Abstract
This review covers the role of three-dimensional (3D) echocardiography in the diagnosis of heart valve disease. Several factors have contributed to the evolution of this technique, which is currently a simple and routine method: rapid evolution in probe and computer technologies, demonstration that 3D data sets allowed more complete and accurate evaluation of cardiac structures, emerging clinical experience indicating the strong potential particularly in valve diseases, volume and function of the two ventricle measurements and several other fields. This report will review current and future applications of 3D echocardiography in mitral, aortic and tricuspid valve diseases underlying both qualitative (morphologic) and quantitative advantages of this technique.
KEY WORDS: Three-dimensional transthoracic echocardiography, Three-dimensional transesophageal echocardiography, Valve disease
INTRODUCTION
Progress in three-dimensional (3D) echocardiography was rather slow in the 1980s and 1990s mainly due to technical reasons. Recently, along with the rapid evolution in probe and computer technologies 3D has grown into a well-developed technique able to display images of the heart that contain important new tissue and morphologic information. This new technique is now simple and rapid, and allows additional clinical information in different fields including valve diseases, volume and function of the two ventricles, intracardiac masses, monitoring new procedures and several other topics (1–3).
This review details the current status of 3D technology with its clinical applications in valve disease.
METHODS AND NEW TECHNOLOGIES
Several methods including random and sequential scanning, free-hand techniques by both transthoracic and transesophageal echocardiography have been proposed in the past and all of them required an off-line reconstruction. Real-time volumetric echocardiography developed in the early 1990s by von Ramm et al of Duke University (4) was based on novel matrix phased array transducer technology. However, the first generation instrument had several practice limitations despite its potential but very promising clinical applications were clearly demonstrated. In 2000, the second generation real-time live 3D (and currently the new probe and software technologies) allowed a true routine application of the method in different fields: it is simple, rapid and can be easily integrated and associated with the standard 2D examination. In brief, these new transducers (with 3000/4000 ultrasound elements) generate multidirectional beam steering and signal processing, which take place automatically in the scanning probe itself. This technology generates true pyramidal volumes of data in the display and creates on-line rendered images; since this pyramidal data set is still limited in terms of volume (generally 60° × 30°), it is possible to obtain a larger volume data set of up to 100° × 100° (the so-called full volume data set) acquiring together up to seven (generally five) sub volumes over consecutive cardiac cycles. Images acquired can be immediately sliced in several planes and rotated in order to visualize cardiac structures from any plane and multiple perspectives (5).
Concerning the “old technologies” 3D sequential transesophageal acquisition (multiplane transesophageal echocardiography (TEE) with ECG and respiratory gating or new techniques with very fast acquisition without gating) is still utilized and very useful in several clinical settings (6–9). Therefore, in this review we refer to real-time transthoracic (3DTTE) and transesoephagel sequential acquisition (3DTEE).
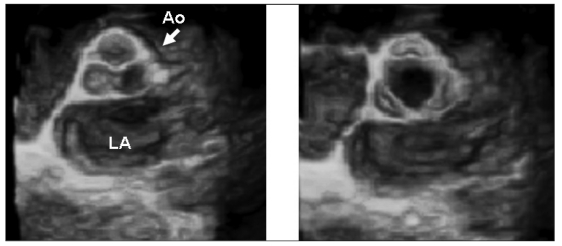
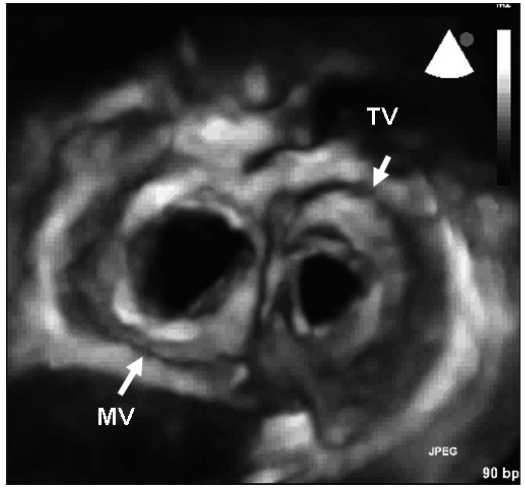
Figures 1 and 2 show 3D examples of normal aortic, mitral and tricuspid valves.
Fig. 1.
- 3DTTE reconstruction of a normal aortic valve: diastolic (left panel) and systolic (right panel) frames of the valve showing accurate evaluation of the leaflets and commissures.
AO = aortic valve; LA = left atrium.
Fig. 2.
- Surgical view of the mitral and tricuspid valves (diastolic frame). MV = mitral valve; TV= tricuspid valve.
MITRAL VALVE DISEASE
Both qualitative and quantitative evaluation of valvular heart disease can be improved by 3D echocardiography. Anyplane and paraplane analysis of a stenotic valve allows an accurate planimetry of the smallest orifice area. Zamorano et al (10) demonstrated that 3DTTE is a feasible, accurate and highly reproducible technique for assessing the mitral valve (MV) area in patients with rheumatic MV stenosis. In a consecutive series of 80 patients, MV area was assessed by conventional echo-Doppler methods and by 3DTTE, and results were compared with those obtained invasively. Compared with all other echo-Doppler methods, 3DTTE had the best agreement with the invasively determined MV area, and intra- and inter-observer variability of the method was very good. Zamorano et al (11) also studied 29 patients undergoing percutaneous balloon mitral valvuloplasty. 3DTTE had the best agreement with the invasively determined MV area, particularly in the immediate post procedural period; therefore, the method could be proposed as an ideal one throughout this procedure and could make invasive evaluation unnecessary in this setting.
As part of these very important quantitative data, 3DTTE can be integrated with 2D evaluation in the qualitative morphology assessment of the MV. Commissures, leaflets, annulus calcifications and subvalvular structures can be visualized from different and unique planes facilitating the understanding of this complex apparatus. Each of the atrioventricular valves can be depicted both from the atrium or the ventricle with access to “en face” views or from any other angle. Vegetations, commissural diseases, subvalvular pathologies (tip of the leaflets/chordae/papillary muscles), clefts can be accurately diagnosed.
MV prolapse is visualized as a bulge or protrusion on the atrial site, and in this regard 3D is the ideal method to demonstrate the pathology. Several studies by 3DTEE and 3DTTE have demonstrated the importance of 3D methods in this field (12–18). Recent data have demonstrated that 3D (either transthoracic or transesophageal) echocardiography is superior in comparison with the corresponding 2D techniques in the description of MV pathology. In particular, since real-time 3DTTE has an accuracy similar to that of 2D transesophageal echocardiography, this new technique (which is also simple and rapid) can be integrated in the standard 2D examination and should be regarded as an important examination in decisions regarding MV repair. MV prolapse is the most frequent etiology of mitral regurgitation in industrialized countries. Since the 1970s, MV repair has become preferential to replacement, and it is now possible in the overwhelming majority of patients with MV prolapse (19, 20). Recent studies and guidelines have underlined the importance of early surgical intervention to preserve long-term left ventricular function in severe mitral regurgitation. In this regard, a non-invasive pre-operative assessment of MV anatomy is essential to define the feasibility and complexity of repair, differentiating cases with simple or complex lesions and to plan the ideal surgical strategy. For these reasons, 3DTTE and 3DTEE should be regarded as an important examination in decisions regarding MV repair, particularly in cases with complex diseases and in the light of these new early surgical strategies. Recently, a large series of cases with MV prolapse evaluated with different echo methods was published by our group (21). One hundred and twelve consecutive patients with severe mitral regurgitation due to degenerative MV prolapse underwent a complete 2D and 3DTTE the day before surgery, and a complete 2D and 3DTEE in the operating theater. Echocardiographic data obtained by the different techniques (including scallops, commissures, chordal rupture identification) were compared with MV surgical inspection. 3D techniques were feasible in a relatively short time (3DTTE 7 ± 4 min; 3DTEE 8 ± 3 min), with good (3DTTE 55%; 3D TEE 35%) and optimal (3DTTE 21%; 3DTEE 45%) imaging quality in the majority of cases. 3DTEE allowed more accurate identification (95.6% accuracy) of all MV lesions in comparison with other techniques. 3DTTE and 2DTEE had similar accuracies (90 and 87%, respectively), while the accuracy of 2DTTE (77%) was significantly lower. Therefore, these data showed that 3DTTE and 3DTEE are feasible, not time consuming and useful methods in identifying the location of MV prolapse in patients undergoing MV repair. They were superior in the description of pathology in comparison with the corresponding 2D techniques and should be regarded as an important adjunct to standard 2D examinations in decisions regarding MV repair.
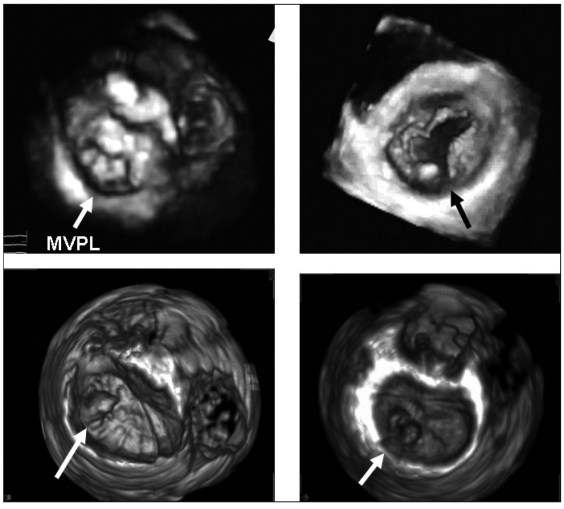
Figure 3 shows four cases of MV prolapse examined by 3DTTE and 3DTEE, respectively.
Fig. 3.
- Four cases of mitral valve disease. Upper left: 3DTTE reconstruction in a patient with mitral valve prolapse of the entire posterior leaflet (Barlow disease) with the three prolapsing scallops (arrow). Upper right: 3DTTE reconstruction in a mitral valve with a central cleft of the posterior leaflet (arrow). Lower left: 3DTEE reconstruction in a patient with an isolated prolapse of the anterolateral scallop of posterior leaflet (P1) (arrow). Lower right: 3DTEE reconstruction in a mitral valve with a cleft and prolapse of the medial scallop (P2) (arrow). MVPL = mitral valve posterior leaflet.
AORTIC VALVE DISEASE
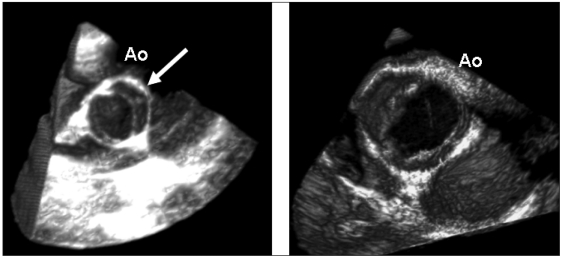
The aortic valve can be easily evaluated by 3DTTE or 3DTEE. The morphology of the valve can be defined with high accuracy demonstrating the normality of the cusps, congenital abnormalities (bicuspid aortic valve) or acquired pathologies (Fig. 4). Few data have concentrated on the accuracy of 3D in the assessment of the severity of aortic valve stenosis (22). Recently Goland et al (23) evaluated the reproducibility and accuracy of real-time 3DTTE in 33 patients with aortic stenosis. Aortic valve area by 3DTTE was compared with 2DTTE planimetry, 2DTEE planimetry and in 15 cases with invasive measurements. Statistical analysis showed good agreement and small absolute differences in aortic valve area between all planimetric methods. Inter-observer variability was better for 3D techniques. Therefore, the authors suggested that this very rapid and novel method provided an accurate and reliable quantitative assessment of aortic stenosis.
Fig. 4.
- Two examples of bicuspid aortic valves. Left panel: 3DTTE reconstruction of a bicuspid valve in a systolic frame showing the two cusps and the rafe (arrow). Right panel: 3DTEE reconstruction (systolic frame) of a bicuspid valve. AO = aortic valve.
TRICUSPID VALVE DISEASE
3D echocardiography offers a direct view to evaluate the leaflet surface of the tricuspid valve, and is a unique method to visualize the three leaflets simultaneously. Potentially, this offers the opportunity to study every tricuspid pathology from different perspectives such as from the right ventricle, from the right atrium or from the oblique planes. Leaflet coaptation and separation can be visualized easily. Even though consecutive series of cases with tricuspid valve disease have not been published, and no data demonstrate an additional clinical value of the method, several case reports have demonstrated the importance of this technique in Ebstein’s anomaly (24), tricuspid stenosis (25) and other tricuspid pathologies. This potential in different tricuspid pathologies could be further completed by the measurement of right ventricular volumes and function (ejection fraction) by off-line 3DTTE analysis. In all tricuspid pathologies a crucial point is represented by right ventricular function and the calculation of right ventricular volumes is not feasible by conventional 2D echocardiography. Full volume analysis by 3DTTE allows, in a few seconds, the acquisition of a complete data set of the right ventricle and off-line calculation of right ventricular volume, as well as recognition of tricuspid disease.
FUTURE CLINICAL APPLICATION OF 3D ECHOCARDIOGRAPHY IN VALVE DISEASES
Standard 2D echocardiography is a well-established method to evaluate infective endocarditis and its complications. However, 3D echocardiography can facilitate the description of any cardiac mass site, and in this field could provide information regarding the attachment and morphology of the vegetations as well as their dimensions. Moreover, through 3D and color Doppler 3D, the perforation and severity of lesions can be studied better. Further studies are needed to define whether these new technologies could also increase the accuracy in the differential diagnosis between vegetations and other valve masses such as fibroelastomas, mixomas or degenerative diseases.
Stress echocardiography may be very useful in valve disease. 3D echocardiography offers the unique possibility to visualize the left ventricle during one breath hold; and therefore, peak exercise (or peak dobutamine) volumes and function can be calculated. Simultaneously, all other information (Doppler data, 3D volumes of regurgitant jets) can be acquired. Therefore, potentially this method could be considered ideal for the stress evaluation of patients with MV regurgitation, MV stenosis and aortic valve regurgitation.
FUTURE TECHNICAL DEVELOPMENTS
Several technical advancements will improve 3D technologies further. Size of matrix transducers, spatial and temporal resolution could be optimized increasing the feasibility of the examination in all clinical settings and increasing the resolution and quality of the examinations.
New software is going to be developed facilitating the morphologic and quantitative evaluation of structures. New methodologic approaches to 3D evaluation of MV annulus (26), MV tracking and left and right ventricular functional analysis (27), have been recently proposed. All these new systems could increase the rapidity and feasibility and clinical value of routine examination, stress echocardiography and contrast studies.
3D color Doppler flow mapping is already implemented in the ultrasound 3D units; however, even though several studies have already been published concerning the clinical value of this method (28) in the quantitative approaches to valve regurgitant jets and shunt lesions, further technical improvements are needed to include this technology in a routine exam.
A new and revolutionary method will very soon be implemented in the cardiological examination. Due to advances in both micro industrial design and size of matrix transducers, prototypes of 3D transesophageal real-time transducers have been integrated in new equipment. Images from these new transducers (TEE probes with dimensions similar to a standard multiplane probe) can be obtained from any transesophageal position in real time, just with one touch of the keyboard that converts the standard 2D image into a rendering 3D/4D view of the visualized structures (without any cropping or reconstruction of the data set). Therefore, we could postulate that in a few years all 2DTEE studies might be integrated by 3DTEE imaging with obvious clinical value in all clinical settings. Preliminary data show that intra-operative monitoring of cardiac surgery, monitoring of PFO or interatrial septal defect, mitral and aortic valve diseases could be several fields in which this technology could become the standard echocardiographic examination procedure.
REFERENCES
- 1.Badano LP, Dall’Armellina E, Monaghan MJ, et al. Real-time three-dimensional echocardiography: technological gadget or clinical tool? J Cardiovasc Med. 2007;8:144–62. doi: 10.2459/JCM.0b013e3280116b50. [DOI] [PubMed] [Google Scholar]
- 2.Roelandt JR, Yao J, Kasprzak JD. Three-dimensional echocardiography. Curr Opin Cardiol. 1998;13:386–96. doi: 10.1097/00001573-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Levine RA, Weyman AE, Hand Shumacher MD. Three-dimensional echocardiography: techniques and applications. Am J Cardiol. 1992;69:121–34. doi: 10.1016/0002-9149(92)90656-j. [DOI] [PubMed] [Google Scholar]
- 4.Kisslo J, Firek B, Ota T, et al. Real-time volumetric echocardiography: the technology and the possibilities. Echocardiography. 2000;17:773–9. doi: 10.1111/j.1540-8175.2000.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 5.Pepi M, Tamborini G, Pontone G, et al. Initial experience with a new on-line transthoracic three-dimensional technique: assessment of feasibility and of diagnostic potential. Ital Heart J. 2003;4:544–50. [PubMed] [Google Scholar]
- 6.Wang XF, Li ZA, Cheng TO, et al. Clinical application of three-dimensional transesophageal echocardiography. Am Heart J. 1994;128:380–8. doi: 10.1016/0002-8703(94)90492-8. [DOI] [PubMed] [Google Scholar]
- 7.Abraham TP, Warner JG, Jr, Kon ND, et al. Feasibility, accuracy, and incremental value of intra-operative three-dimensional transesophageal echocardiography in valve surgery. Am J Cardiol. 1997;80:1577–82. doi: 10.1016/s0002-9149(97)00783-2. [DOI] [PubMed] [Google Scholar]
- 8.Magni G, Hijazi ZM, Pandian NG, et al. Two- and three-dimensional transesophageal echocardiography in patient selection and assessment of atrial septal defect closure by the new DAS-Angel Wings device. Circulation. 1997;96:1722–8. doi: 10.1161/01.cir.96.6.1722. [DOI] [PubMed] [Google Scholar]
- 9.Tamborini G, Pepi M, Susini F, et al. Comparison of two and three-dimensional echocardiography in patients undergoing atrial septal closure with the amplatzer septal occluder. Am J Cardiol. 2002;90:1025–8. doi: 10.1016/s0002-9149(02)02695-4. [DOI] [PubMed] [Google Scholar]
- 10.Zamorano J, Cordeiro P, Sugeng L, et al. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004;43:2091–6. doi: 10.1016/j.jacc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Zamorano J, Perez De Isla L, Sugeng L, et al. Non-invasive assessment of mitral valve area during percutaneous balloon mitral valvuloplasty: role of real-time 3D echocardiography. Eur Heart J. 2004;25:2086–91. doi: 10.1016/j.ehj.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 12.De Castro S, Salandin V, Cartoni D, et al. Qualitative and quantitative evaluation of mitral valve morphology by intra-operative volume-rendered three-dimensional echocardiography. J Heart Valve Dis. 2002;11:173–80. [PubMed] [Google Scholar]
- 13.Godoy IE, Bednarz J, Sugeng L, Mor-Avi V, Spencer KT, Lang RM. Three-dimensional echocardiography in adult patients: comparison between transthoracic and transesophageal reconstructions. J Am Soc Echocardiogr. 1999;12:1045–52. doi: 10.1016/s0894-7317(99)70100-8. [DOI] [PubMed] [Google Scholar]
- 14.Macnab A, Jenkins NP, Ewington I, et al. A method for the morphological analysis of the regurgitant mitral valve using three-dimensional echocardiography. Heart. 2004;90:771–6. doi: 10.1136/hrt.2003.013565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed S, Nanda NC, Miller AP, et al. Usefulness of transesophageal three-dimensional echocardiography in the identification of individual segment/scallop prolapse of the mitral valve. Echocardiography. 2003;20:203–9. doi: 10.1046/j.1540-8175.2003.03010.x. [DOI] [PubMed] [Google Scholar]
- 16.Macnab A, Jenkins NP, Bridgewater BJ, et al. Three-dimensional echocardiography is superior to multiplane transesophageal echo in the assessment of regurgitant mitral valve morphology. Eur J Echocardiogr. 2004;5:212–22. doi: 10.1016/j.euje.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Delabays A, Jeanrenaud X, Chassot PG, Von Segesser LK, Kappenberger L. Localization and quantification of mitral valve prolapse using three-dimensional echocardiography. Eur J Echocardiogr. 2004;5:422–9. doi: 10.1016/j.euje.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Fabricius AM, Walther T, Falk V, Mohr FW. Three-dimensional echocardiography for planning of mitral valve surgery: current applicability? Ann Thorac Surg. 2004;78:575–8. doi: 10.1016/j.athoracsur.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Yacoub FR, Cohn MD. Novel approaches to cardiac valve repair. From structure to function: Part I. Circulation. 2004;109:942–50. doi: 10.1161/01.CIR.0000115633.19829.5E. [DOI] [PubMed] [Google Scholar]
- 20.Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in non rheumatic mitral valve insufficiency. Circulation. 2001;104(suppl 1):S8–11. [PubMed] [Google Scholar]
- 21.Pepi M, Tamborini G, Maltagliati A, et al. Head-to-head comparison of two-dimensional and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol. 2006;48:2524–30. doi: 10.1016/j.jacc.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 22.Kasprzak JD, Youssef FM, Dall’Agata A, et al. Quantification of the aortic valve area in three-dimensional echocardiographic data sets: analysis of orifice overestimation resulting from suboptimal cut-plane selection. Am Heart J. 1998;135:995–1003. doi: 10.1016/s0002-8703(98)70064-x. [DOI] [PubMed] [Google Scholar]
- 23.Goland S, Trento A, Iida K, et al. Assessment of aortic stenosis by three-dimensional echocardiography: an accurate and novel approach. Heart. 2007;93:801–7. doi: 10.1136/hrt.2006.110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acar P, Abadir S, Roux D, et al. Ebstein’s anomaly assessed by real-time 3D echocardiography. Ann Thorac Surg. 2006;82:731–3. doi: 10.1016/j.athoracsur.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Faletra F, La Marchesina U, Bragato R, De Chiara F. Three-dimensional transthoracic echocardiography images of tricuspid stenosis. Heart. 2005;91:499–499. doi: 10.1136/hrt.2004.041459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veronesi F, Tamborini G, Lang RM, et al. Quantitative assessment of mitral annulus dynamic geometry before and after mitral valve repair using real-time 3D echocardiography. Eur J Echocardiogr. 2007;11 abstract in press. [Google Scholar]
- 27.Tamborini G, Brusoni D, Torres J, et al. Three-dimensional evaluation of right ventricular volumes and function: preliminary data in a series of 70 cases. Eur J Echocardiogr. 2007;11 abstract in press. [Google Scholar]
- 28.de Simone R, Glombitza G, Vahl C, Albers J, Meinter H, Hagl S. Three-dimensional color Doppler: a clinical study in patients with mitral regurgitation. J Am Coll Cardiol. 1999:1646–54. doi: 10.1016/s0735-1097(99)00041-8. [DOI] [PubMed] [Google Scholar]