arterial branch points at P15 also co-localized with Jag1 (Fig. 7A, arrowheads). The merged images indicated that co-localization of Jag1 and αSMA antibodies was highest along the edge of the cell that interacts with the vessel. In contrast to Jag1, Dll4 did not appear to be expressed in VSMC at P7 (Fig. 7B, P7 merged), nor at arterial branch points at P15 (Fig. 7B, asterisk) where αSMA was expressed (Fig. 7B, arrowhead). However, some overlapping αSMA and Dll4 expression was seen at P15 in mature arteries (Fig. 7B, P15 merged). Further confirmation of co-localization staining was achieved by evaluation of the specimens on a multiphoton Zeiss microscope equipped with a Meta Detector. The analysis integrates pixel location and excitation individually to avoid any bleed-through concerns (Supplemental Figure).
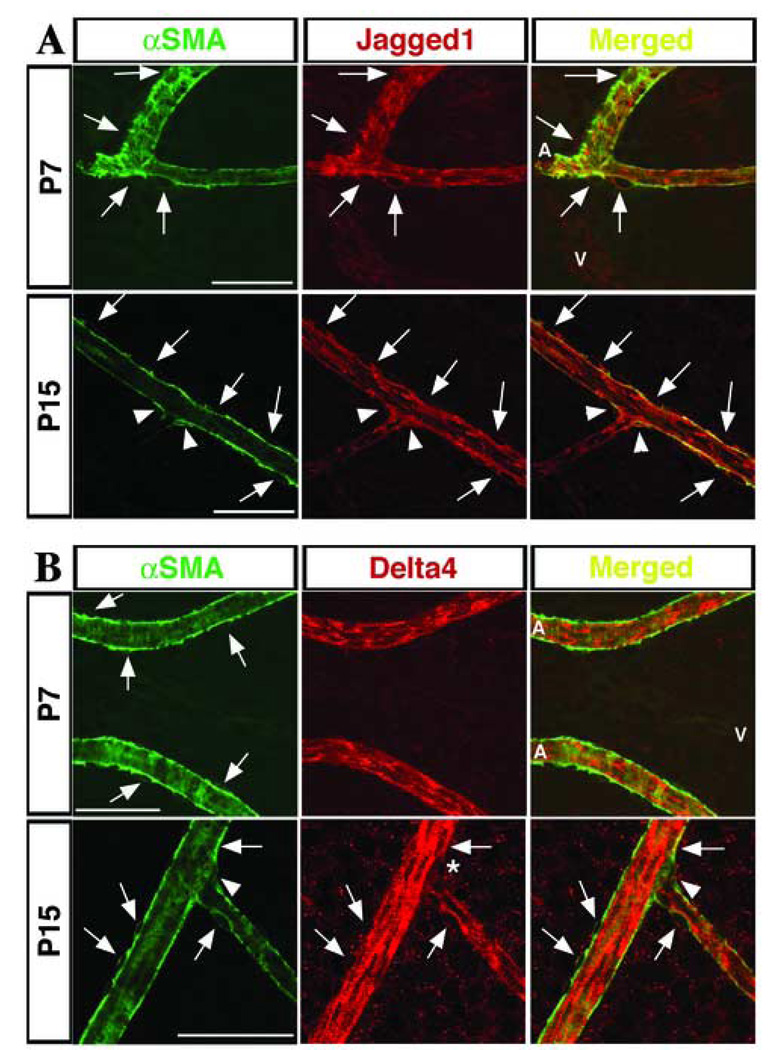
Figure 7. Expression of Jagged1 and Delta-like4 in vascular smooth muscle cells of mature arteries.
(A) Co-localization of -smooth muscle actin (αSMA; in green) and Jag1 (red) at P7 and P15. Arrows point to cells positive for both markers. At P15, Jag1 expressing smooth muscle cells are seen at arterial branch points (P15; arrowheads). (B) Dll4 was not detected in VSMC at P7 but it is expressed in some smooth muscle cells at P15. Arrows indicated cells positive for each marker. The arrowheads at P15 indicate the presence of αSMA at an arterial branch point, while the asterisk marks the lack of Dll4 in that same area. A, artery; V, vein. Scale bars: 50 µm.
Precedence for differences in Notch1 signaling induced by specific ligands has been reported recently. For example, lateral inhibition and prosensory functions of Notch signaling in the mouse inner ear are distinctly mediated by Jag1 and Dll1 respectively (Brooker et al., 2006). Also, during T lymphocyte development, the activation and proliferation of T helper cells is differentially regulated by Dll1, Dll4, and Jag1 (Rutz et al., 2005). The exclusivity of the expression patterns observed for these ligands could explain the differences in embryonic lethality and in phenotypes observed in the Jag1 and Dll4 null mice. Jag1 knockout mice have remodeling defects in the vasculature of the embryo and the yolk sac, resulting in hemorrhage and death by E11.5, indicating the contribution of this ligand at later stages of angiogenesis (Xue et al., 1999). Three separate studies of Dll4 deletion exhibited severe cardiovascular abnormalities including aortic stenosis, defective branching, arteriovenous connections, and pericardial edema (Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004). These mutant mice exhibit an array of defects far more extensive than those displayed by Jag1 and result in lethality between E9.5–10.5 with varying degrees of haploinsufficiency depending on the genetic background.
Although Jag1 and Dll4 appear to be the major ligands involved in vascular development, further investigation of Dll1 is warranted. It has been previously reported that Dll1 is expressed in the endothelium of vessels during development (Beckers et al., 1999), yet its function has not been clearly delineated. Based on the expression pattern of Dll1 in both arteries and veins reported here, as well as the death of Dll1 knockout mice with hemorrhaging at E12 (Hrabe de Angelis et al., 1997), it would appear that Dll1 is also an important contributor to Notch activation in blood vessels. The presence of Dll1 in veins might also explain the sporadic distribution of active Notch in venular endothelium. Together, these results provide new information regarding the temporal and spatial distribution of the DSL ligands during vascular morphogenesis and further suggest exclusive signaling roles for these ligands in endothelial and mural cells.
2. Materials and methods
2.1. Retina collection
Eyes were isolated from P3, P5, P7, and P15 C57/Bl6J pups and fixed in 2% (w/v) paraformaldehyde (PFA) in 1X phosphate buffered saline (PBS) for 10 minutes. The cornea, sclera, lens, vitreous, and hyaloid vessels were removed in 1X PBS and retinas were then dissected. Radial incisions were made at equal intervals along the edge of the retinas. Specimens were fixed for 2 hours in 2% PFA before storage in 1X PBS. At least 3 retinas were examined for each antibody at each time point.
2.2. Immunohistochemistry on whole-mount retinas
Whole-mount retinas were incubated in 1X PBS containing 5% donkey serum (Jackson ImmunoReserach) and 0.3% Triton X-100 for 1 hr at room temperature. Co-immunolocalization of Notch1, Jagged1, Delta-like1, or Delta-like4 with PECAM was performed by co-incubation of primary and secondary antibodies conjugated with Cy3 and fluorescein isothiocyanate (FITC). For primary antibodies, cleaved Notch1 (Valine1744, Cell Signaling Technology, MA) was diluted 1:500, anti-Jagged1 (PCR8)(Nehring et al., 2005) was diluted 1:500, anti-Jagged1(C-20, Santa Cruz Biotechnology, Inc., CA) was diluted 1:200, anti-Delta-like1 (148G)(Nehring et al., 2005) was diluted 1:500, anti-Delta-like4 (mDLL4, R&D Systems, MN) was used at 1µg/ml, anti-EphB4 (mEphB4, R&D Systems, MN) was used at 1µg/ml (data not shown), anti-actin, α-Smooth Muscle-FITC (Sigma-Aldrich, MO) was diluted 1:1000, and anti-PECAM-1 was used at 1µg/ml (CD31, BD Pharmingen, CA) in 1X PBS containing 5% donkey serum and 0.3% Triton X-100 overnight at 4°C. After washing specimens for 1 hr with 1X PBS and 0.05% Tween-20, incubations with secondary antibodies were performed for 3 hrs at 4°C. For nuclear visualization, TO-PRO-3 (Molecular Probes, OR) was diluted 1:500 (data not shown). After a final wash in 1X PBS, samples were observed by confocal microscopy on a BioRad MRC1024 using BioRad LaserSharp2000 acquisition software (BioRad, Hercules, CA). For co-localization analysis we examined the samples on a Zeiss LSM 510 Meta NLO two-photon laser scanning confocal microscope. To obtain true co-localization information we also employed the Meta Detector of the Zeiss multiphoton confocal system that can deconvolve the contribution of each fluorochrome to each pixel by spectral analysis, using information obtained from the reference spectra (FITC and Cy3 alone). The true overlapping pixels were subsequently designated as white (Supplemental Figure).
Supplementary Material
Confocal images obtained on a Zeiss LSM 510 Meta NLO two-photon laser scanning confocal microscope revealed areas of co-localization (shown in white) between Jag1 (A, red) or Dll4 (B, red) and αSMA (green) expression. The graphs display the intensity of excitation (in a color scale) for each channel (x-axis, green; y-axis, red). The areas of overlap/co-localization that appear in white in the photomicrograph correspond to the gradient marked as 3 (area of intersection of both channels).
Acknowledgements
The following antibodies were generously provided by Dr. Gerry Weinmaster (Jag1-PCR8 and Dll1-148G). This study was supported by funds awarded to L.I-A. by the National Institutes of Health (NIH - HL074455) and J.H. (USPHS National Research Service Award GM07104). The authors would also like to thank Drs Gerry Weinmaster and Ann Zovein for critical review of this manuscript and for valuable comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alva JA, Iruela-Arispe ML. Notch signaling in vascular morphogenesis. Curr Opin Hematol. 2004;11:278–283. doi: 10.1097/01.moh.0000130309.44976.ad. [DOI] [PubMed] [Google Scholar]
- Beckers J, Clark A, Wunsch K, Hrabe De Angelis M, Gossler A. Expression of the mouse Delta1 gene during organogenesis and fetal development. Mech Dev. 1999;84:165–168. doi: 10.1016/s0925-4773(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Benedito R, Duarte A. Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr Patterns. 2005;5:750–755. doi: 10.1016/j.modgep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Claxton S, Fruttiger M. Periodic Delta-like 4 expression in developing retinal arteries. Gene Expr Patterns. 2004;5:123–127. doi: 10.1016/j.modgep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25:277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43:522–527. [PubMed] [Google Scholar]
- Fruttiger M, Calver AR, Richardson WD. Platelet-derived growth factor is constitutively secreted from neuronal cell bodies but not from axons. Curr Biol. 2000;10:1283–1286. doi: 10.1016/s0960-9822(00)00757-0. [DOI] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabe de Angelis M, McIntyre J, 2nd, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Karsan A. The role of notch in modeling and maintaining the vasculature. Can J Physiol Pharmacol. 2005;83:14–23. doi: 10.1139/y04-125. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- Nehring LC, Miyamoto A, Hein PW, Weinmaster G, Shipley JM. The extracellular matrix protein MAGP-2 interacts with Jagged1 and induces its shedding from the cell surface. J Biol Chem. 2005;280:20349–20355. doi: 10.1074/jbc.M500273200. [DOI] [PubMed] [Google Scholar]
- Rutz S, Mordmuller B, Sakano S, Scheffold A. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol. 2005;35:2443–2451. doi: 10.1002/eji.200526294. [DOI] [PubMed] [Google Scholar]
- Shawber CJ, Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. Bioessays. 2004;26:225–234. doi: 10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987;255:35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Weinmaster G. Notch signal transduction: a real rip and more. Curr Opin Genet Dev. 2000;10:363–369. doi: 10.1016/s0959-437x(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confocal images obtained on a Zeiss LSM 510 Meta NLO two-photon laser scanning confocal microscope revealed areas of co-localization (shown in white) between Jag1 (A, red) or Dll4 (B, red) and αSMA (green) expression. The graphs display the intensity of excitation (in a color scale) for each channel (x-axis, green; y-axis, red). The areas of overlap/co-localization that appear in white in the photomicrograph correspond to the gradient marked as 3 (area of intersection of both channels).