Introduction
Infective endocarditis caused by a zoonotic micro-organism is a rare clinical condition.
Capnocytophaga canimorsus is a bacterium of the normal oral flora of dogs and cats. It can be transmitted to humans by bite, scratch or others close contacts with dogs, and occasionally cats. It has been implicated in a spectrum of disorders including septicemia, meningitis and septic arthritis. Splenectomy and alcoholism have been reported to be risk factors, but infection may occur in previously healthy patients.
We reported the case of a 65-year-old man with bicuspid aortic valve endocarditis and multiple abscesses of the aortic wall caused by the canine bacteria Capnocytophaga canimorsus. This case highlights the capacity of Capnocytophaga canimorsus to produce endocarditis whose incidence may be underestimated due to slow growth and incorrect identification of the organism.
Case report
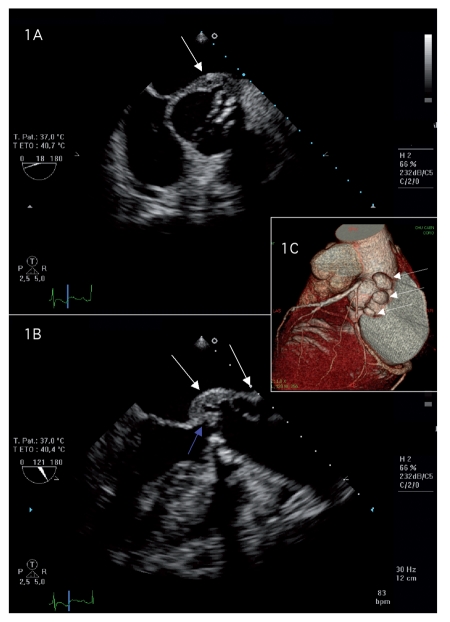
A 65-year-old man was referred to our hospital for suspicion of endocarditis. His medical history included a cardiac follow-up for asymptomatic significant aortic stenosis. He adopted an antibiotic prophylaxis by amoxicillin (3g per os in the hour before a risk procedure). Cardiovascular risk factors were nicotinism, dyslipidemia and hypertension. He did not present other relevant pathologies. For two weeks, the patient presented intermittent fever associated with major asthenia. On initial examination, findings were fever at 39°C, tachycardia and 3/6 systolic murmur in the aortic area. Laboratory investigation revealed increased inflammatory indexes (C reactive protein: 175 ng/mL; white blood cells: 19×109/L/mm3 with 85% neutrophils), normocytic anemia (10.5 g/dL), renal insufficiency (creatininemia: 150 micromoles/L, clearance: 43 mL/min). Transthoracic echocardiogram revealed a high suspicion of aortic endocarditis with vegetation. As the aortic valve was calcified and given the echogenicity, the analysis of the lesion was limited and not optimal. We then realized a transoesophageal echocardiogram for better delineation of the pathology and to exclude an extension to the other valves. Transoesophageal echocardiography showed a calcified bicuspid aortic valve with a vegetation on the posterior hemivalve (8 × 9 mm) and large abscesses affecting left coronary sinus, and the aortic root (Figure 1A and B). A multidetector computed tomographic angiography (MCTA) confirmed on 3D volume rendering showed three additional images on the aortic root, behind the main left coronary artery suggesting mycotic aneurysm or abscesses (Figure 1C). There was no significant lesion on the coronary arteries. Blood cultures were negative. The patient started an empirical parenterally antibiotic therapy with gentamycin (160 mg/24 h) plus ampicillin (12 g/24 h then 6 g/24 h after deterioration of creatinin clearance secondary to gentamycin and contrast perfusion during MTCA). He rapidly became afebrile and the inflammatory syndrome decreased. After 25 days, three anaerobia sets of blood cultures (chocolate agar plate incubated in a carbon dioxide-enriched environment) grew a Gram negative bacillus, oxydase and catalase positive, that was identified as Capnocytophaga species. The identification of Capnocytophaga canimorsus was made using 16S rDNA gene sequencing. The patient revealed he had four dogs at home. He did not report any bite but he made a habit of kissing these dogs. The patient underwent aortic valve and root replacement (intervention of Bentall) with the implantation of a valve conduit (Carbomedics n. 23). Intraoperatively, the surgical view confirmed the existence of three abscesses located in the left coronary sinus around the left coronary ostium and corroborated the ultra sound and MCTA findings. The patient had an uneventful post-operative recovery. He was discharged after a total of six weeks antibiotic treatment based on susceptibility test (organism sensitive for ampicillin).
Figure 1.
Transoesophageal echocardiography short-axis view demonstrated calcified bicuspid aortic valve with a paravalvular abscess (A). A vegetation on the posterior hemivalve (blue arrows) and a large abscess affecting left coronary sinus and the aortic root (white arrows) was documented from the transoesophageal echocardiography long-axis view (B). The multidetector computed tomographic angiography demonstrated three images on the aortic root, behind the main left coronary artery suggesting mycotic aneurysm or abscesses (white arrows) (C).
Discussion
Capnocytophaga canimorsus is a commensal gram negative bacillus living in the saliva of dogs and cats. It is known to cause severe infection, mainly septicemia, meningitis, septic arthritis, and more rarely, infective endocarditis with less than 15 cases in the published literature since its identification in 1976.1 Capnocytophaga canimorsus is transmitted though bites from dogs (rarely cats) and close contacts with animals as in our observation. Significant risk factors for Capnocytophaga canimorsus infection are immunocompromised states including asplenism or chronic alcoholism.2 However, 40% of septicemia occurred in patients with no classical predisposing conditions.3 For infective endocarditis, underlying cardiac condition at risk for endocarditis seems to be the most important risk factor. One ethird of cases of endocarditis had pre-existing cardiac lesions including mitral leaflet prolapse, aortic stenosis, mechanical valve, myxoma, heart murmure.4 Isolation of this slow-growing bacterium is difficult. Blood cultures (aerobic or anaerobic flasks) may be negative or the latter positive. The use of specific culture media (agar chocolate, brain heart infusion), CO2 and enriched atmosphere enhanced growth. PCR performed on the valve was recently used with success for the diagnosis of Capnocytophaga canimorsus endocarditis.5 In the case of culture negative endocarditis, it is important to carefully research animal contact and to inform the microbiology laboratory of the possibility of unusual bacteria such as Capnocytophaga canimorsus. There is no consensus about the choice of antibiotics and the length of treatment.6 Antibiotic therapies are often initiated empirically as Capnocytophaga canimorsus are slow growing bacteria. Most patients are successfully treated with broadspectrum antibiotics, as many strains are still susceptible to all antibiotics. High dose of β lactams remains a treatment of choice7,8 also there is a high rate of resistance to these. The efficacy of antibiotic therapy, must be confirmed after isolation and in vitro susceptibility testing of the strain. The difficulty in diagnosing Capnocytophaga canimorsus endocarditis may delay diagnosis and appropriate treatment and may explain the high mortality rate of 25% in spite of its low virulence.4 In conclusion, Capnocyto phaga canimorsus produces rarely reported endocarditis whose incidence may be underestimated, considering its failure to grow on standard media. The combination of animal contact, underlying disease or predisposition to endocarditis and apparently culture negative endocarditis should raise the suspicion of Capnocytophaga canimorsus infection.
References
- 1.Bobo RA, Newton EJ. A previously undescribed gram-negative bacillus causing septicemia and meningitis. Am J Clin Pathol. 1976;65:564–9. doi: 10.1093/ajcp/65.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Kooter AJ, Derks A, Vasmel WL. Rapidly progressive tricuspid valve endocarditis caused by Capnocytophaga canimorsus infection in an immunocompetent host. Clin Microbiol Infect. 1999;5:173–5. doi: 10.1111/j.1469-0691.1999.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol. 1996;12:521–33. doi: 10.1007/BF00144007. [DOI] [PubMed] [Google Scholar]
- 4.Sandoe JA. Capnocytophaga canimorsus endocarditis. J Med Microbiol. 2004;53:245–8. doi: 10.1099/jmm.0.05274-0. [DOI] [PubMed] [Google Scholar]
- 5.Wareham DW, Michael JS, Warwick S, et al. The dangers of dog bites. J Clin Pathol. 2007;60:328–9. doi: 10.1136/jcp.2006.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolivet-Gougeon A, Sixou JL, Tamanai-Shacoori Z, Bonnaure-Mallet M. Antimicrobial treatment of Capnocyto-phaga infection. Int J Antimicrob Agents. 2007;29:367–73. doi: 10.1016/j.ijantimicag.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Krol-van Straaten MJ, Landheer JE, de Maat CEM. Capnocytophaga canimorsus (formerly DF-2) infections: review of the literature. Neth Jmed. 1990;36:304–9. [PubMed] [Google Scholar]
- 8.Baddour LM, Wilson WR, Bayer AS, et al. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]