Abstract
Objective:
The aim of our study was to evaluate the effect of cardiac resyncronization therapy (CRT) on QT dispersion (QTd), JT dispersion (JTd) and transmural dispersion of re-polarization (TDR), markers of heterogeneity of ventricular repolarization in a study population with severe heart failure.
Methods and Results:
Fifty patients (43 male, 7 female, aged 60.2 ± 3.1 years) suffering from congestive heart failure (N = 39 NYHA class III; N = 11 NYHA class IV) as a result of coronary artery disease (N = 19) or of dilated cardiomyopathy (N = 31), sinus rhythm, QRS duration >130 ms (mean QRS duration >156 ± 21 ms), an ejection fraction < 35%, left ventricular end-diastolic diameter >55 mm, underwent permanent biventricular DDDR pacemaker implantation. A 12-lead standard electrocardiogram was performed at baseline, during right-, left-, and biventricular pacing and QTd, JTd and TDR were assessed. Biventricular pacing significantly reduced QTd (73.93 ± 19.4 ms during BiVP vs 91 ± 6.7 ms at sinus rhythm, p = 0.004), JTd (73.18 ± 17.16 ms during BiVP vs 100.72 ± 39.04 at baseline p = 0.003), TDR (93.16 ± 15.60 vs 101.55 ± 19.08 at baseline; p<0.004), as compared to sinus rhythm. Right ventricular endocardial pacing and left ventricular epicardial pacing both enhanced QTd (RVendoP 94 ± 51 ms, p<0.03; LVepiP 116 ±71 ms, p<0.02) and TDR (RVendoP 108.13 ± 19.94 ms; p<0.002; LVepiP 114.71 ± 26.1; p<0.05).There was no effect on JTd during right and left ventricular stimulation.
Conclusions:
Biventricular pacing causes a statistically significant reduction of ventricular heterogeneity of ripolarization and has an electrophysiological antiarrhythmic influence on arrhythmogenic substrate of dilatative cardiomiopathy.
KEY WORDS: Biventricular pacing, QT dispersion, JT dispersion, TDR, Heart failure
INTRODUCTION
Congestive heart failure (CHF) remains a major health problem worldwide, despite considerable progress in its management. The incidence and prevalence of this disease continues to increase due to an aging population, which, in part, is related to the use of new pharmacologic and non pharmacologic therapies. An estimated 6–7 million people have CHF in the United States and Europe, and approximately 1 million patients are diagnosed with CHF every year (1, 2). The cost involved in the management of this problem is enormous and continues to climb. Despite a progressive prognosis improvement thanks to the therapy with ace-inhibitors (3, 4), beta-blockers (5) and spironolactone (6), the quality of life in patients with advanced heart failure remains poor and mortality, due to risks for ventricular arrhythmias and sudden cardiac death, can increase. Patients with advanced heart failure frequently have conduction disturbances (atrioventricular, inter- or intraventricular conduction delay with QRS interval > 120 ms) that play an important role in worsening cardiac systolic function and in increasing risk of death (7, 8). In recent years a new therapeutic approach, biventricular pacing (BiVP), has been introduced as an adjunctive therapy to pharmacological treatment in patients with severe CHF and intraventricular conduction delay. Biventricular pacing stimulates the right atrium and both the ventricles simultaneously to resynchronize their action. Many studies have demonstrated that BiVP in patients with advanced CHF significantly improves ventricular hemodynamics, quality of life, exercise capacity and reduces mortality and hospitalization rates (9–11). Little is known yet about the influence of cardiac resynchronization therapy on the heterogeneity of ventricular repolarization (11, 12), which represents the arrhythmogenic substrate of dilatative cardiomyopathy and is identified on the electrocardiogram by QT dispersion (QTd), JT dispersion (JTd) and transmural dispersion of repolarization (TDR). The aim of our study was to evaluate the effect of biventricular pacing on electrocardiographic parameters of heterogeneity of ventricular repolarization, both spatial (QTd, JTd) and transmural (TDR).
METHODS
Selection of patients
The study population consisted of fifty patients (43 male, 7 female, aged 60.2 ± 3.1 years) suffering from congestive heart failure (N = 39 NYHA class III; N = 11 NYHA class IV) as a result of coronary artery disease (N = 19) or of dilated cardiomyopathy (N = 31). All patients were in sinus rhythm and demonstrated a left bundle branch block (QRS duration > 130 ms; mean QRS duration >156 ± 21 ms) in the standard 12-lead electrocardiogram. All patients included in the evaluation showed an ejection fraction < 35 and left ventricular end-diastolic diameter > 55 mm. All patients were taking heart failure medications (Ace-inhibitors, beta-blockers, diuretics, spironolactone), which remained unchanged during the previous six weeks before the permanent biventricular DDDR pacemaker implantation. None of the patients was on class I or III antiarrhythmic drug therapy.
Implantation of pacemaker
All patients underwent first time permanent biventricular pacemaker implantation. All leads were implanted transvenously. The atrial lead was placed high in the right atrium, the right ventricular lead was placed in the right ventricular apical endocardium, and the left ventricular lead was placed in an epicardial posterolateral vein via coronary sinus access similar to the methods described by Daubert et al (13). A venogram helped to optimize the position of the lead. The pacemakers were triple-output devices that made use of standard dualchamber technology, with built-in adapters to synchronize the pacing of the two ventricles. Results of the implantations were assessed from the positions of the leads on chest X-ray films and from changes in the width of the QRS interval on 12-lead surface electrocardiogram.
Protocol study
Each patient underwent a twelve-lead surface electrocardiogram, recorded at a speed of 50 mm/sec and performed before pacemaker implantation and at the end of follow-up period, twelve months after the procedure. The ECG recording was performed during sinus rhythm (SR), right ventricular endocardial pacing (RVendoP), left ventricular epicardial pacing (LVepiP) and biventricular pacing (BiVP). Each stimulation continued for 3 minutes. Special attention was paid to eliminate all correctable causes of noise, such as muscle tremor, and to minimize electromagnetic frequency interference generated by electrical sources next to lead array. The pacing sequences were randomly switched between the patients and no effects on the results were observed. The study was approved by the local ethics committee and written informed consent was obtained from all patients prior to enrolment.
Data acquisition and electrocardiogram measurements
Twelve-lead body surface electrocardiograms were recorded at a speed of 50 mm/sec. The analysis was performed by one investigator only. QRS duration, QT interval, JT interval and Tpeak-end interval measurements were performed with the use of computer software (Configurable Measurement System) using digitizer 34180 (Calcomp, Anaheim, California). The variability of the measurements was 0.36 ± 5 ms, non statistically significant. The standard correlation was 95% (95% confidence intervals −6.73 to 6.01). In each electrocardiogram lead the analysis included three consecutive heart cycles, whenever possible. The leads were excluded from analysis when the end of the T-wave was not clearly distinguishable or the signal quality was too poor for analysis. The QRS interval was measured from the start of the Q wave or, in absence of the Q wave, from the start of R wave to the end of S, that is to its return to the isoelectric line. The QT interval was measured from the initial deflection of the QRS complex to the end of T wave, that is, to the point where T wave returned to the isoelectric line. In case there was a U wave, the QT interval was measured to the lowest part of the curve between the T and U waves. The JT interval was derived by subtracting the QRS duration from the QT interval. The Tpeak-end interval was defined as the interval from the maximum T-wave amplitude to the end of the T-wave (14). QT dispersion (QTd) was the difference between the maximal and the minimal QT value in all leads (15). The difference between the maximal and the minimal JT value in all leads was defined JT dispersion. The transmural dispersion of repolarization (TDR) was defined as the interval between the peak to the end of the T wave (16, 17), which for our analysis we have considered the mean value. All measurements were corrected for heart rate using Bazett’s formula.
Statistical analysis
Statistical analysis was done using Student’s t test for paired data and one-way analysis of variance (ANO-VA) coupled with Fisher’s LSD test among three or more groups. Data are presented as mean ± standard deviation. Differences were considered to be significant at a p value < 0.05. Analyses were performed using the statistical package SPSS 9.0 software for Windows.
RESULTS
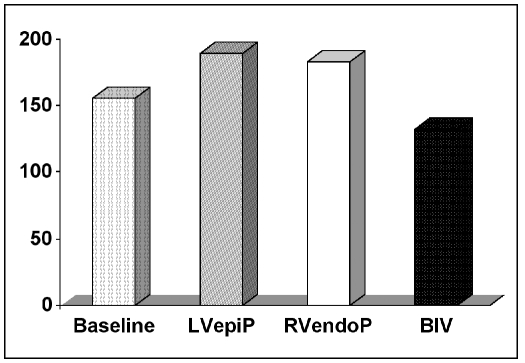
Biventricular pacing resulted in a statistically significant reduction in patient QRS duration (132 ± 16 ms during BiVP versus 156 ± 21 ms at baseline, n = 50, p = 0.02). Right ventricular endocardial pacing and left ventricular epicardial pacing, on the other hand, led to a marked prolongation in ventricular activation time (183 ± 12.3 ms during RVendoP; 190 ± 15 ms during LVepiP versus 156 ± 21 ms at baseline, p<0.05) (Fig. 1).
Fig. 1.
QRS duration at baseline rhythm (156±21 ms) and during left ventricular epicardial pacing (LVepiP; 190±15 ms, p<0.05), right ventricular endocardial pacing (RVendoP; 183±12.3 ms, p<0.05) and biventricular pacing (BiVP; 132±16 ms, p<0.02).
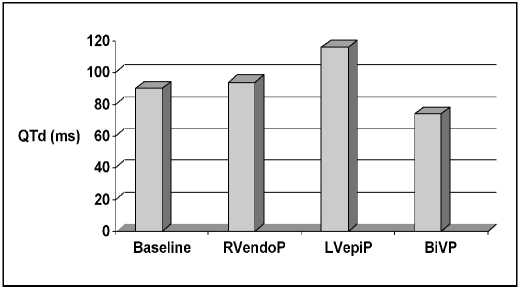
The effects of pacing site on QT dispersion values are shown in Figure 2. Biventricular pacing significantly reduced QT dispersion, compared to sinus rhythm (73.93 ± 19.4 ms during BiVP vs 91 ± 36.7 at sinus rhythm, n = 50, p = 0.004). Right ventricular endocardial pacing and left ventricular epicardial pacing both caused a significant increase in QTd value (RVendoP 94 ± 51 ms, p<0.03; LVepiP 116 ± 71 ms, p<0.02).
Fig. 2.
Pacing site dependent changes in QT dispersion (QTd) values. During sinus rhythm (91±36.7 ms) right ventricular endocardial pacing (RVendoP, 94±51 ms, p<0.03 ), left ventricular epicardial pacing (LVepiP; 116±71 ms, p<0.02), and biventricular pacing (BiVP 73.93±19.4 ms; p=0.004 ).
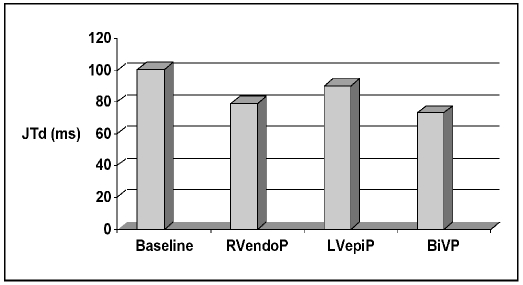
There was no statistically significant effect on JT dispersion during RVendoP and LVepiP as compared to sinus rhythm, whereas biventricular pacing significantly decreased the JT dispersion (73.18 ± 17.16 ms during BiVP vs 100.72 ± 39.04 at baseline n = 50, p = 0.003; Fig. 3).
Fig. 3.
Pacing site dependent changes in JT dispersion values. During sinus rhythm (100.72±39.04 ms), right ventricular endocardial pacing (RVendoP; 79.18±30.83 ms; p= NS), left ventricular epicardial pacing (LVepiP; 89.95±43.60 ms; p=NS), and biventricular pacing (BiVP 73.18±17.16 ms; p=0.003).
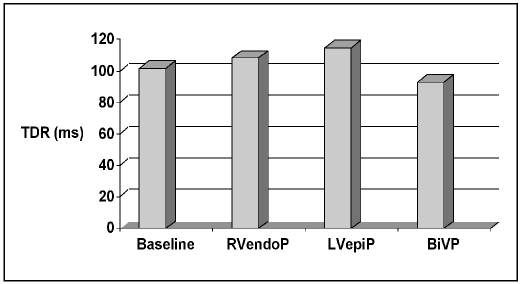
The transmural dispersion of repolarization (TDR) was significantly increased during RVendoP (108.13±19.94 ms; p<0.002) and LVepiP (114.71 ± 26.1 ms; p<0.05), whereas biventricular pacing significantly decreased the transmural dispersion of repolarization (93.16 ± 15.60 ms; p<0.004), as compared to sinus rhythm (101.55 ± 19.08 ms; Fig. 4).
Fig. 4.
Pacing site dependent changes in transmural dispersion of repolarization (TDR) values during sinus rhythm (101.55±19.08 ms), right ventricular endocardial pacing (RVendoP; 108.13±19.94 ms; p<0.002), left ventricular epicardial pacing (LVepiP; 114.71±26.1 ms; p<0.05), and biventricular pacing (BiVP, 93.16± 15.60 ms; p<0.004).
DISCUSSION
In our study, we evaluated the pacing-site dependent change in markers of ventricular repolarization heterogeneity in a population of fifty patients with congestive heart failure. The ventricular repolarization heterogeneity is an expression of regional (QTd, JTd) and transmural (TDR) differences in cellular action potential duration and in ventricular recovery time. In heart failure patients, the preferential prolongation of the M cell action potential results in the development of a transmural dispersion of repolarization (TDR), which can be estimated from the electrocardiogram as the interval between the peak and the end of the T wave (18). Previous electrophysiological studies (19–21), have highlighted the role of ventricular repolarization heterogeneity on the genesis of ventricular malignant arrhythmias in heart failure patients. In failing myocardium, the enhancement of QTd, JTd and TDR values increases the risk for development of malignant ventricular arrhythmias, probably via two mechanisms. First, it facilitates transmural differences early after depolarization propagation; second, it could cause intramural functional conduction blocks that predispose to re-entrant polymorphic ventricular tachyarrhythmia (22). Several studies suggested that TDR and JTd are clinically useful in assessing arrhythmia risk (23, 24), because they are parameters less dependent on ventricular depolarization and reflect better than QTd the ventricular repolarization heterogeneities in patients with intraventricular conduction abnormalities (25, 26). Medina-Ravell and coworkers in a recent experimental study have suggested (12) a potentially harmful influence of cardiac resynchronization therapy on ventricular repolarization. The authors found that biventricular pacing or left ventricular epicardial pacing prolong QT interval, increase transmural dispersion of repolarization, and may be a potential risk for development of torsade de pointes in a subset of patients. A following electrophysiological study by Fish et al (27) confirmed this hypothesis. Nevertheless, Abraham WT et al and Bristow MR et al in two distinct prospective, randomized studies (28, 29) did not find any excess mortality due to sudden death in a population with chronic heart failure and biventricular pacing. However, in the study of Medina-Ravell, QTd, JTd and Tpeak-end was not estimated at baseline and during biventricular pacing. In our opinion, this information is really important. In our study, differences in various parameters of ventricular repolarization heterogeneity (QTd, JTd, TDR) during right endocardial ventricular, left epicardial ventricular and biventricular pacing in heart failure patients have been obtained using a standard 12-lead ECG. Our data, in contrast to Medina-Ravell’s findings, showed that biventricular pacing reduced the QTd, JTd and TDR and, with them, the relative risk of malignant ventricular arrhythmias. Nevertheless, in accordance with Medina-Ravell et al findings and those of Fish et al, epicardial pacing of left ventricle has been shown to increase the ventricular heterogeneity of repolarization, probably because the physiological ventricular activation was reversed. Normally, ventricular activation starts with endocardium via subendocardial Purkinje network and spreads across the ventricular wall. Although the epicardium is activated last, it repolarizes first, producing a repolarization sequence opposite to activation (14). Furthermore, our data showed that sole right endocardial ventricular pacing increased QTd and TDR less than left epicardial ventricular stimulation.
LIMITATIONS
QT interval and Tpeak-end measurements were conducted in 12-lead ECGs, with the use of computer software and digitizer by an experienced observer. Despite all that, the reliability of the measurements remains uncertain to the extent that the lack of a consensus has as a consequence the absence of indisputable, generally accepted criteria for the definition of the end of T interval.
CONCLUSIONS
Biventricular pacing causes a stastically significant reduction of all parameters proposed to estimate the heterogeneity of ventricular repolarization (QTd, JTd, TDR), as compared to sinus rhythm, whereas both right ventricular endocardial and left ventricular epicardial pacing cause an increment of heterogeneity of ventricular repolarization. In conclusion our data show that cardiac resynchronization therapy could have an electrophysiologic antiarrhythmic influence on arrhythmogenic substrate of dilatative cardiomyopathy. Further long-term studies have to asses the utility of surrogate parameters of ventricular dispersion of repolarization.
REFERENCES
- 1.Eriksson H. Heart failure: A growing public health problem. J Intern Med. 1995;237:135–41. doi: 10.1111/j.1365-2796.1995.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 2.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol. 1993;22:6–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 3.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 4.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 5.MERIT Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure(Merit-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 6.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomised aldactone evaluation study investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 7.Baldasseroni S, Opasich C, Gorini M, et al. Italian Network on Congestive Heart Failure Investigators. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: A report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 8.Shamim W, Francis DP, Yousufuddin M, et al. Intraventricular conduction delay: A prognostic marker in chronic heart failure. Int J Cardiol. 1999;70:171–8. doi: 10.1016/s0167-5273(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 9.Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–73. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 1.Leclercq C, Cazeau S, Ritter P, et al. A pilot experience with permanent biventricular pacing to treat advanced heart failure. Am Heart J. 2000;140:862–70. doi: 10.1067/mhj.2000.110570. [DOI] [PubMed] [Google Scholar]
- 11.Abraham WT, Fiser WG, Smith AL, Delurgio DB, Leon AR MIRACLE study group. Multicenter InSync randomized clinical evaluation: Cardiac reynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 12.Medina-Ravell VA, Lankipalli RS, Yan GX, et al. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: Does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation. 2003;107:740–6. doi: 10.1161/01.cir.0000048126.07819.37. [DOI] [PubMed] [Google Scholar]
- 13.Daubert JC, Ritter P, Le Breton H, et al. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin Electrophysiol. 1998;21:239–45. doi: 10.1111/j.1540-8159.1998.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 14.Fuller MS, Sandor G, Punske P, et al. Estimates of repolarization dispersion from electrocardiographic measurements. Circulation. 2000;102:685–91. doi: 10.1161/01.cir.102.6.685. [DOI] [PubMed] [Google Scholar]
- 15.De Bruyne MC, Hoes AW, Kors JA, Hofman A, Van Bemmel JH, Grobbee DE. QT dispersion predicts cardiac mortality in the elderly: The Rotterdam Study. Circulation. 1998;97:467–72. doi: 10.1161/01.cir.97.5.467. [DOI] [PubMed] [Google Scholar]
- 16.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–36. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 17.Antzelevitch C, Shimizu W, Yan GX, et al. The M cell: Its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–52. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 18.Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96:517–27. doi: 10.1007/s003950170002. [DOI] [PubMed] [Google Scholar]
- 19.Lubinski A, Kornacewicz-Jach Z, Wnuk-Wojnar AM, et al. The terminal portion of the T wave: A new electrocardiographic marker of risk of ventricular arrhythmias. Pacing Clin Electrophysiol. 2000;23:1957–9. doi: 10.1111/j.1540-8159.2000.tb07061.x. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu M, Ino H, Okeie K, et al. T-peak to T-end may be a better predictor of risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335–9. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spodick DH. Reduction of QT-interval imprecision and variance by measuring the JT interval. Am J Cardiol. 1992;70:628–9. doi: 10.1016/0002-9149(92)91399-o. [DOI] [PubMed] [Google Scholar]
- 22.Das G. QT interval and repolarization time in patients with intraventricular conduction delay. J Electrocardiol. 1990;23:49–52. doi: 10.1016/0022-0736(90)90150-z. [DOI] [PubMed] [Google Scholar]
- 2.Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanisms of ventricular arrhythmia dependent on the dispersion of action potential duration. Circulation. 1983;67:1356–67. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- 24.Vassallo JA, Cassidy DM, Kindwall KE, Marchlinski FE, Josephson ME. Non uniform recovery of excitability in the left ventricle. Circulation. 1988;78:1365–72. doi: 10.1161/01.cir.78.6.1365. [DOI] [PubMed] [Google Scholar]
- 25.Merx W, Yoon MS, Han J. The role of local disparity in conduction and recovery time on ventricular vulnerability to fibrillation. Am Heart J. 1977;94:603–10. doi: 10.1016/s0002-8703(77)80130-0. [DOI] [PubMed] [Google Scholar]
- 26.Kuo CS, Munakata K, Reddy CP, Surawicz B. Mechanism of ventricular arrhythmias caused by increased dispersion of repolarization. Eur Heart J. 1985;6(Suppl D):63–70. doi: 10.1093/eurheartj/6.suppl_d.63. [DOI] [PubMed] [Google Scholar]
- 27.Fish JM, Di Diego JM, Nesterenko V, Antzelevitch C. Epicardial activation of left ventricular wall prolongs QT interval and transmural dispersion of repolarization. Implication for biventricular pacing. Circulation. 2004;109:2135–41. doi: 10.1161/01.CIR.0000127423.75608.A4. [DOI] [PubMed] [Google Scholar]
- 28.Abraham WT, Fisher WG, Smith AL, et al. MIRACLE Study Group Multicenter InSync randomized clinical evaluation. N Engl J Med. 2002;346:1845–53. [Google Scholar]
- 29.Bristow MR, Saxon LA, Feldman AM. Comparison of Medical Therapy, Pacing and Defibrillation in Chronic Heart Failure(COMPANION) Investigators. Cardiac resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]