Abstract
The aims of this study were to evaluate through Color Doppler Myocardial Imaging (CDMI) echocardiography if atrial or ventricular myocardial alterations could be detectable in patients with thalassemia major (THAL) and if these alterations could be considered as predictive elements for supra-ventricular arrhythmic events. Twenty-three patients with THAL underwent clinical and electrocardiographic evaluation; patients were grouped in THAL1 (9 with supra-ventricular arrhythmias) and THAL2 (14 without arrhythmias); 12 healthy subjects were considered as control group (C). We examined through conventional 2D Color Doppler echocardiography some morphological and functional parameters regarding left ventricular (LV) systolic and diastolic function, and through CDMI the velocities at mitral annulus level, the regional LV and left atrial (LA) strain and strain rate. All THAL patients had LV dimension (p<0.05), LA area (p<0.01) and E/Em ratio (p<0.001) to be significantly higher than controls. The mitral annulus longitudinal velocities were significantly lower in THAL1 than in THAL2 (p<0.001); the E/Em ratio was higher in THAL1 than THAL2 (p<0.001). The THAL1 showed a lower systolic strain rate of atrial wall than THAL2 and C (p<0.05). The multiple regression highlighted a significantly inverse correlation among E/Em and atrial strain (p<0.02). CDMI showed both THAL subgroups had subtle systolic and diastolic left ventricular myocardial alterations, which could represent the onset of developing “iron cardiomyopathy” and are related to supra-ventricular arrhythmia. Monitoring these parameters in the THAL patients could contribute to decisions about follow-up and therapy.
Key words: thalassemia, echocardiography, myocardial dysfunction.
Introduction
Iron overload represents the most important cause of mortality and morbility in patients affected by thalassemia major (THAL).1 A previous Italian case study recognized in the “iron overload cardiomyopathy” the main cause of mortality in THAL (63.3%), followed by infections and hepatic diseases.2 More recent studies confirmed these results and discovered a higher percentage of survival among those patients born after the introduction of the chelation therapy with desferoxamine.3 However, even patients treated with periodical transfusions and intensive chelation suffer at a certain point from cardiomyopathy4 and of arrhythmic symptoms, most frequently ventricular, which still constitute a recurrent complication in the natural development of the disease.2,3
Traditional morphological and functional qualitative analysis of the heart through M-mode, B-mode and color Doppler echocardiography can now be greatly improved by a relatively new ultrasonic methodology, the Color Doppler Myocardial Imaging (CDMI) that allows a better quantitative description of myocardium regional longitudinal function.5,6 Furthermore, CDMI could be able to detect early alterations of intramyocardial function, crucial in our complex physiopathological model, in which iron intramyocardial deposit is considered the most responsible for cardiomyopathy in these patients. CDMI allows the analysis of myocardial strain and strain rate, which are not affected by rotational movement of the heart, by tethering effect, or by load conditions.6 On the other hand, the analysis of left atrial function through CDMI is still to be fully explored.7–10 In our study, the analysis of atrial deformation could have a significant incremental value both integrating the diagnostic findings of myocardial CMDI functional analysis and adding prognostic information about the potentiality of generating atrial arrhythmias.
The aims of the study were: a) to test, in THAL patients, if CDMI is able to detect the presence of even early, sub clinical structural and functional alterations of the atrial and ventricular myocardium, which could be referred to as “iron overload cardiomyopathy”; and b) to obtain predictive elements of supraventricular arrhythmias.
Materials and Methods
Twenty-three patients (13 males and 10 females) with THAL underwent a clinical evaluation, electrocardiography and transthoracic conventional echocardiography and CDMI. These patients were grouped into two subgroups: THAL1, 9 pts (6 males and 3 females) who had supra-ventricular arrhythmias (atrial tachycardia, atrial flutter, atrial fibrillation) in the last six months and THAL2, 14 pts (7 males and 7 females) without arrhythmias. All patients in this study were both under regular hematic transfusions, with the last transfusion given seven days before the test and under chelating therapy with desferoxamine in individual doses and were part of 157 THAL patients periodically evaluated in our Echo-Lab. Serum ferritine concentrations were measured to assess the efficacy of iron chelation therapy. Full blood count and liver and renal function assessment were periodically performed. Twelve healthy subjects (9 males and 3 females) were considered as control group(c).
Conventional 2D-Doppler echocardiography
Studies were performed using a commercially available digital echograph GE System Five, equipped with a broadband sector transducer, following the digital echocardiography Guidelines of the American Society of Echocardiography.11 Left ventricular mass was calculated according to Devereux's formula;12 stroke volume was calculated as the difference between end-diastolic volume and end-systolic volume, while the cardiac output was calculated according to the formula: stroke volume x heart rate. With this method we analyzed the following main parameters:
left ventricle internal diastolic and systolic dimensions, respectively LVIDd, LVIDs;
septum and posterior wall thickness in diastole and in systole, respectively IVSd, IVSs, PWd, PWs;
left atrium systolic diameter (LAD) and area (LAarea);
left ventricular systolic (ESV) and diastolic volumes (EDV) and relative ejection fraction (LVEF);
left ventricular mass both in absolute (LVM) and indexed value by BSA (LVM/BSA);
early and late transmitral flow peak velocities and their ratio (PeakE, PeakA, E/A ratio).
Pulsed wave tissue doppler imaging
In the apical four chamber views, the PW Doppler sample volume was subsequently placed in 2 different sites of mitral annulus: septal and lateral. The apical 4-chamber view was chosen to obtain a quantitative assessment of the global diastolic left ventricular longitudinal function, almost simultaneously to the Doppler left ventricular inflow, and to minimize the incidence angle between the Doppler beam and the longitudinal motion of mitral annulus.13,14 The pattern of PW shows a systolic wave (Sm) and two diastolic waves that corresponded to early (Em) and late (Am) diastole, respectively. The following measurements were determined in each place as index of global diastolic function, peak velocities of the Em and Am waves (cm/sec) and their ratio. Peak velocities of Sm (cm/sec) were calculated as indices of global systolic function.14 PW tissue Doppler of Em was used for the measurement of early peak diastolic mitral annulus velocity and left ventricular filling pressures were approximated from the relationship of E/Em (E being the early peak mitral flow velocity).15
CDMI derived-indices: strain rate and strain
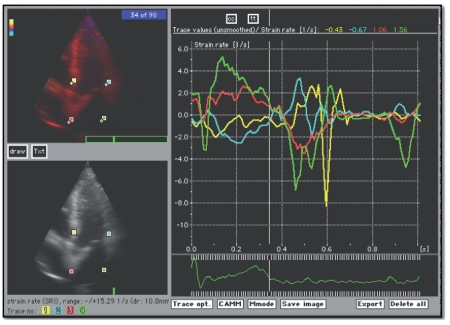
CDMI has been recently introduced as a method to evaluate regional myocardial function by providing a map of color-encoded tissue velocities. This methodology enables us to calculate, from regional mean velocities, strain rate (SR) and the relative strain (ε).5,6 The concept of regional myocardial strain was defined as fractional tissue deformation in response to an applied force (stress) and by definition negative strain means shortening and positive strain elongation. Strain rate is the speed at which deformation (i.e. strain) occurs (expressed in s−1). In echocardiography, SR can be calculated by measuring the instantaneous velocity gradient across a deforming piece of tissue:16,17 strain (ε) and strain rate (SR) both at basal segments of interventricular septum (IVS) and of lateral wall (LVW) and at distal segments of the interatrial septum (IAS) and of atrial lateral wall (LAW), considering the ε and SR systolic peak and the SRpeak in early and late diastole (E and A) (Figure 1).
Figure 1.
Color doppler myocardial imaging: apical 4 chamber views of interested areas at basal segments of interventricular septum and lateral wall, at distal segments of the interatrial septum and atrial lateral wall. In graphics the traces of strain rate at selected points.
Statistical analysis
Continuous variables were expressed as mean values±Standard Deviation. The statistical comparison between groups was made using analysis of variance and unpaired Student's t-test was used for subgroup comparison. The statistical significance level was placed at p<0.05. Variables with a p<0.10 at univariate analysis were entered into multivariate multiple linear regression analysis to identify independent predictors of E/Em ratio regression.
Results
The THAL1 group consisted of 9 patients with previous supra-ventricular arrhythmia: 3 patients with atrial fibrillation episodes, one atrial tachycardia and 5 supra-ventricular parossistic tachycardia. Main demographic characteristics of the study population are shown in Table 1. Thalassemic patients showed a lower BSA compared to group 1 C. THAL1 patients were significantly older compared to group C and THAL2, whereas there were no differences between THAL patient groups in hemoglobin and ferritin values. THAL1 patients showed a significantly higher LVID (also indexed: LVIDd/BSA), LAD and LAarea values if compared to THAL2 and C. No study group showed significant differences in LVEF values. The transmitral flow velocity and myocardial velocity at mitral annulus are shown in Table 2. No differences were found in diastolic function if analyzed by transmitral flow velocities,whereas the myocardial velocities at mitral annulus level, both in systole and in diastole, were significantly lower in THAL1 than those of THAL2 and C, and E/Em ratio was higher in all THAL groups in comparison with C, and significantly higher in THAL1 compared with THAL2. In THAL1, patients presented an E/Em ratio ≥8 and 2 of them >10; no patients had E/Em ratio >15. In THAL2, 6 patients presented an E/Em ratio ≥8 and none >10. The ventricular deformability, expressed by strain and strain rate, is shown in Table 3. THAL1 and THAL2 had a lower ventricular longitudinal deformability, with the lower level of systolic strain if compared to C; early (E) and late (A) diastolic SR peak at IVS and LVW level was significantly lower on THAL1 compared to THAL2 and C. Atrial deformation analysis is shown in Table 4. Strain and the SR peak values at atrial lateral wall were significantly lower in THAL1 if compared both with THAL2 and C, while at interatrial septum only SR peak A values were significantly lower in comparison to THAL2 and C. By performing univariate analysis in the THAL1 population, the E/Em ratio at lateral mitral annulus was significantly related to LAW SR-Epeak (R=0.681, p<0.044, β-coefficient 0.635). While the E/Em ratio at septal mitral annulus was significantly related to LVW SR-Epeak (R=0.785, p<0.027, β-coefficient −1.282). In THAL2 E/Em ratio at septal mitral annulus was significantly related to IVS SR-Epeak (R=0.563, p<0.036, β-coefficient −0.734), LVWε (r=0.480, p<0.027, β-coefficient −0.069).
Table 1. Demographic and conventional echo parameters.
| THAL1 (9) | THAL2 (14) | C (12) | |
|---|---|---|---|
| BSA (m2) | 1.55±0.1°°° | 1.54±0.2ψψ | 1.83±0.2 |
| Age (yrs) | 35.33±6.9§§° | 26.29±5.6 | 27.33±9.0 |
| Hb (g%) | 11.2±1.4 | 10.8±1.3 | - |
| Ferritin (mg/L) | 2144±2132 | 2383±1859 | - |
| LVIDd (cm) | 5.54±0.6§§° | 4.93±0.5 | 4.91±0.6 |
| LVIDd/BSA | 3.58±0.4°°° | 3.26±0.6ψψ | 2.68±0.3 |
| LVIDs (cm) | 3.66±0.7° | 3.17±0.5 | 3.09±0.5 |
| LVEF (%) | 68.89±11.0 | 73.21±6.9 | 74.67±5.5 |
| LAD (cm) | 4.08±0.7§§° | 3.44±0.4 | 3.42±0.5 |
| LAD/BSA | 12.30±3.7°°° | 10.08±1.7ψψψ | 7.51±0.7 |
| LA area (cm2) | 18.97±5.5§°° | 15.27±1.8ψ | 3.74±1.7 |
THAL1 vs THAL2:
p<0.05;
p<0.01;
p<0.001;
THAL1 v.s C:
p<0.05;
p<0.01;
p<0.001;
TAHL2 vs. C:
p<0.05;
p<0.01;
p<0.001;
BSA: Body surface area; Hb: Hemoglobin; LVIDd: Left ventricle internal diastolic dimensions; LVIDs: Left ventricle internal systolic dimensions; LVEF: Left ventricular ejection fraction; LAD: Left atrium systolic diameter; LAarea: Left atrium area.
Table 2. Transmitral flow velocities and PW-TDI doppler at mitral annulus level.
| THAL1 (9) | THAL2 (14) | C (12) | ||||
|---|---|---|---|---|---|---|
| PeakE (m/s) | 0.94±0.1 | 0.96±0.2 | 0.82±0.1 | |||
| PeakA (m/s) | 0.47±0.1 | 0.54±0.1 | 0.48±0.1 | |||
| E/A | 2.25±1.2 | 1.81±0.4 | 1.79±0.5 | |||
| Annulus side | Septal | Lateral | Septal | Lateral | Septal | Lateral |
| Sm (cm/s) | 6.3±1.3§°° | 6.9±1.9§°° | 7.7±1.2ψ | 8.6±2.4 | 9.2±2.2 | 9.6±2.1 |
| Em (cm/s) | 9.5±1.4§§§°° | 12.1±2.9§§§° | 12.8±2.1 | 16.9±3.1 | 13.8±4.2 | 17.1±5.0 |
| Am (cm/s) | 5.1±1.4§§°° | 4.9±1.8§§§° | 6.9±1.6 | 8.1± 2.2 | 7.6±1.6 | 7.5±3.0 |
| E/Em | 10.3±2.5§§°°° | 9.0±2.2§§§°°° | 7.6±1.3ψ | 5.8±1.3ψ | 5.9±3.4 | 4.8±2.8 |
THAL1 vs THAL2:
p<0.05;
p<0.01;
p<0.001;
THAL1 vs C:
p<0.05;
p<0.01;
p<0.001;
TAHL2 vs. C:
p<0.05;
p<0.01;
p<0.001;
Transmitral flow velocities: PeakE: early peak velocities; PeakA: late peak velocities; E/A: ratio between PeakE and PeakA Pulsed Wave Tissue Doppler Imaging; Sm: systolic wave; Em: early diastolic wave; Am: late diastolic wave; E/Em ratio between PeakE and Em.
Table 3. Ventricular deformation.
| THAL1 (9) | THAL2 (14) | C (12) | ||
|---|---|---|---|---|
| IVS SRpeak | S | −1.38±0.9 | −1.39±0.6 | −1.53±0.9 |
| E | 2.17±0.9§°∞ | 2.54±1.0ψ | 2.98±1.2 | |
| A | 0.91±0.8§°∞ | 1.41±1.0 | 1.26±1.0 | |
| IVS ϵ | S | 15.5±5.4§° | 19.8±7.4ψ | 24.6±9.2 |
| LVW SRpeak | S | −1.66±1.0 | −1.58±1.0 | −1.34±0.6 |
| E | 2.05±1.4°∞ | 2.58±1.0 | 2.41±0.7 | |
| A | 0.85±1.0°∞ | 0.71±0.7ψ | 1.03±0.4 | |
| LVW ϵ | S | 18.1±10.7° | ∞20.0±9.0 | 23.4±9.7 |
THAL1 vs THAL2:
p<0.05;
p<0.01;
p<0.001;
THAL1 vs C:
p<0.05;
p<0.01;
p<0.001;
TAHL2 vs. C:
p<0.05;
p<0.01;
p<0.001;
IVS SRpeak: strain rate systolic (S), early (E) and late (A) diastolic peak at basal segments of interventricular septum; IVSϵ: strain systolic (S) peak at basal segments of interventricular septum; LVW SRpeak: strain rate systolic (S), early (E) and late (A) diastolic peak at basal segments of left ventricular lateral wall; LVWϵ: strain systolic (S) peak at basal segments of left ventricular lateral wall.
Table 4. Atrial deformation.
| THAL1 (9) | THAL2 (14) | C (12) | ||
|---|---|---|---|---|
| IAS SRpeak | S | 2.94±1.7 | 2.66±1.7 | 2.97±1.7 |
| E | −4.00±2.6 | −4.18±1.8 | −3.15±2.5 | |
| A | −1.38±1.9§ | −2.30±1.1 | −2.10±0.9 | |
| IAS ϵ | S | 39.1±24.5 | 46.8±13.1 | 46.8±16.4 |
| LAW SRpeak | S | 1.09±0.9§§° | 2.71±1.5 | 2.56±1. |
| E | −2.87±2.4 | −4.12±1.7 | −4.11±1.9 | |
| A | −1.12±1.5§ | −2.58±1.4 | −2.17±2.0 | |
| LAW ϵ | S | 21.3±13.2§ | 36.2±15.6 | 37.1±14.9 |
THAL1 vs THAL2:
p<0.05;
p<0.01;
p<0.001;
THAL1 vs C:
p<0.05;
p<0.01;
p<0.001;
TAHL2 vs. C:
p<0.05;
p<0.01;
p<0.001;
IAS SRpeak: strain rate systolic (S), early (E) and late (A) diastolic peak at distal segments of interatrial septum; IASϵ: strain systolic (S) peak at distal segments of interatrial septum LAW SRpeak: strain rate systolic (S), early (E) and late (A) diastolic peak at distal segments of left atrial lateral wall; LAWϵ: strain systolic (S) peak at distal segments of left atrial lateral wall.
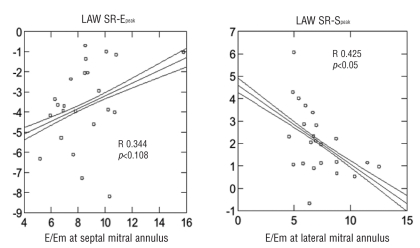
By performing multiple linear regression analysis in an overall thalassemic population E/Em ratio at lateral mitral annulus showed the best correlation to LAW SR-Speak (R=0.425 p<0.05, β-coefficient −0.695). E/Em ratio at septal mitral annulus showed the best correlation to LVW SR-Epeak (R= 0.599 p<0.003, β-coefficient −1.056) (Figures 2 and 3). No significant differences in hemoglobin and ferritin were found among any thalassemic group and no significant correlations were identified in comparison with echocardiographic parameters.
Figure 2.
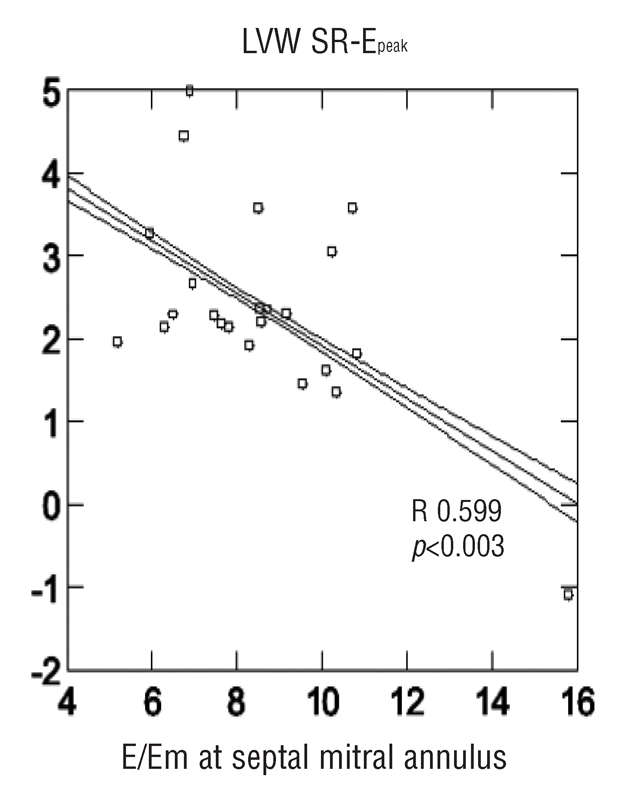
Correlation between the ratio of early transmitral flow velocity/early diastolic velocity at mitral septal annulus (E/Em, x axis) and the left ventricular wall strain rate at peak E (LVW SR-E, y axis) in all the Thalassemic population. A statistically significant relation is observed.
Figure 3.
Left panel: correlation between the ratio of early transmitral flow velocity/early diastolic velocity at mitral septal annulus (E/Em, x axis) and the left atrial wall strain rate at peak E (LAW SR-E, y axis) in all the Thalassemic population. Right panel: correlation between the ratio of early transmitral flow velocity/early diastolic velocity at mitral lateral annulus (E/Em, x axis) and the left atrial wall strain rate at peak S (LAW SR-S, y axis) in all the Thalassemic population. A statistically significant relation is observed.
Discussion
The main findings of the present study are :
the early detection through CDMI of systolic left ventricular dysfunction (significantly lower myocardial deformability, i.e. strain); these alterations are more pronounced in the THAL1 subgroup, which showed a higher estimated left ventricular end-diastolic pressure;
the coexistence of a lower rate of myocardial atrial deformability (strain rate), essentially in the THAL1 subgroup;
the strict relationships between left atrium systolic and diastolic strain rate parameters and the estimated end-diastolic left ventricular pressure.
Relatively recent profound changes have been made in the management of patients with THAL. Regular red blood cell transfusions eliminate the complications of anemia and compensatory bone marrow expansion, permit childhood development, and extend survival.18 In parallel, repeated transfusions determine a “second disease” while treating the first,19 the inexorable accumulation of iron in different tissues (also myocardium) that, with no treatment, is deadly in the second decade of life. Furthermore, the prognosis of THAL over the last 20 years has improved side by side with the development of iron-chelating therapy for iron overload. Without any chelating therapy, myocardial disease remains the life-limiting complication of transfusional iron overload. As highlighted over 30 years ago, if irregularly transfused, unchelated children frequently develop left ventricular hypertrophy and conduction disturbances by late childhood, and ventricular arrhythmias and refractory congestive failure by their mid-teens.20
Comparison with previous studies
Within the heart, even small amounts of unbound iron may generate harmful reactive oxygen metabolites and toxicity, while both chronic pulmonary hypertension21 and myocarditis22 may accelerate iron-induced cardiac failure23 in thalassemia. These observations may explain the unsettled correlation observed between the severity of myocardial iron deposition and that of cardiac fibrosis. Two trials, both covering over ten years, have demonstrated unequivocally that effective long-term use of deferoxamine in THAL is associated with long-term survival free of iron overload complications.24,25 Both studies identified the magnitude of body iron burden as the principal determinant of clinical outcome. Evidence that established iron-induced dysfunction of heart26–28 and liver29,30 might improve during intensive deferoxamine therapy has been presented in several reports. Cardiac biopsy or NMR remain the main procedures for detecting iron deposits in heart. Demonstrating hemosiderin in cardiac biopsy material is used in the diagnosis of cardiac iron overload; the hemosiderin deposition is greatest in the subepicardial myocardium. Myocardial fibrosis is, at most, mild. On the other hand conventional echocardiogram may show advanced iron-induced cardiac disease, but it is not sufficiently sensitive for the early detection of iron-induced cardiac dysfunction.31,32 In fact, previous echocardiographic studies of patients with THAL and secondary iron overload disclosed alterations of left ventricular diastolic function, which precedes any systolic dysfunction and increased myocardial reflectivity. Our data can be added to previous observations confirming what is already shown in literature: with same age, hemoglobin and ejection fraction, THAL patients present an enlargement of left atrium and ventricle, a faster peak of early diastolic flow and an increase of the traditional parameters depending on preload.33 The use of a highly sensitive method such as CDMI, has enabled us to detect very early and subclinical intra-myocardial alterations both at ventricular and atrial level.
With PW-TDI, we found lower myocardial velocities at mitral annulus level both in systole (global systolic longitudinal left ventricular dysfunction) and in diastole (impaired early left ventricular diastolic function). Furthermore, an E/Em ratio>8, considered the indicative cut-off of end-diastolic filling pressure of the left ventricle,15 was found in 65% of thalassemic patients. These data were confirmed by the significantly impaired systolic regional longitudinal function with CDMI both at septum and at lateral wall level (significantly lower strain both at septum and lateral wall). It is relevant that these systolic alterations are more significant in the THAL1 group. We could hypothesize that such myocardial alterations of both systolic and diastolic function could be the expression of the early onset of an “iron cardiomyopathy” and not only depend on volume alterations, as previously described by Chrysohoou.34
It is interesting to analyze the data shown by the two sub-groups of THAL patients: patients presenting supra-ventricular arrhythmia (THAL1) showed a significant reduction of systolic and diastolic myocardial velocity at mitral annulus level and an increased E/Em ratio and were older than the thalassemic patients without arrhythmias (THAL2). These findings could give force to the hypothesis of a more advanced structural cardiomyopathy in the THAL1 subgroup. Moreover, the THAL1 group showed a significant reduction of strain rate value of the atrial wall in two of its components (S and A). This reduction of the strain rate of left atrium external wall, analyzed following Wang's recent studies on patients with return of the sinus rhythm after atrial fibrillation, represents a parameter able to identify those at risk of relapsing arrhythmias.35 In fact our data, focusing on the analysis of the deformation of left atrium lateral wall, showed that patients with lesser deformation speed are those who were more frequently subject to supra-ventricular arrhythmias. CDMI derived parameters, such as E/Em and atrial strain and strain rate, identified different behaviour between the two groups of THAL patients: the group with arrhythmias showed significantly lower values of atrial strain and strain rate in relation to a higher E/Em value.
Conclusions
Color Doppler myocardial imaging, a relatively novel echocardiographic technique, has an incremental value in the follow-up analysis of myocardial function in patients with THAL who underwent iron-chelating therapy. With this method it is possible to detect some subtle atrial and ventricular myocardial alterations of both systolic and diastolic function, not shown by conventional echocardiography, which could represent the onset of the development of an “iron cardiomyopathy”. More specifically the strain rate of the free wall of left atrium seems to be related to the development of supra-ventricular arrhythmias. The monitoring of these parameters in the thalassemic population can help identify those at risk of developing a cardiomyopathy and/or supra-ventricular arrhythmia and can promote earlier decision-making on therapy and follow-up.
Study limitation
Our study has two limitations: the small sample size and demographic differences between the two study groups. In fact the patients in the THAL1 group were significantly older than patients in the THAL2 group. Age difference, and consequently a more longstanding disease and disease-related therapy history, may account for the subtle differences in myocardial structure and function observed in the study. This, may suggest echo monitoring and a more aggressive chelating therapy (i.e. with new oral chelating therapy) in older thalassemic patients.
References
- 1.Rund D, Rachmilewitz E. β-Thalassemia. N Engl J Med. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 2.Zurlo MG, De Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989;2:27–30. doi: 10.1016/s0140-6736(89)90264-x. [DOI] [PubMed] [Google Scholar]
- 3.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and disease complications in thalassemia major. Ann N Y Acad Sci. 1998;850:227–31. doi: 10.1111/j.1749-6632.1998.tb10479.x. [DOI] [PubMed] [Google Scholar]
- 4.Aessopos A, Farmakis D, Hatziliami A, et al. Cardiac status in well-treated patients with thalassemia major. Eur J Haematol. 2004;73:359–66. doi: 10.1111/j.1600-0609.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 5.Edvardsen T, Gerber BL, Garot J, et al. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans. Circulation. 2002;106:50–56. doi: 10.1161/01.cir.0000019907.77526.75. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland GR, Di Salvo G, Claus P, et al. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004;17:788–802. doi: 10.1016/j.echo.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Sirbu C, Herbots L, D'hooge J. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in Cal subjects. Eur J Echocardiogr. 2006;7:199–208. doi: 10.1016/j.euje.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Monte I, Licciardi S, Modica G. Myocardial strain rate in Cal subjects. Ital Heart J. 2005;6:604–11. [PubMed] [Google Scholar]
- 9.Di Salvo G, Drago M, Pacileo G. Atrial function after surgical and percutaneous closure of atrial septal defect: a strain rate imaging study. J Am Soc Echocardiogr. 2005;18:930–3. doi: 10.1016/j.echo.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Inaba Y, Yuda S, Kobayashi N. Strain rate imaging for Noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr. 2005;18:729–36. doi: 10.1016/j.echo.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JD, Adams DB, Devries S, et al. Guidelines and recommendations for digital echocardiography. J Am Soc Echocardiogr. 2005;18:287–97. doi: 10.1016/j.echo.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Lutas ME, Casale PN. Standardization of M-mode echocardiographic measurements. JACC. 1984;4:1222–30. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 13.Issaz K, Munoz del Romeral L, Lee E. Quantization of the motion of the cardiac base in Cal subjects by Doppler echocardiography. J Am Soc Echocardiogr. 1993;6:166–76. doi: 10.1016/s0894-7317(14)80487-2. [DOI] [PubMed] [Google Scholar]
- 14.Oki T, Tabata T, Yamada H. Clinical application of pulsed Doppler tissue imaging for assessing abCal left ventricular relaxation. Am J Cardiol. 1997;79:921–8. doi: 10.1016/s0002-9149(97)00015-5. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh S, Middleton K, Koplen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 16.D'hooge J, Heimdal A, Jamal F. Regional strain and strain rate measurements by cardiac ultrasound principles, implementation and limitations. Eur J Echocardiography. 2000;1:154–70. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 17.Pislaru C, Abraham TP, Belohlavek M. Strain and strain rate echocardiography. Curr Opin Cardiol. 2002;17:443–54. doi: 10.1097/00001573-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Weathrall DJ, Clegg JB. The Thalassemia Syndromes. Oxford, UK: Blackwell Scientific Publications; 1981. [Google Scholar]
- 19.Cohen AR. Management of iron everload in the pediatric patient. Hematol Oncol Clin North Am. 1987:521–521. [PubMed] [Google Scholar]
- 20.Engle MA, Erlandson ST, Smith CH. Late cardiac complications of chronic, severe, refrectory anemia with hemochromatosis. Circulation. 1964;30:689–689. doi: 10.1161/01.cir.30.5.698. [DOI] [PubMed] [Google Scholar]
- 21.Grisaru D, Rachmilewitz FA, Mosseri M. Cardiopulmonary assessment in β-thalassemia major. Chest. 1990;98:1138–1138. doi: 10.1378/chest.98.5.1138. [DOI] [PubMed] [Google Scholar]
- 22.Kremastinos DTh, Tiniakos G, Theodorakis GN. Myocarditis in β-thalassemia major: A cause of heart failure. Circulation. 1995;91:209–209. doi: 10.1161/01.cir.91.1.66. [DOI] [PubMed] [Google Scholar]
- 23.Inati A, Zeineh N, Isma'ell H. β-thalassemia: the Lebanese experience. Clin Lab Haematol. 2006;28:217–27. doi: 10.1111/j.1365-2257.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 24.Brittnham GM, Griffith PM, Nienhius AW. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994:331–567. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 25.Olivieri NF, Nathan DG, Lesser ML. Survival of medically treated patients with homozygous β-thalassemia. N Engl J Med. 1994;331:574–574. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 26.Daar S, Pathare V. Combined therapy with desferrioxamine and deferiprone in β-thalassemia major. Patients with trasfusional iron overload. Ann Hematol. 2006;85:315–9. doi: 10.1007/s00277-005-0075-z. [DOI] [PubMed] [Google Scholar]
- 27.Aldouri MA WB, Hoffbrand AV, Flynn DM. High incidence of cardiomyopathy in beta-thalassemia patients receiving transfusion and iron chelation: reversal by intensified chelation. Acta Haematol. 1990;84:113–113. doi: 10.1159/000205046. [DOI] [PubMed] [Google Scholar]
- 28.Marcus RE, Davies SC, Bantock HM. Desferrioxamine to improve cardiac function in iron-overloaded patients with thalassemia major. Lancet. 1984;1:392–392. doi: 10.1016/s0140-6736(84)90439-2. [DOI] [PubMed] [Google Scholar]
- 29.Aldouri MA, Wonke B, Hoffbrand AV. Iron state and hepatic disease in patients with thalassaemia major treated with long-term subcutaneous desferoxamine. J Clin Pathol. 1987;40:1352–1352. doi: 10.1136/jcp.40.11.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen A, Mizanin J, Schwartz E. Treatment of iron overload in Colley's anemia. Ann NY Acad Sci. 1985;445:374–374. doi: 10.1111/j.1749-6632.1985.tb17197.x. [DOI] [PubMed] [Google Scholar]
- 31.Cecchetti G, Binda A, Piperno A. Cardiac alterations in 36 consecutive patients with idiopathic haemochromatosis: polygraphic and echocardiografic evaluation. Eur Heart J. 1991;12:224–224. doi: 10.1093/oxfordjournals.eurheartj.a059873. [DOI] [PubMed] [Google Scholar]
- 32.Benson L, Liu P, Olivieri N. Left ventricular function in young adults with thalassemia. Circulation. 1984;80:274–274. [Google Scholar]
- 33.Iarussi D, Di Salvo G, Pergola , et al. Pulsed Doppler tissue imaging and myocardial function in thalassemia major. Heart Vessels. 2003;18:1–6. doi: 10.1007/s003800300000. [DOI] [PubMed] [Google Scholar]
- 34.Chrysohoou C, Greenber M, Ptsavos C, et al. Diastolic function in young patients with Beta-thalassemia major: an echocardiographic study. Echocardiography. 2006;23:38–44. doi: 10.1111/j.1540-8175.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Wang M, Fung JW, et al. Atrial strain rate echocardiography can predict success or failure of cardioversion for atrial fibrillation: a combined transthoracic tissue Doppler and transoesophageal imaging study. Int J Cardiol. 2006:3–3. doi: 10.1016/j.ijcard.2006.01.051. [DOI] [PubMed] [Google Scholar]