Abstract
We review and compare our experience with tricuspid ring annuloplasty between usage of the Cosgrove-Edwards flexible band and the MC3 rigid ring for repair of functional tricuspid regurgitation to determine the efficacy and mid-term durability of tricuspid annuloplasty. 117 patients with functional tricuspid regurgitation undergoing open heart surgery and tricuspid valve repair from May 2005 to December 2007 were reviewed. The flexible bands were used in thirty five patients before October 2006. Since then, the rigid rings were used in the next consecutive eighty two cases. Echocardiographic evaluation of tricuspid regurgitation was performed preoperatively and postoperatively in follow-up schedule. The degree of tricuspid regurgitation was reduced from 2.80±0.67 to 0.71±1.0 (regurgitation severity grade: 0 to 4) in the patients with flexible bands at discharge. It was from 2.68±0.70 to 0.22±0.60 in the patients with rigid rings. At thirty six months postoperative period, tricuspid regurgitation grades in patients with flexible bands and rigid rings were 0.80±0.95 and 0.36±0.77, respectively. Freedom from recurrent tricuspid regurgitation (grade 2 or 3) in patients with flexible bands and rigid rings were 68.6% and 87.8%, respectively. Recurrent tricuspid regurgitation was significantly lower in the patients with rigid rings. Although both flexible band and rigid ring annuloplasty provide low rate of recurrent tricuspid regurgitation, rigid ring annuloplasty might be more effective than flexible band annuloplasty for decreasing functional tricuspid regurgitation in immediate and mid-term postoperative periods.
Key words: tricuspid valve, annuloplasty, valve heart valve.
Introduction
Functional tricuspid regurgitation (TR) is a common finding in adult patients with concomitant aortic or mitral valve disease. Functional TR occurs mainly from annular dilation and right ventricular enlargement, which is often secondary to left heart failure from myocardial or valvular causes, right ventricular volume and pressure overload, and dilation of cardiac chambers.1–4 The basic concept of repair for functional TR is reducing the annular size by suturing or placing an annuloplasty ring. Suture annuloplasty such as Kay method and De Vega method have been performed historically as surgical techniques of tricuspid annuloplasty and nowadays still employed for surgeons’ preference. Suture annuloplasty can plicate the annulus by bicuspidization or reduce annular size semicircularly.5,6 For last three decades, ring annuloplasty (i.e., flexible band, semi-rigid ring, and rigid ring) has been used with commercially available several prosthetic annuloplasty rings.7–9 In terms of clinical outcomes, a relatively high recurrence rate for suture annuloplasty has been reported.10 A number of series have reported ring annuloplasty conferred significant improvement over suture annuloplasty in long-term survival and eventfree survival as well as recurrence of TR.11–13 Ring annuloplasty with a flexible band can reduce the dilated annulus.14 Therefore, it is called reduction annuloplasty. Another concept of repair for functional TR is remodeling the dilated annulus by placing a semi-rigid or a rigid ring.7,15 These rings have a two-dimensional or three dimensional shapes, which allows the surgeon to adapt the ring shape to the physiological configuration of annulus but they restrict the physiological motion of annulus during implantation. There was no data available regarding comparison of clinical outcomes between the use of a flexible band and a rigid ring in performing tricuspid annuloplasty for functional TR. In this study, we review and compare our experience with ring annuloplasty using the Cosgrove-Edwards flexible annuloplasty system (Edwards Life Sciences, CA, USA) and the Edwards MC3 tricuspid annuloplasty system (Edwards LifeSciences) that is a latest three dimensional rigid annuloplasty ring, for repair of functional TR to determine the efficacy and mid-term durability of tricuspid annuloplasty.
Materials and Methods
Prosthetic annuloplasty ring
We performed a retrospective review of 117 patients with functional TR undergoing tricuspid ring annuloplasty in concomitant with open heart surgeries from May 2005 to December 2007. The indications for tricuspid annuloplasty were moderate TR or more with annular dilatation, and pulmonary hypertension (systolic pulmonary artery pressure >60 mmHg) with annular dilatation irrespective of the TR grade. From May 2005 to September 2006, the Cosgrove-Edwards annuloplasty system was used in the consecutive 35 patients (Flexible group). This system provides a C-shape flexible band which covers the anterior portion and the posterior portion of the tricuspid annulus. The flexible band adapts to three-dimensional contour of the annulus while providing support against dilation and enables preservation of native saddle shape and natural function of the tricuspid valve. This procedure is known as reduction annuloplasty. Since October 2006, the Edwards MC3 tricuspid annuloplasty system has become available commercially in Japan and then this new system was used in the latter consecutive 82 patients (Rigid group) instead of using the Cosgrove-Edwards annuloplasty system. This system provides a rigid ring which is constructed of titanium alloy and has a sewing ring that consists of a layer of silicone rubber covered with polyester velour cloth. Its anatomically correct design conforms to the three dimensional tricuspid orifice and minimizes stress on sutures. This procedure is known as remodeling annuloplasty.
Surgical procedures
Operations were performed through median full sternotomy or partial sternotomy using standard or vacuum assisted cardiopulmonary bypass with bicaval and aortic cannulation under tepid temperature. Tricuspid ring annuloplasty was performed through right atriotomy following concomitant valve procedures and/or closure of atrial septal defect. Tricuspid valve procedures were achieved during cardiac arrest or beating heart following aortic declamp under cardiopulmonary bypass. Tricuspid ring annuloplasty using the Cosgrove-Edwards annuloplasty system was performed with eight to ten 2–0 Ethibond Excel sutures (Ethicon, Inc, Somerville, NJ, USA) placed on the annulus along the anterior and posterior leaflets. A flexible Cosgrove-Edwards annuloplasty ring was tied down with the sutures and placed on the annulus under cardiac arrest or beating heart. Using the Edwards MC3 tricuspid annuloplasty system, tricuspid ring annuloplasty was performed with ten to fourteen 2–0 Ethibond Excel sutures placed on the annulus from the anteroseptal commissure to the middle of the septal leaflet along the anterior and posterior leaflets. A rigid Edwards MC3 annuloplasty ring was tied down with the sutures and placed on the annulus under beating heart. The ring size was determined by the length between the commissures along the septal leaflet. One or two smaller size of the ring than the measured annulus size under beating heart was chosen and placed for dilated annulus.
Tricuspid regurgitation grade evaluation
TR was graded as none, grade 1 (mild), grade 2 (moderate), grade 3 (moderate to severe), and grade 4 (severe) based on the severity of TR by the two-dimensional transthoracic echocardiographic findings with the color Doppler regurgitation distance on the four-chamber view from the cardiac apex.16 Transthoracic echocardiography was performed preoperatively and postoperatively. Postoperative echocardiographic evaluations were performed on postoperative day seven and regularly every six to twelve months. The patients were followed at outpatient clinic in our hospital or related facilities. Patient clinical status and most recent echocardiographic results were obtained from the patients cardiologists. Follow-up was 100% complete with a mean follow-up period of 24.5±9.3 months (range, 8 to 48 months).
Data analysis
Continuous variables are reported as the mean ± standard deviation unless otherwise specified. Categorical patient variables were compared using the χ2 test or Fisher’s exact test where appropriate. Continuous variables were compared by Student’s t-test or Mann-Whitney U test. The Kaplan-Meier curves for statistical analysis of TR recurrence were used. Logistic regression and univariate analysis were used to examine and identify predictors of TR recurrence. The differences were considered statistically significant at a P<0.05.
Results
Patient characteristics
Preoperative characteristics of the patients in each group are given in Table 1. The patients of 91.4% and 84.1% had mitral valve disease in Flexible group and Rigid group, respectively. The patients of 85.7% and 72.0% had chronic or paroxysmal atrial fibrillation in Flexible group and Rigid group, respectively. There was no significant difference between the Flexible group and the Rigid group patients in age, gender, NYHA functional class, prevalence of pulmonary hypertension, and atrial fibrillation. The degrees of preoperative tricuspid regurgitation were 2.80±0.67 and 2.68±0.70 in Flexible group and Rigid group, respectively. There was no significant difference between the two groups (P=0.44).
Table 1. Preoperative characteristics of patients with functional tricuspid regurgitation undergoing tricuspid ring annuloplasty.
| Parameter | Flexible group | Rigid group | P |
|---|---|---|---|
| (n=35) | (n=82) | ||
| Age (years) | 72.6±11 | 72.4±10 | 0.92 |
| Male gender | 17 (48.6) | 29 (35.4) | 0.18 |
| NYHA functional class | 3.08±0.7 | 2.92±0.8 | 0.66 |
| Aortic stenosis | 3 (8.6) | 5 (6.1) | 0.63 |
| Aortic regurgitation | 3 (8.6) | 13 (15.9) | 0.37 |
| Mitral stenosis | 4 (11.4) | 2 (2.4) | 0.12 |
| Mitral regurgitation | 28 (80.0) | 67 (81.7) | 0.83 |
| Atrial septal defect | 2 (5.7) | 9 (11.0) | 0.58 |
| Sinus rhythm | 5 (14.3) | 23 (28.0) | 0.11 |
| Atrial fibrillation | 30 (85.7) | 59 (72.0) | 0.11 |
| Chronic hemodialysis | 2 (5.8) | 5 (6.0) | 0.73 |
| Pulmonary hypertension | 23 (65.7) | 46 (56.1) | 0.33 |
| LVEF (%) | 56.0±8.0 | 57.5±13 | 0.83 |
| Preoperative TR grade | 2.80±0.67 | 2.68±0.70 | 0.44 |
| 0 (none, trivial) | 0 | 0 | |
| 1 (mild) | 0 | 6 | |
| 2 (moderate) | 10 | 25 | |
| 3 (moderate to severe) | 22 | 45 | |
| 4 (severe) | 3 | 6 |
Age is reported as means±standard deviation; Values in parentheses are percentages.
Patient operative characteristics
Patient operative characteristics, ring size, concomitant procedures, and mortality in each group were shown in Table 2. There was no significant difference between the Flexible group and the Rigid group patients in surgery time, cardiopulmonary time, cardiac arrest time, and concomitant procedures. The median ring size was 28 mm in Flexible group and that was 26 mm in Rigid group. Smaller rings (26 mm) were used in the Rigid group than the Flexible group (P<0.001).
Table 2. Patient operative characteristics, ring size, concomitant procedures, and mortality.
| Flexible group | Rigid group | P | |
|---|---|---|---|
| (n= 35) | (n=82) | ||
| Surgery time (min) | 270±54 | 257±61 | 0.44 |
| CPB time (min) | 126±25 | 131±38 | 0.64 |
| Cardiac arrest time (min) | 110±22 | 101±33 | 0.35 |
| Annuloplasty ring size | <0.001 | ||
| 30 mm | 6 (20.0) | 4 (4.9) | |
| 28 mm | 16 (45.8) | 9 (11.0) | |
| 26 mm | 13 (34.3) | 69 (84.1) | |
| Concomitant procedure | |||
| Mitral valve repair | 28 (80.0) | 62 (75.6) | 0.61 |
| Mitral valve replacement | 4 (11.4) | 8 (9.8) | 0.78 |
| Aortic valve replacement | 6 (17.1) | 18 (22.0) | 0.56 |
| CABG | 5 (14.3) | 12 (14.6) | 0.96 |
| MAZE procedure | 30 (85.7) | 59 (72.0) | 0.11 |
| Atrial septal defect closure | 2 (5.7) | 9 (11.0) | 0.58 |
| MICS | 1 (2.8) | 12 (14.6) | 0.12 |
| Mortality | 0.18 | ||
| Hospital death | 4 (11.4) | 2 (2.4) | 0.12 |
| Late death | 2 (5.7) | 4 (4.9) | 0.86 |
Postoperative degree of tricuspid regurgitation
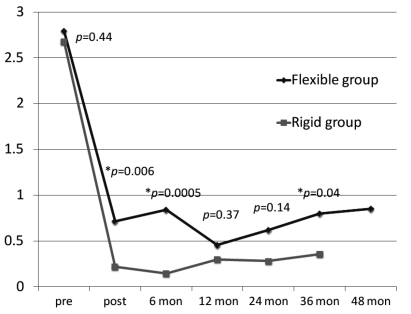
Changes of preoperative and postoperative TR grade in Flexible group and Rigid group were shown in Figure 1. Mean postoperative follow-up periods in Flexible group and Rigid group were 34.6±9.0 months and 21.±7.0 months, respectively. There were no prosthetic-related complications (i.e., prosthetic endocarditis, ring dehiscence, thromboembolic event, and conduction system injury) during follow-up period. There was statistical difference of postoperative TR grade between the two groups at postoperative day 7, 6 months, and 36 months but there was no difference at 12 months and 24 months.
Figure 1.
Changes of pre and postoperative TR grade in Flexible group and Rigid group. *Statistically significant.
Recurrent tricuspid regurgitation
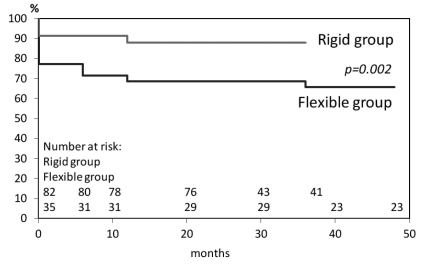
Postoperative TR grade 2 and 3 were defined as recurrent TR. Twelve (68.6%) out of 35 patients developed recurrent TR in Flexible group and 9 (87.8%) out of 82 patients in Rigid group during follow-up period. In those patients, three patients in Flexible group and three patients in Rigid group showed grade 2 TR on postoperative day 7 and the TR continued during the follow-up period. These six persistent TR patients were included in the patients with recurrent TR. Freedom from recurrent TR in the both groups was shown in Figure 2. There was statistical difference between the two groups (P=0.002). Freedom from recurrent TR in Flexible group is lower than Rigid group in each postoperative follow-up period. 28 mm or 30 mm ring use, pulmonary hypertension, or MAZE procedure was not a predictor for recurrent TR identified by univariate analysis of the patients with recurrent TR Table 3.
Figure 2.
Freedom from recurrent TR (grade 2 or 3) in Flexible group and Rigid group.
Table 3. Univariate analysis of patients with recurrent tricuspid regurgitation.
| Odds ratio | 95%CI | P | |
|---|---|---|---|
| 28 or 30 mm ring use | |||
| Flexible group | 1.43 | 0.46–3.43 | 0.93 |
| Rigid group | 0.64 | 0.09–4.87 | 0.94 |
| Pulmonary hypertension | |||
| Flexible group | 1.07 | 0.24–4.66 | 0.77 |
| Rigid group | 0.98 | 0.24–3.93 | 0.75 |
| MAZE procedure | |||
| Flexible group | 1.65 | 0.15–17.8 | 0.89 |
| Rigid group | 1.41 | 0.27–7.37 | 0.98 |
Discussion
The most common mechanism of functional TR is annular dilatation and subsequent failure of leaflet coaptation. Tricuspid annular dilatation occurs primarily in its anterior and posterior aspect, which can result in significant functional TR as a result of leaflet malcoaptation.4 Dreyfus and colleagues suggested that tricuspid annular dilatation is an ongoing process that will lead to severe TR with time, and they warrant early surgical correction regardless of the severity of TR.17 Because uncorrected TR even non TR without severe annular dilatation may worsen or persist after mitral valve surgery, leading to progressive heart failure and poor survival.13,18,19
When we treat annular dilatation by ring annuloplasty technique using either a flexible or rigid ring, we should know how the tricuspid valve works following ring annuloplasty. In recent studies using 3-dimensional echocardiography regarding the geometry of tricuspid annulus, health subjects had a nonplanar-shaped tricuspid annulus with homogenous contraction.20,21 In the other hand, the annulus in patients with functional TR had a more planar annulus with annular dilatation. Contraction of the annulus was then asymmetrically decreased. The nonplanar and non-single-plane structure of the annulus was observed in patients with TR, but the more severe TR, the more planar the annulus. The highest point of the annulus toward the right ventricular apex from the right atrium was in the anteroseptal segment, which was close to the right ventricular outflow tract and the aortic valve. The lowest point was the posteroseptal segment, where the coronary sinus. They concluded that the geometry of the tricuspid annulus was complicated and showed the physiological and optimal ring shape for tricuspid valve annuloplasty.22,23 The design concept and the ring shape of the Edwards MC3 ring system came from the idea based on the geometry of the tricuspid annulus. It has adopted the concept of this remodeling annuloplasty. This ring has a 3-dimensional design and is preconfigured to accommodate the saddle shape of the annulus. In contrast, a flexible band follows physiological motion of the tricuspid annulus during cardiac cycle, but may not keep the optimal saddle shape of the tricuspid annulus. Although it is not well known that how important physiological tricuspid annulus motion and shape during cardiac cycle are, the only difference between a rigid ring and a flexible band is flexibility of the plicated annulus. Regarding the size reduction of annulus, there is no difference between the two modalities. Our study results showed that the rigid ring was more effective than the flexible band for controlling functional TR in immediate and mid-term postoperative periods. We did not examine the geometry of the tricuspid annulus following ring implantation by echocardiography. It is interesting to see how the flexible band behaves and whether the annulus keeps 3-dimensional geometry.
Although we are not going to deny this result about the superiority of the rigid ring for controlling functional TR, ring size might affect the result because smaller rings were used in Rigid group than Flexible group. The median ring size was 28 mm in Flexible group and it was 26 mm in Rigid group. Therefore, the smaller ring might reduce functional TR in Rigid group. There was no difference between the two groups in preoperative characteristics, but this result tended to undersize the annulus more in the Rigid group than the Flexible group. There was procedural difference between the two groups for ring size measurement during ring annuloplasty. Ring size is usually determined by measuring the area of the anterior leaflet or the length between the commissures along the septal leaflet. If needed, one to two smaller size of the ring will be placed for reducing the dilated annulus more. However there is quite difference of the annular size in measuring between the annulus under an arrested heart and a beating heart. The length between the commissures along the septal leaflet is always measured shorter on a beating heart than an arrested heart. It is more physiological on beating heart than arrested heart. Therefore it is favorable that measurement of the annulus for suitable ring size is performed under heart beating. For this reason, we changed the strategy of ring annuloplasty, doing from on arrested heart to beating heart in 2006. Most patients in Flexible group underwent ring annuloplasty under arrested heart, but beating heart procedure was performed in all patients of Rigid group. This resulted that smaller ring was used in Rigid group than Flexible group. Meanwhile, McCarthy and colleagues reported that the size of the ring was not identified as a risk factor for recurrent TR.12 They concluded that the strategy of undersizing the tricuspid annulus for functional TR using small rings could not be validated as protection against late recurrent TR. On the other hand, Filsoufi and colleagues commented that systematic downsizing of the prosthetic ring than the type of the ring had probably played an important role in decreasing the incidence of significant residual or recurrent TR, or both, after ring annuloplasty.15 In our study, large rings (28 mm or 30 mm) use was not a risk factor for recurrent TR in both Flexible and Rigid ring groups.
There were some reports regarding risk factors for recurrent TR following tricuspid annuloplasty. Regardless the types of annuloplasty ring, recurrence of residual TR early after the procedure, which was associated with preoperative tethering of tricuspid valve leaflets, and postoperative left ventricular dysfunction predict mid-term outcome of tricuspid annuloplasty. Increased right ventricular pressure results in worse TR during mid-term follow-up. However, they did not mention about the difference between the types of annuloplasty ring.24,25 We did not examine preoperative tethering and we could not identify predictors for recurrent TR. Rigid ring annuloplasty certainly provided better result for controlling TR in our study. Although further echocardiographic evaluation is warranted, we assume that rigid ring annuloplasty might reduce tethering effectively by fixing the annular shape during systolic phase.
Limitations
This study has several limitations. The retrospective nature of the study is a major limitation. The sample size was relatively small and the follow-up period was short in some patients. This preliminary result must be verified in a more numerous population of patients, and on a long-term basis. There were major differences in the surgical approach. A flexible Cosgrove-Edwards annuloplasty ring is designed to cover the annulus along the anterior and posterior leaflets. However, a rigid Edwards MC3 annuloplasty ring covers the annulus along the anterior, posterior, and middle of the septal leaflet. Even if there was no difference between the two groups in preoperative characteristics, there was procedural difference between the two groups for ring size measurement during ring annuloplasty. The annuloplasty in the Flexible group was performed under an arrested heart and a beating heart procedure was performed in Rigid group. This resulted that the median ring size was 28 mm in Flexible group and it was 26 mm in Rigid group.
Conclusions
Ring annuloplasty effectively corrects functional TR with excellent early clinical outcomes. Although both flexible band annuloplasty and rigid ring annuloplasty provide low rate of recurrent tricuspid regurgitation, rigid ring annuloplasty might be more effective than flexible band annuloplasty for decreasing functional TR in immediate and mid-term postoperative periods. Continued follow-up should be mandatory for confirming the effectiveness of these tricuspid annuloplasty techniques.
References
- 1.Cohn LH. Tricuspid regurgitation secondary to mitral valve disease: when and how to repair. J Card Surg. 1994;9:237–41. doi: 10.1111/j.1540-8191.1994.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 2.Simon R, Oelert H, Lichtlen PR. Influence of mitral valve surgery on tricuspid incompetence concomitant with mitral valve disease. Circulation. 1980;62:I-152–I-157. [PubMed] [Google Scholar]
- 3.Kuwaki K, Morishita K, Tsukamoto M, Abe T. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left-sided valvular disease. Eur J Cardiothorac Surg. 2001;20:577–82. doi: 10.1016/s1010-7940(01)00786-2. [DOI] [PubMed] [Google Scholar]
- 4.Seo HS, Ha JW, Moon JY, et al. Right ventricular remodeling and dysfunction with subsequent annular dilatation and tethering as a mechanism of isolated tricuspid regurgitation. Circ J. 2008;72:1645–1649. doi: 10.1253/circj.cj-08-0237. [DOI] [PubMed] [Google Scholar]
- 5.De Vega NG, De Rábago G, Castillón L, et al. A new tricuspid repair. Short-term clinical results in 23 cases. J Cardiovasc Surg. 1973:384–6. Spec No: [PubMed] [Google Scholar]
- 6.Kay GL, Morita S, Mendez M, et al. Tricuspid regurgitation associated with mitral valve disease: repair and replacement. Ann Thorac Surg. 1989;48:S93–5. doi: 10.1016/0003-4975(89)90656-5. [DOI] [PubMed] [Google Scholar]
- 7.Carpentier A, Deloche A, Dauptain J, et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg. 1971;61:1–13. [PubMed] [Google Scholar]
- 8.Carrier M, Pellerin M, Guertin MC, et al. Twenty-five years' clinical experience with repair of tricuspid insufficiency. J Heart Valve Dis. 2004;13:952–6. [PubMed] [Google Scholar]
- 9.McCarthy JF, Cosgrove DM., 3rd. Tricuspid valve repair with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg. 1997;64:267–8. doi: 10.1016/s0003-4975(97)00348-2. [DOI] [PubMed] [Google Scholar]
- 10.Rivera R, Duran E, Ajuria M, Liu F. Carpentier’s flexible ring versus DeVega’s annuloplasty: A prospective randomized study. J Thorac Cardiovasc Surg. 1985;89:196–203. [PubMed] [Google Scholar]
- 11.Matsuyama K, Matsumoto M, Sugita T, et al. De Vega annuloplasty and Carpentier-Edwards ring annuloplasty for secondary tricuspid regurgitation. J Heart Valve Dis. 2001;10:520–4. [PubMed] [Google Scholar]
- 12.McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: Durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674–85. doi: 10.1016/j.jtcvs.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Tang GL, David TE, Singh SK, Maganti MD, Armstrong S, Borger MA. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation. 2006;114:577–81. doi: 10.1161/CIRCULATIONAHA.105.001263. [DOI] [PubMed] [Google Scholar]
- 14.Gatti G, Maffei G, Lusa AM, Pugliese P. Tricuspid valve repair with the Cosgrove-Edwards annuloplasty system: Early clinical an echocardiographic results. Ann Thorac Surg. 2001;72:764–7. doi: 10.1016/s0003-4975(01)02830-2. [DOI] [PubMed] [Google Scholar]
- 15.Filsoufi F, Salzerg SP, Coutu M, Adams DH. A three-dimensional ring annuloplasty for the treatment of tricuspid regurgitation. Ann Thorac Surg. 2006;81:2273–8. doi: 10.1016/j.athoracsur.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 17.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair. Ann Thorac Surg. 2005;79:127–32. doi: 10.1016/j.athoracsur.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 18.Filsouri F, Anyanwu AC, Salzberg SP, et al. Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg. 2005;80:845–50. doi: 10.1016/j.athoracsur.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Matsunaga A, Duran CM. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation. 2005;112:I53–7. doi: 10.1161/CIRCULATIONAHA.104.524421. [DOI] [PubMed] [Google Scholar]
- 20.Hiro ME, Jouan J, Pagel MR, et al. Sonometric study of the normal tricuspid valve annulus in sheep. J Heart Valve Dis. 2004;13:452–60. [PubMed] [Google Scholar]
- 21.Jouan J, Pagel MR, Hiro ME, et al. Further information from a sonometric study of the normal tricuspid valve annulus in sheep: geometric changes during the cardiac cycle. J Heart Valve Dis. 2007;16:511–8. [PubMed] [Google Scholar]
- 22.Ton-Nu TT, Levine RA, Handschumacher MD, et al. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation. 2006;114:143–9. doi: 10.1161/CIRCULATIONAHA.106.611889. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda S, Saracino G, Matsumura Y, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: A real-time, 3-dimensional echocardiographic study. Circulation. 2006;114:1492–8. doi: 10.1161/CIRCULATIONAHA.105.000257. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda S, Gillinov AM, McCarthy PM, et al. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2006;114:I582–7. doi: 10.1161/CIRCULATIONAHA.105.001305. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda S, Song JM, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005;111:975–9. doi: 10.1161/01.CIR.0000156449.49998.51. [DOI] [PubMed] [Google Scholar]