Abstract
The combination of Simvastatin and Ezetimibe allows dual inhibition of both cholesterol production and absorption. This treatment approach allows achieving same low serum cholesterol levels with the administration of much lower doses of statins. This should reduce side effects, compared to statin only therapy, enabling more patients to achieve their LDL cholesterol treatment goals. With ezetimibe/simvastatin therapy, reductions of about 60% from baseline in LDL cholesterol have been shown. Concomitant improvement in other lipid fractions have also been demonstrated. The ezetimibe/simvastatin combination has been well tolerated, with a safety profile similar to that of statin therapy. This article will review clinical experience with ezetimibe/simvastatin combination, commenting upon its place and potential value in the prevention of cardiovascular disease.
KEY WORDS: Ezetimibe, Hypercholesterolemia, Statins
INTRODUCTION
Although the efficacy of statin based treatments in the control of cholesterolemia has been extensively demonstrated, the persistence of an excess in cardiovascular risk is still evident; therefore, also associated events in patients treated with this category of drugs.
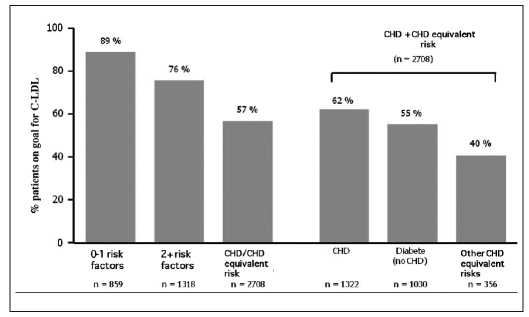
In particular, as emerged from a survey (1) conducted on more than 4800 US patients, published in the American Journal of Cardiology (2005), only 57% of high cardiovascular risk patients achieved the target for low-density lipoprotein (LDL) cholesterol (Fig. 1).
Fig. 1.
- Results of the NEPTUNE (National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology) II survey: hypolipemic treatment does not reach the LDL cholesterol target levels fixed by ATP-III (1).
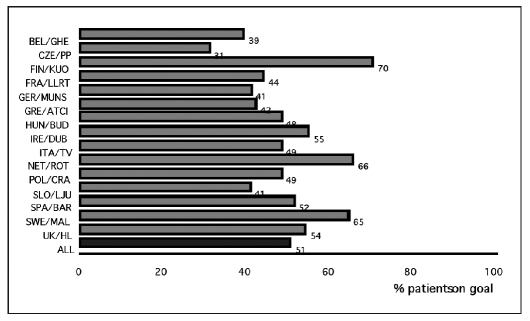
The EUROASPIRE registries (2) present the prevalence and the therapeutic management of cardiovascular risk in Europe. From the analysis of the EUROASPIRE II data, among 15 European countries, including Italy, a high prevalence of incorrect life styles, changeable risk factors, and the inappropriate use of antihypertensive and hypocholesterolemic therapy were evident. The percentage of patients receiving hypolipemic drug therapy on target for LDL levels, fluctuated between 31 and 70% (average 51%).
A low percentage of patients are treated with statins and in this group the guideline based targets are rarely reached (Fig. 2).
Fig. 2.
- EUROASPIRE II: in Europe only 51% of patients undergoing hypocholesterolemic therapy attain the cholesterol target (<5 mmol/l; <190 mg/dl) (2).
In 2005, the results of a survey (3) were published with retrospective data regarding more than 58000 European patients treated with statins. Although the data source was different between the countries (Italy used data from a government database from Ravenna), it was again demonstrated that the percentage of patients on target is still unsatisfactory, and in particular in Italy (only 14%).
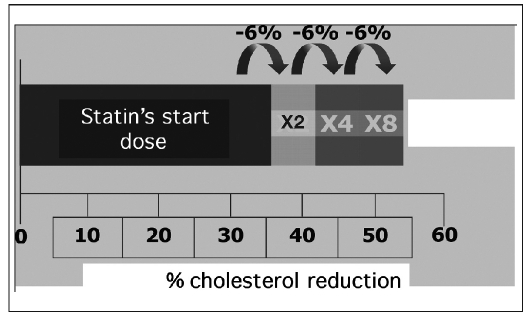
A possible explanation for the missed target for LDL cholesterol could be related with the “rule of six” (4) (Fig. 3). Every doubling of statin dose develops, on average, a further 6% reduction in LDL cholesterol. Against this moderately therapeutic effect, often many dose increments are necessary to reach the therapeutic goal. Moreover, if the baseline cholesterol level is high (≥180 mg/dl or 4.6 mmol/L), some statins, at the maximum approved dose, are unable to maintain cholesterol levels within the target recommended by the guidelines.
Fig. 3.
- Effect of therapy with statins on LDL cholesterol levels: the “rule of six” (see text for details).
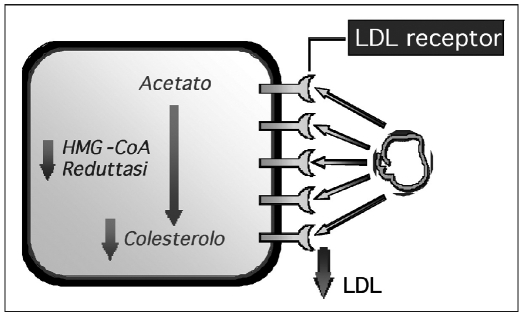
So which strategies can be used to control cholesterolemia? Serum cholesterol levels can be maintained under control with different strategies: treatment with statins acts on the reaction in the cholesterol synthesis, particularly in the transformation of HMG-CoA reductase in mevalonic acid. This causes a growth of LDL receptor expression in the hepatocytes and boosts the receptor-mediated removal of LDL cholesterol from the circulation (5) (Fig. 4).
Fig. 4.
- Statins: method of action (see text for details).
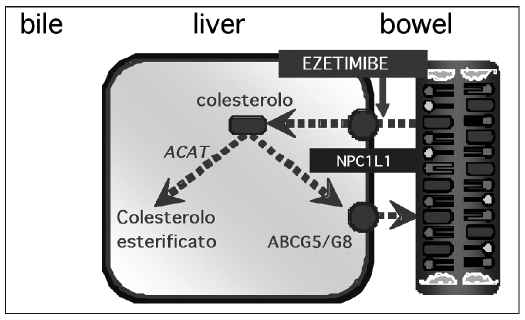
A second approach is the block of cholesterol uptake in the bowel with a selective uptake inhibitor, such as ezetimibe. Consequently, less cholesterol arrives at the liver: this means an up-regulation of LDL receptors and an increased clearance of LDL cholesterol from the circulation (6). Usually, cholesterol is absorbed in the small intestine due to the action of the cholesterol transporting protein Niemann-Pick C1 Like 1 (NPC1L1) (7). Ezetimibe inhibits the absorption of biliar and food-derived cholesterol, selectively interfering with the activity of the entherocytic NPC1L1 protein (8) (Fig. 5).
Fig. 5.
- Cholesterol intake inhibition mediated by ezetimibe.
Ezetimibe is fast converted to its glucoronide metabolite in the bowel (9, 10), where both the original drug and the metabolite inhibit cholesterol uptake. In addition, the metabolite is more powerful than the original drug due to a multiple transit in the entherohepatic circle. This means a longer effect in the bowel.
Ezetimibe has a good safety profile: there are no clinically relevant interactions with other hypolipemic drugs, such as statins and fibrates; moreover, there are no significant effects in sieric levels of digoxin, glypizide and warfarin. However, it is also known that the simultaneous administration of ezetimibe and cyclosporin could modify the sieric cyclosporin levels and increase the bioavailability of ezetimibe (9).
Studies with ezetimibe only or in addition to other statins have demonstrated a good tolerability profile: sometimes a small increase in ALT (≥3 than the normal limits) is found when ezetimibe is administrated in association with statins. This is generally an asymptomatic, reversible event, not associated with cholestasis (SPC ZETIA, US 2005). No increase in CPK was found in studies with ezetimibe only or in association with other statins.
In a randomized, double-blind versus placebo study, Sudhop et el estimated the individual amount of absorbed cholesterol after 2 weeks of ezetimibe therapy (10 mg/die) in 18 patients affected by mild hypercholesterolemia. The amount of absorbed cholesterol dosed in the faeces was between 24.9 and 74.7% in the placebo group and between 2.3 and 48.7% in the ezetimibe group. After 2 weeks of therapy, the average uptake of fractioned cholesterol was 22.7% in the ezetimibe group and 49.8% in the placebo group. The reduction in cholesterol uptake in the ezetimibe group was 54% (p<0.001).
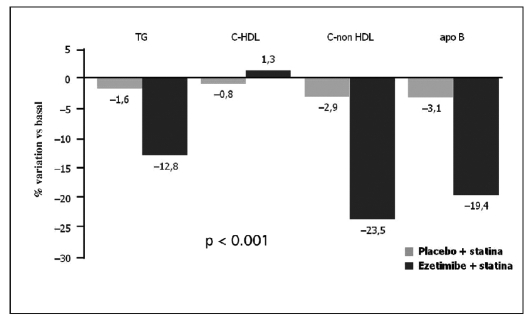
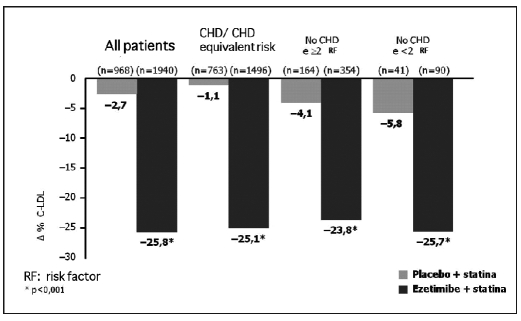
In the EASE (Ezetimibe Add-on to Statin for Effectiveness) (12) study, co-administration of ezetimibe and statins reduced LDL cholesterol 25.8% more than with statin only therapy. This effect has also been shown in all the risk categories defined by the National Cholesterol Education Program (NCEP) (p<0.001) (Fig. 7). In patients with coronary artery disease or with a similar risk for coronaropathy, the reduction in LDL cholesterol levels was 25.1% in the ezetimibe + statin group, and 1.1% in the statin only group. Co-administration of ezetimibe and statin significantly improved the global lipidic profile in comparison with the use of the single statin (p<0.001) (Fig. 6).
Fig. 7.
- Results from the EASE study (ezetimibe add-on to statin for effectiveness) (12): effects of the association of ezetimide + statin on the lipidic profile.
Fig. 6.
- Results from EASE (ezetimibe add-on to statin for effectiveness) study (12).
The sieric levels of triglycerides, non-high-density lipoprotein (HDL) cholesterol, and B apolipoprotein with ezetimibe + statin were reduced 12.8, 23.5 and 19.4%, respectively. HDL cholesterol increased by 1.3% in the ezetimibe + statin group, vs. a 0.8% reduction in the statin only group (Fig. 7).
So what is the main feature of the association of ezetimibe and statin?
The association of ezetimibe 10 mg/day with the starting dose of statin leads to the same reduction in LDL cholesterol reached by the triple dose of the statin alone. It means a dose eight times the starting dose. This association can resolve the limit forced by the “rule of six”, reducing LDL cholesterol similarly as with the maximum recommended statin dose, but with less side effects (13–15).
In a double-blind, randomized study, Goldberg et al (16) showed that the association between simvastatin with ezetimibe, at every dose used (from 10/10 to 10/80), led to a >15% reduction in the average LDL cholesterol level if compared to the same simvastatin dose only (p<0.001).
The same study demonstrated that this association is significantly (p<0.001) more effective in leading patients to the LDL cholesterol goals than the use of simvastatin only: 83% of patients treated with the association ezetimibe + simvastatin (from 10/10 to 10/80) and affected by hypercholesterolemia at baseline reached the goal (LDL cholesterol <100mg/dl or <2.58 mmol/L) until the end of the study vs. 43% of those treated with simvastatin 10–80 mg only.
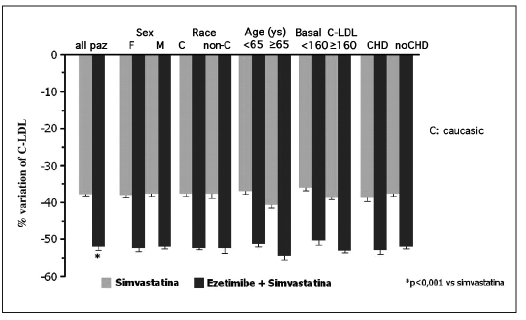
Moreover, the effectiveness of the association ezetimibe + simvastatin in LDL cholesterol lowering seems to be perceptible in the different categories of patients studied (patients with hypertension, obesity, diabetes, independently from age, gender, race, type of dyslipidemia) (Fig. 8).
Fig. 8.
- Variation of LDL cholesterol in the different populations studied (15).
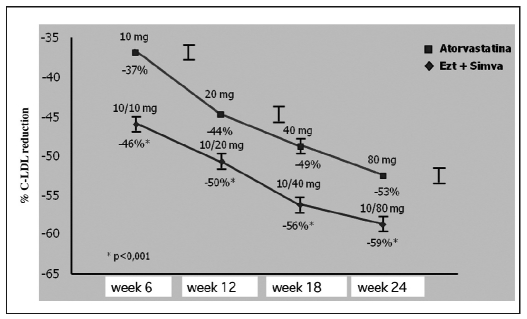
With regard to the comparison with other statins, Ballantyne et al, in a 2004 study (16), demonstrated that after 6 weeks of treatment the association ezetimibe + simvastatin (p<0.001) led to a significant LDL cholesterol lowering higher than with atorvastatin 10 mg only. In general, the association at every dose used is more effective than atorvastatin at every dose (10–80 mg) in LDL cholesterol lowering (Fig. 9).
Fig. 9.
- Ezetimibe + simvastatin: higher reduction of LDL cholesterol vs. atorvastatin in all treatment periods (16).
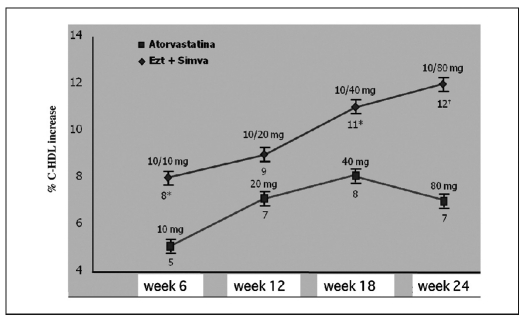
Significantly higher increases in HDL cholesterol than with atorvastatin 10–80 mg (p=0.002) have been demonstrated. At the maximum dose, the average increase in the ezetimibe + simvastatina 10/80 group was almost double that in the atorvastatin only group (12 vs. 6.5%) (Fig. 10).
Fig. 10.
- Ezetimibe + simvastatina: HDL cholesterol increase vs. atorvastatin in all treatment periods (16).
As well as for LDL and HDL cholesterol, the association has also determined significantly higher improvements in other lipidic parameters, such has total cholesterol, B and A-I apolipoproteins, non-HDL cholesterol, and B apolipoprotein/A apolipoproteina ratio than with atorvastatin only (p≤0.05 for every match).
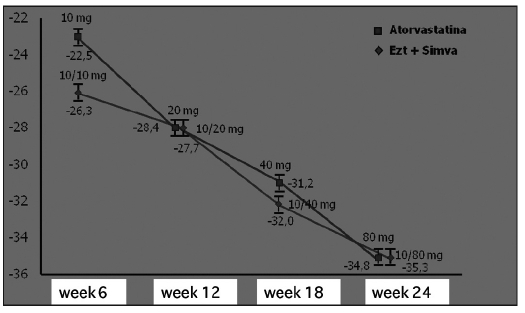
At the end of the study, the triglycerides levels were equally reduced (approximately 35%) in both groups (Fig. 11).
Fig. 11.
- Ezetimibe + simvastatin: triglyceride reduction similar as with atorvastatin (16).
Therefore the data from the studies demonstrate that the combined use of ezetimibe and simvastatin is an effective and well tolerated therapy suitable for reaching desired cholesterol levels, defined as optimal by the guidelines. These results are only rarely reached with a statin only therapy, and often at the cost of a significant incidence of side effects on the liver and muscles.
Clinical studies have not shown important side effects associated with ezetimibe. However, due to the lack of long-term data in a wide group of patients, therapy with ezetimibe seems unadvisable as first choice in all patients, but only in those not adequately controlled with statins only.
References
- 1.Davidson MH, Maki KC, Pearson TA. Results of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96:556–63. doi: 10.1016/j.amjcard.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 2.EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries. Eur Heart J. 2001;22:554–72. doi: 10.1053/euhj.2001.2610. [DOI] [PubMed] [Google Scholar]
- 3.Van Ganse E, Laforest L, Alemao E, Davies G, Gutkin S, Yin D, et al. Lipid-modifying therapy and attainment of cholesterol goals in Europe: The return on expenditure achieved for lipid therapy (REALITY) study. Curr Med Res Opin. 2005;21:1389–99. doi: 10.1185/030079905X59139. [DOI] [PubMed] [Google Scholar]
- 4.Leitersdorf E. Cholesterol absorption inhibition: filling an unmet need in lipid-lowering management. Eur Heart J Suppl. 2001;3(suppl E):E17–23. [Google Scholar]
- 5.Ginsberg HN, et al. Harrison’s Principles of Internal Medicine. McGraw-Hill; 1998. Disorders of lipoprotein metabolism; pp. 2138–49. [Google Scholar]
- 6.Shepard J. The role of the exogenous pathway in hypercholesterolaemia. Eur Heart J Suppl. 2001;3(suppl E):E2–5. [Google Scholar]
- 7.Altmann SW, Davis HR, Jr, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Calvo M, Lisnock J, Bull HG, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1-L1) Pro Natl Acad Sci U S A. 2005;102:8132–7. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB, et al. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interaction. Clin Pharmacokinet. 2005;44:467–94. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 10.Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metabol Dispos. 2002;30:430–7. doi: 10.1124/dmd.30.4.430. [DOI] [PubMed] [Google Scholar]
- 11.Sudhop T, Lütjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–8. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 12.Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005;80:587–95. doi: 10.4065/80.5.587. [DOI] [PubMed] [Google Scholar]
- 13.Stein E. Results of phase I/II clinical trials with ezetimibe, a novel selective cholesterol absorption inhibitor. Eur Heart J Suppl. 2001;3(suppl E):E 11–6. [Google Scholar]
- 14.Mikhailidis DP, Wierzbicki AS, Daskalopoulou SS, et al. The use of ezetimibe in achieving low density lipoprotein lowering goals in clinical practice. Curr Med Res Opin. 2005;21:959–69. doi: 10.1185/030079905x48447. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg AC, Sapre A, Liu J, Capece R, Mitchel YB, Ezetimibe Study Group Efficacy and safety of ezetimibe co-administrated with simvastatin in patients with primary hypercholesterolemia: a randomized, double blind, placebo controlled trial. Mayo Clin Proc. 2004;79:620–9. doi: 10.4065/79.5.620. [DOI] [PubMed] [Google Scholar]
- 16.Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administrated with atorvastetin in adults with hypercholesterolemia. Am J Cardiol. 2004;93:1487–94. doi: 10.1016/j.amjcard.2004.02.060. [DOI] [PubMed] [Google Scholar]