INTRODUCTION
The endothelium is a dynamic organ with many properties that takes part in the regulation of the principal mechanisms of vascular physiology. Its principal functions include the control of blood-tissue exchange and permeability, the vascular tonus, and the modulation of inflammatory or coagulatory mechanisms. Many vasoactive molecules, produced by the endothelium, are involved in the control of these functions. The most important is nitric oxide (NO), a gaseous molecule electrically neutral with an odd number of electrons that gives the molecule chemically reactive radical properties. Already known in the twentieth century, NO, sometimes considered as a dangerous molecule, recently valued as an important endogenous vasodilator factor. Recently, it was discovered that it is involved in several physiological mechanisms of endothelial protection (Tab. I). In 1992, Science elected it as “molecule of the year”; 6 yrs later three American researchers (Louis Ignarro, Robert Furchgott and Fried Murad) obtained a Nobel Prize for Medicine and Physiology “for their discoveries about NO as signal in the cardiovascular system”.
TABLE I. EFFECTS OF ENDOTHELIAL NITRIC OXIDE ON THE CARDIOVASCULAR SYSTEM.
|
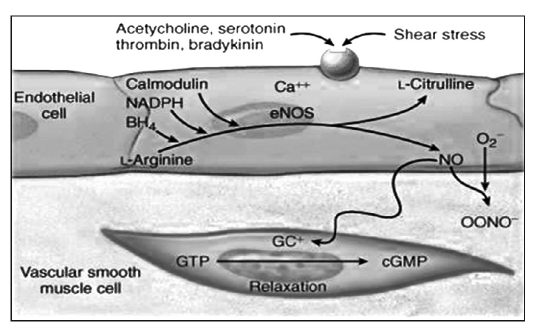
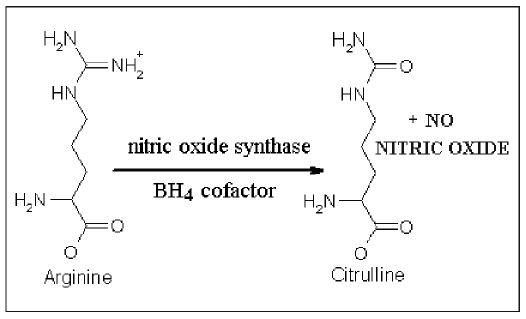
NO is synthesized by endothelial cells from L-argynine and oxygen (Fig. 1). Blood flow and laminar shear stress induce the activation through phosphorilation of NO synthase (NOS), that catalyzes the conversion reaction from L-arginine to citrullin and NO, through two cofactors: calmodulin and pteridin-thetraidrobiopterine (BH4) (Fig. 2). There are at least three isoforms of constitutive NOS: the endothelial form (eNOS), the neuronal form (nNOS) and the inducible form (iNOS); eNOS, the calcium-dependent form of the enzyme, is in many cellular types and it is responsible for NO production in healthy blood vessels. nNOS is a special type of eNOS, expressed in the central nervous system. iNOS, a form induced by immunological stimuli (1), is expressed in the myocytes, in the macrophages and in the endothelial cells. NOS are formed by two distinct catalytic subunits, as terminal C-reductase and terminal N-oxygenase domain. In the presence of sufficient amounts of BH4, these domains work together and synthesize NO. Otherwise in case of increased oxidative stress they cause the production of peroxynitrites. The NO produced induces guanilate cyclase to the synthesis of cGMP from cGTP. The last molecule causes cellular hyperpolarization due to the activation of the potassium canals. These reactions cause the inhibition of the entrance of calcium and, in this way, the vasodilatation in the cardiovascular system (Tab. I).
Fig. 1.
Fig. 2.
1
ENDOTHELIAL DYSFUNCTION AND NO
Normal vascular tonus depends on the equilibrium between the vasoconstrictor and vasodilator molecules released from the endothelium. In healthy endothelium, the balance is shifted towards vasodilatation due to NO (Tab. II). Endothelial dysfunction is synonymous with the insufficiency of endothelium dependent vasodilatation and results in the failure of vasoactive, anticoagulant and anti-inflammatory effects of healthy endothelium. The most important mechanism for endothelial dysfunction is the reduction in NO availability. The substrate insufficiency such as the reduction in L-arginine in endothelial cells or any defect in the transport of L-arginine into the cell, the existence of NOS inhibitors such as asymmetrical dimethylarginine (ADMA) and G-monomethyl-L-arginine (L-NM-MA), increase in the reactive oxygen molecules, reduction in the diffusion of NO due to intimal thickening, the mutations in the eNOS gene expression, increase in the catabolism of NO, cofactor insufficiency and increase in the vasoconstrictor molecules released from the endothelium are the other mechanisms that must be considered in endothelial dysfunction. Endothelial dysfunction coexists with many disease states in the cardiovascular system and is known as the first stage of atherosclerosis, which is probably the most important disease of the modern age. In the cardiovascular system, other clinical conditions, which are related with endothelial dysfunction are hypertension, are hyperglycemia-insulin resistance, dyslipidemia, menopause, heart failure, variant angina, cardiac syndrome X and hyperhomocysteinemia.
TABLE II. EFFECTS OF ENDOTHELIAL NITRIC OXIDE ON THE CARDIOVASCULAR SYSTEM.
|
NO oppose the atherogenical stimuli preventing vascular structural modifications; it can inhibit the adhesion of platelets and monocytes, the migration of the smooth muscular cells and the endothelial apoptosys. It was demonstrated that NO, for example, inhibits, through S-nytrosation, key-enzymes of the apoptotic chart (Caspases 6, 7, 8) (2).
I. Aging
Data obtained in humans and in animal experiments indicate that aging alters the endothelial dependent vasodilatation in the big arteries and in the resistance vessels (3). Moreover, aging is associated with a progressive remodeling of the vascular wall, which includes the thickening of the tunica intima and of the tunica media and the increase in its rigidity (4). With advancing old age, smooth muscular cells migrate progressively from the tunica media and accumulate in the intima (5). This fact is associated with a progressive decline in endothelial functionality, which causes a reduced response to the vasodilating factors, consequent to the alteration of the expression and/or the activity of NOS and with an increased formation of free radicals. Aging is associated with an alteration in the equilibrium between vasoconstrictor and vasodilating factors released by the endothelium, or rather with a progressive reduction in NO and endothelial derived hyperpolarizing factor (EDHF) associated with an increase in oxygen free radicals and prostanoids derived from cycle-oxygenase. In addition, it was recently demonstrated that, with aging, there is an inferior proliferation and migration of the endothelial cells from the sites near the atherosclerotic lesion, preventing the preservation of intimal integrity, which is normally favored by a healthy endothelium (6).
II. Hypertension
In the case of hypertension, the release of vasoconstrictor mediators from the endothelium increases. Hypertension is characterized by an increase in the production and activity of angiotensin II; it induces endothelin production by way of the mitogen activated protein kinase (MAPK) pathway. Hypertension stimulates the production of superoxide anions and reactive oxygen radicals (7) and increases the consumption of BH4, and inhibits NO production. In the case of normotension, L-NMA reduces the vasodilatation effect of acetylcholine, but it is inefficacious in patients with hypertension (8). This discovery indicates a decreased NO-bioavailability, maybe caused by a reduction in NOS activity due to L-Arginine deficiency, inhibition by ADMA or BH4 deficiency. An increase in reactive oxygen radicals that produce with NO peroxynitrates could also be an important factor. Oxidative stress is responsible for this alteration. Then a compensatory mechanism produces hyperpolarizant factor. When the oxidative mechanism ends, NO bioavailability increases and the compensatory hyperpolarizant mechanism vanishes. The important role of NO in vascular function regulation is explained by the interaction with other vasoactives substances, for example, endothelin-1 (ET-1) (9). ET-1 acts on specific receptors called ETA and ATB. ETA receptors are situated on smooth muscular cells and they cause contraction and cellular growth. ETB receptors are situated either on smooth muscular cells, where they induce contraction, or on the endothelium, where they induce dilatation that produces NO. NO reduces ET-1 synthesis. Decreased NO bioavailability plays an important role in vascular homeostasis due to its direct effects and its ability to regulate other important factors, for example, ET-1.
III. Dyslipidemia
Low-density lipoprotein (LDL) causes the occurrence of a situation characterized by an increase in angiotensin II, surface adhesion molecules and reactive oxygen molecules, which results in a low grade inflammation. This situation provides a base for endothelial dysfunction. Moreover, the oxygen radicals react with NO and cause the production of peroxynitrite in the existence of oxidized LDL. Peroxynitrite inhibits eNOS production and also changes the mission of eNOS from synthesis of NO to synthesis of oxygen radicals (10). The increase in LDL and decrease in high density lipoprotein (HDL) causes the disruption of the caveola complex, which are the specialized invaginations of the endothelial membrane, containing eNOS (11). Since the cofactors, essential for NO synthesis, are oxidized due to dyslipidemia, the function of eNOS is negatively affected.
IV. Diabetes mellitus
Metabolic abnormalities such as hyperglycemia, increase in free fatty acids and insulin resistance cause endothelial dysfunction by inhibiting NO synthesis or increasing the catabolism of NO. In healthy humans, insulin increases NOS activity by stimulating phosphotidylinositol-3 kinase and Akt kinase. In insulin resistant patients, the signal transduction by insulin through phosphotidylinositol-3 kinase pathway is impaired. Insulin stimulates NOS less and NO production decreases. However, the signal transduction by insulin through MAPK remains intact. As a result of this pathway, more endothelin is produced and inflammation and thrombosis increase (12). The phosphotidylinositol-3 kinase pathway is also responsible for the insulin-mediated glucose uptake in the cells. So insulin resistance is aggravated in the case of endothelial dysfunction resulting in a vicious cycle. It is shown that, after administration of L-NMMA, which is a NOS inhibitor, both endothelium-dependent vasodilatation and insulin mediated glucose uptake are impaired (13). Clinical studies with angiotensin-converting enzymes (ACE) inhibitors and statins demonstrated that these agents did not only reduce coronary artery disease and death due to cardiovascular events, but also prevented the occurrence of type II diabetes mellitus (14, 15). These findings suggest a role for endothelial dysfunction in the pathophysiology of insulin resistance. Hyperglycemia increases the production of the superoxide anion due to mitochondrial electron transport (17). Superoxide activates protein kinase C. The activation of protein kinase C, stimulates membrane bound NAD(P)H oxidase to produce more superoxide. The reactivity between superoxide and NO results in peroxynitrite production, peroxynitrite oxidises the BH4, which is a cofactor for NOS. This situation causes NOS to produce superoxide instead of NO. Superoxide anion also increases the production of advanced glycation end products (AGEs) (19). The AGEs increase superoxide and reactive oxygen radical production. Moreover, the oxidative stress caused by hyperglycemia inhibits DDAH (20). This increases ADMA levels. As a result, NO synthesis decreases. The increase in the amount of free fatty acids seen in diabetes mellitus and insulin resistance, affects the NO balance in an opposite manner by increasing free oxygen radicals, activating protein kinase C and causing dyslipidemia. Another mechanism for endothelial dysfunction in diabetes mellitus and insulin resistance is the increase in the release of vasoconstrictor prostanoids and endothelin (21). Even, in healthy humans, the administration of insulin resulted in an increase in plasma concentrations of endothelin-1 (20, 22).
V. Cigarette smoke
Cigarette smoke is a known risk factor for coronary pathology. In humans it has been shown that in vivo nicotine reduces endothelial dependent dilation. Most qualified hypotheses attribute the endothelial damage to the large number of oxygen free radicals, present in cigarette smoke: oxidative stress determines compromised NO production. In chronic smokers endothelial trouble in the brachial and coronary arteries and also damage in the coronary microcirculation has been seen (16).
VI. Obesity
Central obesity, in addition to being an important component of the metabolic syndrome, is a potent predictor of coronary disease in humans. Many studies evidenced an independent association between obesity and endothelial damage. In obese patients, endothelial trouble is due to two mechanisms: the resistance to insulin and the production of adipokines and pro-inflammatory cytokines, which promote oxidative stress and a reduction in NO concentrations (17). High BMI levels have been associated with the increased expression of adhesion molecules such as E-selectin, a precocious endothelial damage marker.
VII. Hyperhomocysteinemia
Homocysteine is a thiol amino acid that lies at a critical branch point in methionine metabolism. There are two main metabolic pathways that involve homocysteine. In most tissues, methionine is activated by adenosine triphosphate (ATP) to form S-adenosylmethionine (SAM), which serves as a donor for methyl transferases, including protein arginine methyl transferases that produce methylarginines. S-adenosylhomocysteine (SAH) is a major product of SAM-dependent methyl transfer reactions, and which can undergo hydrolysis to form homocysteine. Homocysteine can either undergo condensation with serine to form cystathionine, or undergo remethylation to form methionine. Condensation of homocysteine to cystathionine is catalyzed by the vitamin B6-dependent enzyme cystathionine β-synthase (CBS). In vascular tissue, the major pathway for homocysteine remethylation is catalyzed by the vitamin B12-dependent enzyme methionine synthase (MS) (18). This reaction utilizes 5-methyltetrahydrofolate, which is generated by 5,10-methylene tetrahydrofolate reductase (MTHFR).
Hyperhomocysteinemia is characterized by increased production of reactive oxygen radicals, and folate deficiency, which leads to reduced bioavailability of NO and endothelial dysfunction. Homocysteine increases the production of pro-inflammatory cytokines and the expression of adhesion molecules and chemotactic factors. This effect is caused by the stimulation of the activation of transcription factors such as nuclear factor-κB (NF-κB) and sterol regulatory element binding protein (SREBP), and the inhibition of peroxisome proliferator-activated receptors α and γ (PPAR- α and γ) (19). Since increased methionine levels result in increased levels of SAM, hyperhomocysteinemia leads to increased synthesis of ADMA (20). Homocysteine also causes the inhibition of DDAH, which is the enzyme responsible for the catabolism of ADMA (21), leading to a further increase in plasma ADMA levels. Hyperhomocysteinemia is found to be related with coronary artery disease (22).
VIII. A new cardiovascular risk factor: ADMA
Asymmetric dimethylarginine (ADMA) is a naturally occurring component of human blood plasma. It is formed as a metabolic byproduct of continuous protein turnover in all cells of the body. More than 10 yrs ago ADMA was first reported to exert biological effects by inhibiting NO synthesis. It has been recently demonstrated that elevated ADMA concentrations are associated with a higher global cardiovascular risk factor. ADMA competitively antagonizes arginine as a substrate of NOS. Moreover, it increases oxidative stress (possibly via uncoupling of the electron transport between NOS and L-arginine) and decreases the production/availability of endothelium-derived NO (23). As a result, endothelium-dependent vasodilation could be dependent on the plasma L-arginine/ADMA ratio. Elevated serum ADMA concentrations have been observed in patients with other cardiovascular risk factors such as hypercholesterolemia (24), essential hypertension (25) and diabetes (26). Recent studies have demonstrated that elevated ADMA levels represent an independent risk factor for coronary disease in patients with chronic renal failure (27), are predictors of acute coronary syndromes in adults (28) and are associated with higher rates of peri-procedural complications after percutaneous coronary intervention (PCI) (29); on the other hand, a reduction in ADMA levels, months after a PCI procedure, indicates a decreased risk of recurrent cardiovascular events (30). A recent study by Zhang et al (31) showed that smoking increases serum ADMA levels. The metabolism of ADMA seems to be altered by tobacco smoke; this fact could represent an additional link between smoking and endothelial dysfunction (32).
HEART FAILURE AND NITRIC OXIDE
Physiological doses of NO results in positive inotropic, positive chronotropic and positive lusitropic effects in the myocardium. In addition, NO paradoxically reduces the oxygen requirement of the heart by inhibiting the mitochondrial metabolism (33). Cardiac NO release is cyclic, and increases in the early diastolic filling period. When the preload increases, NO release increases too. NO is also effective in the Frank-Starling mechanism (34). Moreover, in low doses, NO increases the β-adrenergic activity in the myocardium. In heart failure, both iNOS and nNOS increases. In idiopathic dilated cardiomyopathy it is shown that 80% of NOS activity in the myocardium is dependent on nNOS (35). The increases in iNOS and nNOS are positively correlated with the increase in oxidative stress in patients with heart failure, and moreover, NO produced by iNOS can result in peroxynitrite production and contractile dysfunction. The high doses of NO released in heart failure results in a negative inotropic and negative chronotropic effect and a reduction in β-adrenergic stimulation (36). Saito et al (37) reported that the administration of selective iNOS inhibitors resulted in a significant reduction in mortality, infarct size and cardiomyocyte hypertrophy in patients with post-infarction heart failure. In patients with dilated cardiomyopathy, it is also shown that the contractile effect of dobutamine is increased with NMMA administration. In cases that developed resistant cardiogenic shock, despite intra-aortic balloon pumping and PCI after myocardial infarction; the administration of L-NMMA significantly reduced the mortality rate (38). These findings suggest that NO could play a major role in the pathogenesis of heart failure and the inhibition of this NO with deleterious effects could be helpful in treatment.
TREATMENT OF ENDOTHELIAL DYSFUNCTION
Due to its role in the pathophysiology and etiology of several life-threatening diseases such as heart disease, hypertension, atherosclerosis, dyslipidemia and diabetes, endothelial dysfunction is becoming an important target in therapeutic approaches. Several treatments have been found to be effective in improving endothelial function.
Aerobic physical exercise has been shown to prevent the aging related to the reduction in endothelial-mediated vasodilation (39). A study investigating patients with coronary heart disease demonstrated that after 4 weeks of aerobic training there was an improvement in endothelial function measured with a reduction in acetylcholine-induced vasoconstriction. The authors proposed that stimulation by shear stress induced by exercise could improve eNOS activity both by promoting its phosphorylation and by inducing its synthesis in endothelial cells.
Polyunsaturated ω-3 fatty acids (40) have been shown to preserve endothelial function and increase NO bioavailability; moreover, some authors have suggested a direct beneficial effect of this molecule on the vascular wall through a decrease in the expression of pro-inflammatory mediators that could contribute to altering the physiological vasodilatory mechanisms.
Dietary supplementation with L-arginine (a substrate of NOS) seems able to rebalance NO production on the dysfunctional endothelium, stimulating NOS activity both directly and indirectly: antagonizing the effects of ADMA and reducing endothelin-1 synthesis (41). Lerman et al (42) found a significant improvement in endothelial function in patients with coronary artery disease after 6 months of L-arginine supplementation. Treated patients showed a better vasodilatory response after acetylcholine induced contraction and reduced endothelin-1 plasmatic levels. In addition, L-arginine supplementation reduced symptoms in both patients with stable angina and heart failure.
Antioxidant vitamins are often thought to be beneficial in the treatment of endothelial dysfunction; however, the results of clinical studies are not clear. The results of a few clinical studies show that the benefit of antioxidant vitamins was positive. The CHAOS study, which is a secondary prevention study, suggested a 47% decrease in non-fatal myocardial infarction incidence, but no effect on mortality with α-tocopherol. The SPACE trial, which included end-stage renal failure patients, showed antioxidant vitamin A treated endothelial dysfunction (40). But most studies do not support these results. The possible explanations for disapproving the results of these studies includes the choice of incorrect vitamin form (α-tocopherol), insufficient dose, combined treatment with β-carotene, improper choice of study population and short treatment period. However, Kinlay et al showed that, long-term, high-dose combined vitamin C and vitamin E treatment failed to improve endothelial function and did not decrease LDL oxidation (43).
Folic acid may represent a useful therapeutic strategy in endothelial dysfunction. Folic acid administration is able to reduce serum homocysteine concentration, to inhibit NOS uncoupling and has itself a direct antioxidant effect. Wilmink et al (44) showed that folic acid inhibited postprandial lipid-induced endothelial dysfunction and caused an increase in urinary excretion of oxygen radicals in healthy volunteers.
An important class of drugs with therapeutic effects on endothelial dysfunction is the ACE-inhibitor. It has been shown that ACE-inhibitors inhibit NADPH oxidase through both bradykinine accumulation and direct effects. NADPH oxidase inhibition leads to a drop in oxygen free radical production. In the BANFF study, quinapril was shown to treat endothelial function in 80 patients with coronary artery disease after 8 weeks of treatment. It has been found that the effect of ACE inhibitors on endothelial function is related to genotype. Quinapril is found to be ineffective on endothelial functions in patients with ACE DD genotype. Ghiadoni et al (45) investigated the effect of different antihypertensive medications on FMD in 168 hypertensive patients and only perindopril was found to increase FMD. The HOPE and EUROPE studies showed a reduction in clinical cardiovascular end-points with ramipril and perindopril (46).
Statins have been found to be effective in endothelial dysfunction both in primary and secondary prevention studies. These benefits seem to be independent of their lipid lowering effects (39) and appear to be linked to their so-called “pleiotropic effects” on the endothelium. Statins inhibit the activity of plasminogen activator inhibitor-1, tissue factor, growth factor and matrix metalloproteinases; terminate smooth muscle cell proliferation and migration and reduce NO levels, reduce apoptosis and inflammation and increase angiogenesis.
Estrogen is another agent thought to be effective on endothelial function preservation. Estrogen increases NO production and NO dependent vasodilation. It inhibits smooth muscle cell proliferation and LDL oxidation. However, despite this evidence, the results of clinical studies like HERS and WHI have suggested an increase in major cardiovascular events with estrogen treatment (47).
Old generation β-blockers are found not useful in the treatment of endothelial dysfunction. However, nebivolol has recently shown a beneficial effect on the endothelium. In addition to its β1 selective antagonist effect, it exerts a direct vasodilation through NO production. Recent experimental evidence suggests that the lipophilic properties of nebivolol could enhance the mechano-sensitive reactivity of the endothelium to shear stress stimulation. This mechanical stress induces eNOS activation through a kinase-dependent mechanism determining calcium ion release.
In a double-blind randomized study on patients with essential hypertension, Tzemos et al (48) demonstrated the efficacy of 8-month treatment with nebivolol (5 mg per day) and a diuretic (bendrofluazide, 2.5 mg) in improving endothelial function. Nebivolol/bendrofluazide increased both stimulated and basal endothelial NO release, whereas for the same degree of blood pressure (BP) control, atenolol/bendrofluazide had no effect on NO bioactivity. Therefore, nebivolol could offer additional vascular protection in treating hypertension.
CONCLUSIONS
As previously explained, endothelial dysfunction results in an impaired NO production, which results in decreased vasodilation and a prothrombotic state. Its presence is associated with most cardiovascular risk factors such as hypertension, smoking, hypercholesterolemia, homocystinuria and diabetes mellitus. One common denominator shared by these diseases is the reduced levels of eNOS in the endothelium. An impaired endothelial function is known to be the first step to atherosclerosis. Several studies have demonstrated that the decrease in vasodilatory capacity NO-mediated of the endothelium (endothelium dependent) is a strong predictor of future cardiovascular events. This major risk seems to be independently associated with impaired endothelial function: young healthy adults with impaired endothelial function are more inclined to develop atherosclerosis than adults with preserved endothelium (49). It has been largely demonstrated that NO represents the most important biological mediator involved in the regulation of endothelial homeostasis. A decrease in bioavailability of this molecule appears to be the final pathway for all cardiovascular risk factors. In a damaged or altered endothelium several factors such as oxidative stress, intima thickness and enzymatic inhibition lead to NO depletion, shifting the vascular balance towards vasoconstriction, inflammation and thrombosis. Most cardiovascular risk factors act in reducing NO bioavailability through many ways, and this could be the main final mechanism that links risk factors to vascular disease and increased odds of cardiovascular events. According to this hypothesis we should consider endothelial dysfunction as an important target of new therapeutic strategies. Moreover, impaired NO availability could be not only the final pathway of all cardiovascular risk factors but also itself be the cause of some of them. For example, impairment in NO bioactivity or endothelial dysfunction involving resistant arteries may increase the systemic BP in susceptible individuals; therefore, giving rise to hypertension. It has been also suggested that insufficient NO production could have a role in the development of insulin resistance. In this way, endothelial dysfunction could represent an important player in the vicious circle of metabolic syndrome development.
References
- 1.Tentolouris C, Tousoulis D, Goumas G, Stefanadis C, Davies G, Toutouzas P. L-Arginine in coronary atherosclerosis. Int J Cardiol. 2000;75:123–8. doi: 10.1016/s0167-5273(00)00320-x. [DOI] [PubMed] [Google Scholar]
- 2.Scarabelli TM, Stephanou A, Pasini E, et al. Different signaling pathways induce apoptosis in endothelial cells and cardiac myocytes during ichaemia-riperfusion. Circ Res. 2002;90(5):745–78. doi: 10.1161/01.res.0000015224.07870.9a. [DOI] [PubMed] [Google Scholar]
- 3.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92:652–62. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–94. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Orlandi A, Bochaton-Piallat ML, Gabbiani G, Spagnoli LG. Aging, smooth muscle cells and vascular pathobiology: implications for atherosclerosis. Atherosclerosis. 2006;188:221–30. doi: 10.1016/j.atherosclerosis.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann N Y Acad Sci. 2007;1100:353–60. doi: 10.1196/annals.1395.038. [DOI] [PubMed] [Google Scholar]
- 7.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 8.Taddei S, Virdis A, Mattei P, Natali A, Ferrannini E, Salvetti A. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation. 1995;92:2911–8. doi: 10.1161/01.cir.92.10.2911. [DOI] [PubMed] [Google Scholar]
- 9.Armas-Padilla MC, Armas-Hernandez MJ, Sosa-Canache B, et al. Nitric oxide and malondialdehyde in human hypertension. Am J Ther. 2007;14:172–6. doi: 10.1097/01.pap.0000249914.75895.48. [DOI] [PubMed] [Google Scholar]
- 10.Kuzkaya N, Weismann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–54. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 11.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 12.De Vriese AS, Verbeuren TJ, van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–74. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin- mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest. 1995;96:786–92. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of the diabetes mellitus: evidence for a protective treatment effect in the West Scotland Coronary Prevention Study. Circulation. 2001;103:357–62. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin- converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann PA, Gnecchi-Ruscone T Camici PG, et al. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–8. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 17.Taddei S, Ghiadoni L, Salvetti G, Virdis A, Salvetti A. Obesity and endothelial dysfunction. G Ital Cardiol. 2006;7:715–23. [PubMed] [Google Scholar]
- 18.Stanger O, Weger M. Interactions of homocysteine, nitric oxide, folate and radicals in the progressively damaged endothelium. Clin Chem Lab Med. 2003;41:1444–54. doi: 10.1515/CCLM.2003.222. [DOI] [PubMed] [Google Scholar]
- 19.Ungvari Z, Csiszar A, Edwards JG, et al. Increased superoxide production in coronary arteries in hyperhomocysteinemia. Role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–23. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- 20.Böger RH, Lentz SR, Bode-Böger SM, Knapp HR, Haynes WG. Elevation of asymmetrical dimethylarginine may mediate endothelial dysfunction during experimental hyperhomocyst(e)inemia in humans. Clin Sci (Lond) 2001;100:161–7. [PubMed] [Google Scholar]
- 21.Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway. Role of asymmetrical dimethylarginine. Circulation. 2001;104:2569–75. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 22.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 23.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–8. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 24.Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–7. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 25.Surdacki A, Nowichi M, Sandmann J, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol. 1999;33:652–8. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Abbasi F, Asagmi T, Cooke JP, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:1201–3. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 27.Zoccali C, Bode-Boger SM, Mallamaci F, et al. Plasma concentrations of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–7. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 28.Valkonen VP, Paiva H, Salonen JT, et al. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–8. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 29.Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J. 2003;24:1912–9. doi: 10.1016/j.ehj.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Krempl TK, Maas R, Sydow K, Meinertz T, Boger RH, Kahler J. Elevation of asymmetric dimethylarginine in patients with unstable angina and recurrent cardiovascular events. Eur Heart J. 2005;26:1846–51. doi: 10.1093/eurheartj/ehi287. [DOI] [PubMed] [Google Scholar]
- 31.Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertension. 2006;48:278–85. doi: 10.1161/01.HYP.0000231509.27406.42. [DOI] [PubMed] [Google Scholar]
- 32.Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. J Mol Cell Cardiol. 2006;41:275–84. doi: 10.1016/j.yjmcc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–41. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 34.Belge C, Massion PB, Pelat M, Balligand JL. Nitric oxide and the heart: update on new paradigms. Ann N Y Acad Sci. 2005;1047:173–82. doi: 10.1196/annals.1341.016. [DOI] [PubMed] [Google Scholar]
- 35.Paulus WJ, Bronzwaer JG. Myocardial contractile effects of nitric oxide. Heart Fail Rev. 2002;7:371–83. doi: 10.1023/a:1020754232359. [DOI] [PubMed] [Google Scholar]
- 36.Gealekman O, Abbasi Z, Rubinstein I, Winaver J, Binah O. Role of myocardial inducible nitric oxide synthase in contractile dysfunction and beta-adrenergic hyporesponsiveness in rats with experimental volume-overload heart failure. Circulation. 2002;105:236–43. doi: 10.1161/hc0202.102015. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Hu F, Tayara L, Fahas L, Shennib H, Giaid A. Inhibition of NOS II prevents cardiac dysfunction in myocardial infarction and congestive heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H339–45. doi: 10.1152/ajpheart.00596.2001. [DOI] [PubMed] [Google Scholar]
- 38.Cotter G, Kaluski E, Blatt A, et al. L-NMMA (a nitric oxide synthase inhibitor) is effective in the treatment of cardiogenic shock. Circulation. 2000;101:1358–61. doi: 10.1161/01.cir.101.12.1358. [DOI] [PubMed] [Google Scholar]
- 39.Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res. 2007;73:326–40. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 40.Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107:1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- 41.Kabat A, Dhein S. L-arginine supplementation prevents the development of endothelial dysfunction in hyperglycaemia pharmacology. Pharmacology. 2006;76:185–91. doi: 10.1159/000091606. [DOI] [PubMed] [Google Scholar]
- 42.Lerman A, Burnett JC, Higano ST. L-Arginine supplementation prevents the development of endothelial dysfunction in hyperglycaemia. Pharmacology. 2006;76:185–95. doi: 10.1159/000091606. [DOI] [PubMed] [Google Scholar]
- 43.Kinlay S, Behrendt D, Fang JC, et al. Long term effect of combined vitamins E and C on coronary and peripheral endothelial function. J Am Coll Cardiol. 2004;43:629–34. doi: 10.1016/j.jacc.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 44.Wilmink HW, Stroes ES, Erkelens WD, et al. Influence of folic acid on postprandial endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:185–8. doi: 10.1161/01.atv.20.1.185. [DOI] [PubMed] [Google Scholar]
- 45.Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, Salvetti A. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281–6. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 46.Dagenais JR, Yusuf S, Bourassa MG, et al. Effects of ramipril on coronary events in high risk persons: result of the heart outcomes prevention evaluation study. Circulation. 2001;104:522–6. doi: 10.1161/hc3001.093502. [DOI] [PubMed] [Google Scholar]
- 47.Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study. Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 48.Tzemos N, Lim PO, MacDonald TM. Nebivolol Reverses endothelial disfunction in essential hypertension. Circulation. 2001;104:511–4. doi: 10.1161/hc3001.094207. [DOI] [PubMed] [Google Scholar]
- 49.Juonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–23. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]