Abstract
The impact of left ventricular hypertrophy (LVH) on survival among patients with established coronary artery disease (CAD) is not well understood. We sought to evaluate the effect of LVH on the survival of patients with CAD following percutaneous coronary intervention (PCI). Three hospitals in New York City contributed prospectively defined data on 4284 consecutive patients undergoing PCI. Allcause mortality at a mean follow-up of three years was the primary endpoint. LVH was present in 383 patients (8.9%). LVH patients had a greater prevalence of hypertension (88% vs. 68%, p<0.001), vascular disease (21% vs. 6.6%, p=0.001), and prior heart failure (10% vs. 5.5%, p<0.001). LVH patients presented less often with one-vessel disease (38% vs. 50%, p=0.040) and more often with two- (34% vs. 29%, p=0.014) or three-vessel (22% vs. 18%, p=0.044) disease. Ejection fractions and angiographic success were similar in both groups. In-hospital mortality did not differ between groups. At three-year follow-up, the survival rate for patients with LVH was 86% vs. 91% in patients without LVH (log-rank p=0.001). However, after adjustment for differences in baseline characteristics using Cox proportional hazards analysis, LVH was found not to be an independent predictor of mortality (hazard ratio, 0.93; 95% confidence interval, 0.68–1.28; p=0.67). We conclude that LVH at the time of PCI is not independently associated with an increase in the hazard of death at three years.
Key words: angioplasty, stent, left ventricular hypertrophy, survival.
Introduction
It is well documented that left ventricular hypertrophy (LVH) detected by 12-lead electrocardiography or echocardiography is a significant risk factor for cardiovascular morbidity and mortality in cohorts ranging from the general population to those with established cardiovascular disease, including coronary artery disease (CAD).1–10 However, the impact of LVH on survival among patients with established CAD following revascularization is largely unknown. The current study was designed to evaluate the effect of LVH on long-term outcomes of patients with CAD following revascularization with percutaneous coronary intervention (PCI).
Patients and Methods
Patient population
The study population was a cohort of 4284 consecutive patients undergoing PCI from January 1, 1998 to October 1, 1999 at three hospitals in New York City.
Clinical data
Prospectively defined data elements were contributed to a central coordinating center for analysis. Data elements included information on demographics, comorbidities, procedural details, complications, and in-hospital outcomes. The same data elements are required to be submitted to the Department of Health on every PCI performed in New York State to make up the Coronary Angioplasty Reporting System database.
Definitions
LVH was defined by physician assessment of the pre-PCI ECG or by echocardiography in documented cases of left bundle branch block and/or pacing. Diabetes included treatment with oral hypoglycemic agents or insulin. Intravenous glycoprotein (GP) IIb/IIIa inhibitors were administered when abciximab, eptifibatide, or tirofiban were given during or within three hours following PCI. The diagnosis of peri-procedural myocardial infarction (MI) required new Q-waves and a rise in creatine kinase to at least 2.5 times the upper limit of normal occurring within 24 hours of the PCI. Heparin therapy indicated treatment with intravenous heparin within 48 hours before the PCI. Nitroglycerin treatment indicated therapy with intravenous nitroglycerin within 24 hours of the procedure for ongoing ischemia or left ventricular failure. Ventricular arrhythmias were those that occurred within seven days of the PCI requiring electrical are pharmacological treatment excluding episodes that occurred within the first 24 hours of an MI. Angiographic success was a reduction of the treated lesion by at least 20% with a residual stenosis of less than 50%.
Percutaneous coronary intervention
All procedural decisions, including device selection and adjunctive pharmacotherapy, were made at the discretion of the individual physician performing PCI. All stents were bare metal. Deployment was at high pressure and patients were maintained on aspirin indefinitely, and ticlopidine or clopidrogel for four weeks following their PCI unless contraindicated. Angiographic assessments were made at the individual hospital by visual assessment.
Endpoints
The primary endpoint was all-cause mortality following discharge from the hospital for the index PCI as determined from the Social Security Death Index. This index has been shown to be highly specific and unbiased.11,12 Follow-up was for a mean of three years.
Statistical analysis
Categorical variables were compared by χ2-analyses. Continuous variables are presented as mean ± SD and were compared using the Student's t-test. All probability values are twotailed. Statistical significance was defined as p<0.05 or confidence intervals that did not include 1.0. Survival curves were constructed by the Kaplan-Meier method with differences in survival assessed with the log-rank test. LVH was related to all-cause mortality using multivariable Cox proportional hazard regression analyses to adjust for differences in baseline characteristics. Potential confounders were entered into models if they were clinically relevant or showed univariable differences between groups with a p<0.10. All analyses were performed using the SPSS (Chicago, IL, USA) statistical analysis program.
Results
Baseline characteristics
LVH was identified in 383 (8.9%) of 4284 patients who underwent PCI for CAD. Baseline characteristics of patients are presented in Table 1. Patients with LVH were older (66 vs. 63 years, p=0.001) and less often characterized as white (66% vs. 79%, p<0.001). LVH patients had a lower prevalence of smoking (9.4% vs. 14%, p=0.019) but greater prevalence of hypertension (88% vs. 68%, p<0.001), diabetes (31% vs. 26%, p=0.041), vascular disease (21% vs. 6.6%, p<0.001), creatinine of >2.5 mg/dL, (3.7% vs. 1.7%, p=0.009), dialysis (3.9% vs. 1.5%, p<0.001), and prior heart failure (10% vs. 5.5%, p<0.001).
Table 1. Baseline characteristics.
| LVH | No LVH | p | |
|---|---|---|---|
| (N=383) | (N=3901) | ||
| Demographics | |||
| Mean age ± SD (years) | 66.6±11.4 | 63.1±11.9 | <0.001 |
| Age > 75 years (%) | 27 | 18 | <0.001 |
| Female (%) | 35 | 31 | 0.06 |
| White (%) | 66 | 79 | <0.001 |
| Body mass index ± SD | 28.1±5.8 | 28.6±6.0 | 0.13 |
| Clinical history (%) | |||
| Obesity | 30 | 33 | 0.14 |
| Smoking | 9.4 | 14 | 0.02 |
| Hypertension | 88 | 68 | <0.001 |
| Diabetes | 31 | 26 | 0.04 |
| Stroke | 0.3 | 0.1 | 0.51 |
| Vascular disease | 21 | 6.6 | <0.001 |
| Creatinine >2.5 mg/dL | 3.7 | 1.7 | 0.009 |
| Dialysis | 3.9 | 1.5 | <0.001 |
| Cardiac history (%) | |||
| Previous CHF | 10 | 6 | <0.001 |
| Prior MI | 37 | 35 | 0.45 |
| Previous cardiac surgery | 18 | 17 | 0.74 |
| Previous PCI | 25 | 26 | 0.84 |
| Clinical presentation (%) | |||
| CHF | 12 | 4.3 | <0.001 |
| MI <6 hours | 2.6 | 4 | 0.17 |
| MI <24 hours | 4.2 | 6.5 | 0.08 |
| Unstable angina | 45 | 42 | 0.27 |
| Heparin <48 hours | 31 | 29 | 0.46 |
| Nitroglycerin <24 hours | 16 | 13 | 0.09 |
| Ventricular arrhythmia | 2.1 | 1.9 | 0.82 |
| Thrombolysis <6 hours | 0.3 | 0.7 | 0.32 |
| Thrombolysis >6 hours | 0.8 | 3.4 | 0.005 |
| Shock | 2.6 | 2.5 | 0.88 |
LVH, left ventricular hypertrophy; CHF, congestive heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Procedural characteristics
Angiographic and procedural characteristics are depicted in Table 2. LVH patients presented less often with one-vessel disease (38% vs. 50%, p=0.04) and more often with two-vessel (34% vs. 29%, p=0.01) and three-vessel disease (22% vs. 18%, p=0.04). Mean ejection fraction was lower in LVH patients (49% vs. 51%, p=0.01). Stent placement did not differ in either group. Angiographic success was similar in both groups (98% vs. 97%, p=0.27).
Table 2. Angiographic and procedural characteristics.
| LVH | No LVH | p | |
|---|---|---|---|
| (N=383) | (N=3901) | ||
| Mean ejection fraction ± SD (%) | 51 | 51 | 0.70 |
| One-vessel CAD (%) | 38 | 50 | <0.001 |
| Two-vessel CAD (%) | 34 | 29 | 0.02 |
| Three-vessel CAD (%) | 22 | 18 | 0.04 |
| IABP (%) | 1.8 | 1.3 | 0.43 |
| Stent (%) | 74 | 77 | 0.13 |
| GP IIb/IIIa inhibitor (%) | 21 | 24 | 0.23 |
| Angiographic success (%) | 98 | 97 | 0.27 |
| Atherectomy (%) | 15 | 13 | 0.41 |
LVH, left ventricular hypertrophy; SD, standard deviation; CAD, coronary artery disease; IABP, intra-aortic balloon pump; GP, glycoprotein.
Outcomes
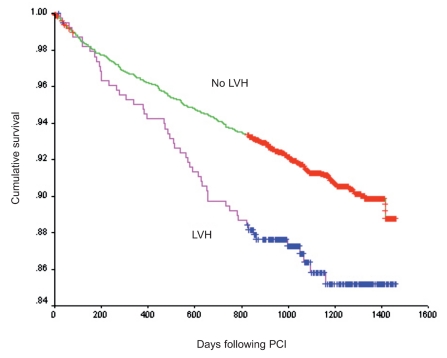
Adverse outcomes following PCI are depicted in Table 3. In-hospital adverse outcomes including death, post-PCI MI, and stent thrombosis were not different between groups. There was a greater rate of emergency bypass surgery in the LVH group (0.5% vs. 0.1%, p=0.004). The mortality at a mean follow-up of three years for patients with LVH was 14% vs. 8.9% in patients without LVH (Figure 1) (log-rank p<0.001). The independent predictors of long-term mortality as determined by Cox proportional hazards analysis are presented in Table 4. LVH was not independently associated with an increase in the hazard of long-term mortality (hazard ratio, 0.93; 95% confidence interval, 0.68–1.28; p=0.67). Clinical characteristics associated with an increased hazard for long-term mortality were age, prior history of heart failure, vascular disease, diabetes, creatinine of >2.5 mg/dL, and dialysis.
Table 3. Adverse outcomes following percutaneous coronary intervention.
| LVH | No LVH | p | |
| (N=383) | (N=3901) | ||
| In-hospital death (%) | 0.8 | 0.4 | 0.34 |
| Emergency bypass surgery (%) | 0.5 | 0.1 | 0.004 |
| Myocardial infarction (new Q-waves) (%) | 0 | 0.2 | 0.41 |
| Myocardial infarction (no new Q-waves) (%) | 1.6 | 1.5 | 0.93 |
| Vascular complication (%) | 0.1 | 0 | 0.48 |
| Abrupt closure (%) | 0.5 | 0.5 | 0.93 |
| Stent thrombosis (%) | 0.8 | 0.6 | 0.69 |
| Out-of-hospital death (%) | 14 | 9 | 0.002 |
LVH, left ventricular hypertrophy.
Figure 1.
Kaplan-Meier analysis of survival following percutaneous coronary intervention (log-rank p<0.001).
Table 4. Cox proportional hazards model for all-cause mortality.
| Variable | Hazard | 95 % Confidence intervals | p | |
|---|---|---|---|---|
| ratio | Lower | Upper | ||
| LVH | 0.93 | 0.68 | 1.28 | 0.67 |
| Age (per one-year increase) | 1.07 | 1.06 | 1.08 | <0.001 |
| Vascular disease | 1.42 | 1.07 | 1.89 | 0.01 |
| Diabetes | 1.47 | 1.18 | 1.84 | 0.001 |
| Previous CHF | 2.04 | 1.53 | 2.73 | <0.001 |
| One-vessel CAD | 0.58 | 0.40 | 0.83 | 0.003 |
| Two-vessel CAD | 0.69 | 0.48 | 0.99 | 0.048 |
| Creatinine of >2.5 mg/dL | 3.41 | 2.38 | 4.90 | <0.001 |
| Dialysis | 4.02 | 2.68 | 6.02 | <0.001 |
| Female | 0.74 | 0.60 | 0.93 | 0.008 |
LVH, left ventricular hypertrophy; CHF, congestive heart failure; CAD, coronary artery disease.
Discussion
The significant findings of this retrospective, observational study are two-fold. First, in an unselected population of patients undergoing PCI for CAD, LVH was associated with an increase in unadjusted three-year mortality. Second, after adjustment for differences in baseline characteristics, LVH was no longer associated with an increased hazard of death.
LVH has long been associated with an increased risk of cardiovascular mortality.1–8 However, there is a paucity of data regarding its impact in patients with CAD and even less information on the effect of LVH on prognosis of patients with CAD following percutaneous revascularization. East and colleagues found echocardiographic LVH to be associated independently with a 56% increase in risk of three-year mortality among patients with CAD.13 However, only 37% of LVH patients underwent revascularization compared to 51% of patients without LVH despite more three-vessel disease in the LVH group. Likewise the Heart and Soul Study found that increased LV mass index determined by echocardiography in patients with CAD increased the risk for both all-cause mortality and sudden or arrhythmic death.9 Again in this study, although all patients had CAD, only 62% with LVH had been revascularized.
LVH may be associated with increased mortality by a number of mechanisms. Activation of the Renin-angiotensin system not only leads to LVH but also may promote the progression of atherosclerosis by the effects of angiotensin II on vasomotor tone, coagulation, and vascular smooth muscle cell proliferation.14–17 Additionally, LVH may predispose to arrhythmic death. Myocardial fibrosis occurs in LVH and may provide the substrate for reentrant ventricular arrhythmias.18–21
It is unclear why the current study found no detrimental effect of LVH on three-year adjusted mortality. One potential explanation is methodological. This database uses primarily the ECG for diagnosis of LVH, which is less sensitive for the detection of LVH than echocardiography.22,23 Thus, if the ECG were insensitive, patients with LVH would be incorrectly assigned to the group without LVH. In such a scenario, if LVH were truly an independent risk factor for mortality, assigning patients with LVH to the no-LVH group would minimize or eliminate any true differences in mortality. Alternatively, it is possible that certain elements of the medical therapy for secondary prevention following PCI, such as β-blockers and angiotensin converting enzyme inhibitors,24 induced regression of LVH, which has been shown to reduce mortality.25 Finally, it is possible that in the setting of CAD, the adverse effect of LVH on survival is magnified in the presence of untreated or unrecognized ischemia. In this scenario, revascularization would eliminate or reduce ischemia and thereby decrease the impact of LVH on mortality to an undetectable level in a cohort of this size.
Several limitations must be borne in mind when analyzing these findings. First, because of its nonrandomized retrospective nature, there may be unrecognized differences between patients with and without LVH in our study. One possible confounder is medication usage, which was impossible to control for because there were no data on medication use in the database. Second, there were no uniform ECG criteria for the diagnosis of LVH. Thus, different methods of LVH determination probably were used by different practitioners. Finally, this study was performed before the introduction of drug-eluting stents. However, it is unlikely that the exclusive use of bare metal stents would have impacted differentially on patients with and without LVH.
Conclusion
The current study demonstrates that, although LVH is a marker for increased mortality, it does not increase independently the hazard of mortality among patients with CAD following PCI.
References
- 1.Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–90. doi: 10.1016/s0735-1097(97)00493-2. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Salomon M, D'Agostino RB, et al. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–93. doi: 10.1161/01.cir.90.4.1786. [DOI] [PubMed] [Google Scholar]
- 3.Mathew J, Sleight P, Lonn E, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–21. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54. doi: 10.1161/01.cir.97.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 6.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 7.Liao Y, Cooper RS, McGee DL, et al. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592–7. [PubMed] [Google Scholar]
- 8.Schillaci G, Verdecchia P, Porcellati C, et al. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35:580–6. doi: 10.1161/01.hyp.35.2.580. [DOI] [PubMed] [Google Scholar]
- 9.Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study) Am J Cardiol. 2008;102:1131–5. doi: 10.1016/j.amjcard.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerhout CM, Lauer MS, James S, et al. Electrocardiographic left ventricular hypertrophy in GUSTO IV ACS: an important risk marker of mortality in women. Eur Heart J. 2007;28:2064–9. doi: 10.1093/eurheartj/ehm223. [DOI] [PubMed] [Google Scholar]
- 11.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131:160–8. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 12.Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4:233–7. doi: 10.1136/jamia.1997.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.East MA, Jollis JG, Nelson CL, et al. The influence of left ventricular hypertrophy on survival in patients with coronary artery disease: do race and gender matter? J Am Coll Cardiol. 2003;41:949–54. doi: 10.1016/s0735-1097(02)03006-1. [DOI] [PubMed] [Google Scholar]
- 14.Goldsmith SR, Hasking GJ, Miller E. Angiotensin II and sympathetic activity in patients with congestive heart failure. J Am Coll Cardiol. 1993;21:1107–13. doi: 10.1016/0735-1097(93)90232-p. [DOI] [PubMed] [Google Scholar]
- 15.Lyons D, Webster J, Benjamin N. Angiotensin II: adrenergic sympathetic constrictor actions in humans. Circulation. 1995;91:1457–60. doi: 10.1161/01.cir.91.5.1457. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan DA, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells: a potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feener EP, Norhtrup JM, Aiello LP, et al. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J Clin Invest. 1995;95:1353–62. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhury L, Mahrholdt H, Wagner A, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–64. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 19.Moon J, McKenna W, McCrohon J, et al. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–7. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 20.Spirito P, Bellone P, Harris K, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–85. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 21.Barbier CE, Bjerner T, Johansson L, et al. Myocardial scars more frequent than expected: magnetic resonance imaging detects potential risk group. J Am Coll Cardiol. 2006;48:765–71. doi: 10.1016/j.jacc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 22.Levy D, Labib SB, Anderson KM, et al. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–20. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 23.Woythaler JN, Singer SL, Kwan OL, et al. Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: comparison with postmortem mass measurements. J Am Coll Cardiol. 1983;2:305–11. doi: 10.1016/s0735-1097(83)80167-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith SC, Jr, Blair SN, Criqui MH, et al. Preventing heart attack and death in patients with coronary disease. Circulation. 1995;92:2–4. [PubMed] [Google Scholar]
- 25.Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2243–349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]