Abstract
Real-time three-dimensional transesophageal echocardiography (RT3DTEE) is now commonly used in daily clinical practice. The transesophageal, compared to the transthoracic approach, allows the visualization of the whole spectrum of the mitral valve apparatus and the posterior cardiac structures. Moreover, images obtained by RT 3D TEE provide a unique and complete visualization of the mitral valve prosthetic elements. Indeed, the possibility to visualize guidewires and catheters in cardiac chambers and their relationship with cardiac structures during percutaneous transcatheter procedures reduces the time of radiation exposure and simplifies the approach becoming the reference method for monitoring. This review aims to underline the potential clinical applications and the advantages of RT3DTEE compared to other methods.
Key words: real-time transesophageal echocardiography
Introduction
The increasing technological development of three-dimensional echocardiography (3DE) in recent years represents one of the major innovative advancements in cardiovascular imaging. Transesophageal echocardiography (TEE) is now commonly used in routine clinical practice due to the significant advancements in probe, processors design and technology.1–2
In recent years, small 3D fully-sampled matrix array probes which can be installed on a gastroscope have been developed from the 3D transthoracic matrix probes. This combination, associated to the use of high frequencies, permits high quality 3D real-time images through a transesophageal approach overcome the limits of transthoracic acoustic windows.
This article aims to analyze the state of the art of Real-time Three-dimensional Transesophageal Echocardiography (RT3DTEE) regarding possible clinical applications, the proprieties and the advantages that this tool offers compared to RT3D with the transthoracic approach.
Technological background
RT3DTEE probe has a similar shape to a 2DTE probe 1.5 cm long, 1 cm thick and 4.5 cm wide with a cross-sectional area of 10×14 mm. It uses a new transducer technology with a matrix of over 2,500 crystals. The probe is connected to the echocardiograph with a dedicated software for 3D image acquisition. This allows both commonly used modalities (Mmode, pulsed and continuous wave Doppler, color Doppler, 2D images) and 3D specific ones (live 3D, zoom 3D, full-volume 3D and color 3D) to be obtained.
The 3D live acquisition mode allows acquisition with a narrow angle (30×60°) with a frame rate of 10–26 Hz. This acquisition modality is very useful to visualize and study structures of interest far from the ultrasound beam (i.e. vegetations).3 In zoom modality, a pyramidal data-set (from 20×20° to 90×90°) is generated with a low frame rate (<10 Hz) and temporal resolution. Images can be turned in all of the three axes and visualized from every angle and view. This acquisition modality is useful to study and visualize the structures near to the ultrasound beam (i.e. mitral valve, interatrial septum).3 In full-volume modality, a wide data-set is acquired in a pyramidal form with a wide-angle (from 65×56° to 102×105°) with a frame rate of 30–40 Hz. The data-set is generated by the acquisition of 4–7 sub-volumes obtained in consecutive cardiac beats using ECG synchronization and containing a wide volume of cardiac structures within. In this modality, the lateral resolution is smaller then real-time modality but it has an higher temporal resolution. Data-set can be processed off-line obtaining an unlimited number of sections of the interested area and the cardiac structures. We can use this acquisition modality to study cardiac structures placed near and far from the ultrasound beam. It is also possible to acquire color Doppler data-sets.3
However, RT3DTEE has some limitations represented by the “low temporal resolution” of the zoom modality and by the possible presence of tissue drop-out artefacts. This obviously represents a huge limitation in the study of structures with high motility such as vegetations and tendineous chordae, as well as drop-out artefacts which can be very frequent especially in patients with poor acoustic windows and suboptimal 2D images. These artefacts can be differentiated from true anatomic defects integrating the images obtained from several planes and finally with color images.3
Clinical application
Three dimensional echocardiography definitely has to be considered an integration of standard 2D echocardiography. Several studies demonstrate the concordance between RT3DTEE images and the real anatomy and the superiority of RT3DTEE over TE 2D.4–5
RT3DTEE provides high quality diagnostic volume-rendered images of the mitral valve apparatus and allows the study of the pathogenic features and the anatomical characteristics of the underlying diseases. At the same time, RT3DTEE provides very accurate images and diagnostic information of prosthetic mitral valve components,6,7 the interatrial septum and left atrial appendage. Interatrial septum is easy to recognize because of its location between the right and left atrium; it can, be studied with an optimal reconstruction and its visualization can simplify percutaneous procedures with better monitoring.
Mitral valve disease
Mitral valve evaluation represents one of the main application fields of 3D echocardiography. Mitral valve apparatus is composed of 6 different elements, each one with a specific function strictly connected to the other: the leaflets, the commissures, the ring, the tendineous chordae, the papillary muscles and the myocardial walls.
A careful evaluation of the function and anatomy of the single parts of the mitral valve apparatus must be performed in order to rule out underlying diseases.
RT3DTEE allows an excellent evaluation of all components of the mitral valve apparatus that can be appropriately evaluated with excellent diagnostic quality images. Even though it is considered a complementary method to transthoracic and transesophageal examination. Several studies suggest that RT3DTEE should be considered the gold standard in mitral valve disease evaluation and identify RT3DTEE as the reference diagnostic tool for surgical planning.4–6 The zoom mode and the full volume are the best acquisition modalities to obtain 3D data-set that includes the mitral valve apparatus for diagnostic analysis. Datasets can be cut according to x, y, z axes to obtain unlimited views of the valve.
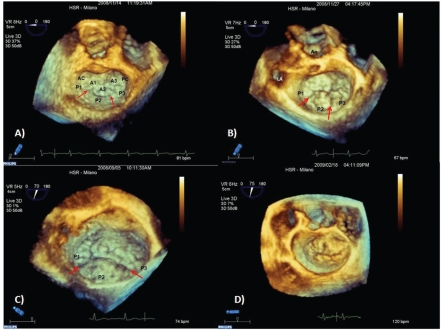
The “surgical view” of the mitral valve is a volume-rendering view obtained from an acquisition data-set in zoom or full-volume modality. It allows a good visualization of the leaflets, annulus and the surrounding structures similar to the surgeon's perspective during the operation. In this view, the observer is placed in the left atrium, looking from the left atrium with the settled image showing the aortic valve at eleven o' clock. In this view, the patient's medial and lateral sides correspond to operator's right and left sides while anterior and posterior leaflets appear in their right position (Figure 1). Evaluation of the subvalvular apparatus is improved by views obtained from full-volume data-sets and displayed in rendering modalities watching the valve from the ventricle. Moreover, we can also obtain quantitative parameters of the mitral valve apparatus (i.e. mitral annulus diameters, surface area of the leaflets) by specific dedicated software.
Figure 1.
Mitral valve en face volume-rendering. (A) Normal mitral valve. Anatomical variants of mitral valve prolapses. (B) Prolapse P2 and P3, that show similar surfaces. (C) Wide anterior and P2–P3 leaflet prolapse with a severe dilatation of the annulus. Anatomical P2 superface is more represented than P3. (D) Complex mixomatous lesion with a wide prolapse of anterior and posterior leaflets. P1,P2,P3: lateral, central and medial scallops of posterior leaflets divided by clefts (arrow). A1, A2, A3: lateral, central and medial segments of anterior leaflet. AC: antero-lateral commissure. PC: posteromedial commissure. LA: left appendage. AO: aorta.
Mitral valve regurgitation
The evaluation of a regurgitant mitral valve includes 3 main steps: i) quantification of the regurgitation; ii) identification of the mechanism; iii) identification of the culprit lesion. Each one of these steps can be faced up by 2D transthoracic and transesophageal approaches. Three dimensional RT has great potential in the study of the mechanism of regurgitation and in the identification of the culprit lesion. Transthoracic 3D-RT has already demonstrated its superiority over transthoracic 2D echocardiography and its non-inferiority over 2D/3D transesophageal echocardiography in the identification of the type 1 (erosion, perforation) and type 2 (flail, prolapse) mechanism; moreover, transthoracic 3DRT has high diagnostic accuracy in the identification of the scallop/ segment responsible for the pathological process.5–8
RT3DTEE is able to overcome the feasibility limitations due to the transthoracic acoustic window, thus providing higher quality diagnostic images than RT3D transthoracic echocardiography. Compared to the surgical findings, RT3DTEE demonstrated higher diagnostic accuracy in the correct identification of the scallop/segment involved in the pathological process.9 RT3DTEE is useful in the quantification of the prolapsed area or flail, in the identification of the anatomical abnormalities, and in the correct definition of the anatomical characteristics of complex mixomatous lesions (Figure 1).
Mitral stenosis
Transthoracic RT3D echocardiography is now commonly considered the gold standard in the evaluation and quantification of the anatomical area of a stenotic mitral valve. It is a complementary method to 2D echocardiography in the anatomical characterization of mitral valve apparatus and in its morphological and functional evaluation. Transthoracic 3D-RT is usually definitive for a full characterization of a stenotic mitral valve,10 while the RT3DTEE approach has to be considered as a secondary tool in the cases in which the transthoracic 3D approach is not conclusive.
Aortic valve disease
Good quality images of the aortic valve by RT3DTEE are obtained in 18–22% of patients.11 This is probably due to the distance between the transducer and the aortic valve, which is an anterior structure. Other reasons are the oblique incidence on the ultrasound beam, the poor tissue amount of the cusps and the presence of calcification which generates drop-out and attenuation artefacts.11 Potential fields of application are the morphological and functional characterization of the bicuspid aortic valve and the endocarditis (evaluation of erosions, cusps perforations, etc.).
Tricuspid valve disease
Tricuspid valve evaluation is not easy to perform using RT3DTEE. In only a small percentage of patients (11%), the tricuspid valve can be studied from the right atrium and ventricular perspective. The reasons for this are the same as those mentioned above for the aortic valve. Transthoracic 3D-RT has to be considered the best approach for a good characterization of tricuspid valve diseases.12
Prosthetics valve
RT3DTEE provides unique images and sections of mitral valve prosthetic elements overcoming the limits of the thoracic window that reduces the feasibility of the 3D transthoracic approach in the study of mitral valve prosthesis. As in native mitral valve studies, the zoom mode is considered the best modality of acquisition because it can include all prosthetic components within the pyramidal volume data-set. All prosthetic elements can be visualized from both atrial and ventricular perspectives. However, the visualization from the left atrium is preferred because it guarantees a whole evaluation of prosthesis.6
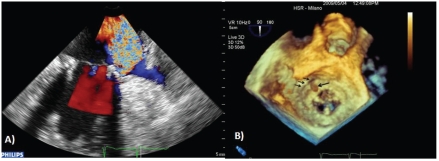
RT3DTEE provides additive details about the prosthetic dehiscence pathology, such as the localization, dimension, shape, and area of the leaks, due to its excellent capability in defining spatial relationships with the adjacent structures (Figure 2).7 This information is very useful in defining a surgery plan. Moreover, compared to transthoracic and transesophageal 2D echocardiography, RT3DTEE provides additive information regarding motility pathology due to prosthetic thrombosis and degenerative processes.
Figure 2.
(A) Two dimensional transesophageal color Doppler demonstrates the presence of severe paravalvular regurgitation on the lateral side. (B) En face volume rendering shows lateral side prosthetic dehiscence (arrows) and an endocarditis vegetation on the prosthesis ring in the region of the dehiscence (arrow).
Like native aortic and tricuspid valves, prostheses in these positions are not optimally visualized.
Percutaneous transcatheter procedure monitoring
Several cardiac pathologies are now commonly treated with a percutaneus interventional approach. The unique diagnostic tool traditionally used to monitor these procedures was fluoroscopy. However, fluoroscopy doesn't provide anatomical images of the cardiac structures, thus for this reason echocardiography became the most commonly used monitoring tool. Intracardiac and 2D transesophageal echocardiography are now commonly used in daily clinical practice.13–16 Recently, RT3DTEE has become the gold standard to monitor several non-coronary interventional procedures due to its capability in the 3D real time contemporary visualization of cardiac structures, catheters and devices. Moreover, RT3DTEE usually allows procedures monitoring in a single view without the need to move the ultrasound beam in order to obtain several 2D planes to visualize the components (cardiac structures and devices).17–18
Mitral valve disease
Percutaneous approaches for the treatment of mitral valve regurgitation have been recently developed but their application is still limited to high surgery risk patients. One of these approaches consists in the application of a clip at the tip of the mitral leaflets generating a percutaneous edge-to-edge and consequently a double mitral valve orifice.17 Transesophageal echocardiography monitoring is essential in several steps of the procedure: during the trans-septal puncture, for a correct alignment of the clip to the mitral valve leaflets and to place the arms of the clip perpendicular to the coaptation line, and before deployment in order to evaluate the residual regurgitant jet.3 RT3DTEE has been shown to be superior to 2D transesophageal echocardiography in the visualization of the wires and clips located in the left atrium and through the valve.3 Moreover, the whole wire length and the clip orientation to the mitral valve leaflets is perfectly visualized from the left atrium with just one view without any manipulation of the probe.3
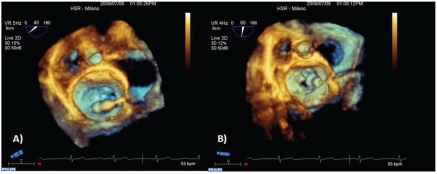
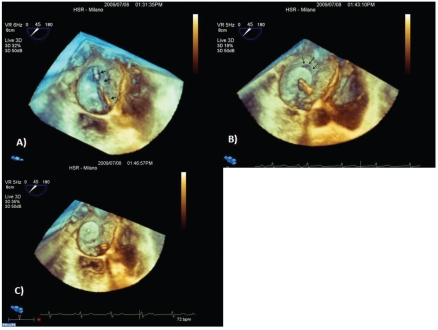
When valve anatomy is favorable, percutaneous balloon valvuloplasty represents the gold standard treatment for mitral valve stenosis. Transesophageal or transthoracic echocardiography monitoring is a decisive factor in guiding trans-septal puncture, the optimal balloon localization in the mitral valve orifice and to evaluate the final result or the possible complications occurring during the procedure. Recent experiences in this field demonstrate that the trans-septal puncture, the orientation of the wires and the correct placement of the balloon between the atrium and the mitral valve orifice are optimized by RT3DTEE3 (Figure 3). Finally, RT3DTEE allows an accurate evaluation of the efficacy of the procedure regarding the commissures split and the assessment of the presence of residual valve regurgitation.3–19
Figure 3.
Mitral valve balloon valvuloplasty. (A) Trans-septal path. Wire and balloon into the left atrium and its correct alignment towards the mitral valve orifice. (B) Good balloon positioning inside the mitral valve orifice.
Paravalvular leaks closure
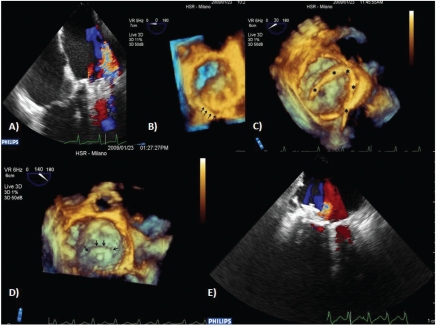
Prosthetic dehiscence is a complication that occurs in 2.5–5% of patients undergoing valve replacement.20 Recently, new closure devices and new percutaneous approaches for leak closure have been developed. RT3DTEE performed before the procedure is crucial in the evaluation of leak dimension, site and shape. All this information is essential to choose the most appropriate device and to plan the best approach. RT3DTEE performed during the procedure provides a high spatial resolution of the wires and the devices and their relationship with respect to the leak. This is due to the possibility to obtain images of the prosthesis from the left atrium, lowering the acoustic shadow generated by the artificial valve (Figure 4).3–21
Figure 4.
(A) Transesophageal color Doppler shows the postero-lateral paraprosthetic mitral valve regurgitation. (B) En face volume rendering shows an area of partial dehi-scence in the posterior site. (C) Whole wire visualization (asterisks): trans-septal path, left atrium and good tip positioning in posterior site. (D) Occluder device deployment correctly placed in the dehiscence area (arrow). (E) Post-procedure color Doppler shows a small residual leak.
Percutaneous aortic valve replacement
Since its introduction, percutaneous aortic valve replacement has been increasingly used. During this procedure, RT3DTEE allows an accurate evaluation of cardiac anatomy, especially the left ventricular outflow tract, and the relationship between wires, stents and cardiac structures.3 At the end of the procedure, RT3DTEE helps confirm the correct positioning of the prosthesis and its normal function.
Left atrial appendage percutaneous closure
Left atrial appendage is a common source of embolism in patients with atrial fibrillation. Surgical or percutaneous obliteration have been proposed for patients with contraindications to anticoagulant therapy. Several devices have been developed for the percuteneous closure. However the efficacy and the long-term results of these devices are still unknown.22 Device advancement and deployment is monitored by fluoroscopy and transesophageal 2D echocardiography that is extremely important for the final result. It is also fundamental to verify the left appendage complete obliteration. The persistence of a residual communication between atrium and left atrial appendage can significantly increase the embolic risk because of the creation of a low flow in the chamber with a significant stasis within and consequently high probability of generating clots.23 RT3DTEE provides additional perspectives during the procedure since it can also show the spatial representation and the spatial relationships of the different structures, including the mitral valve, interatrial septum, left atrial appendage and wires.3–24 The en face visualization of the occluder during the deployment allows a unique visualization of the correct positioning and the left appendage exclusion. This can be confirmed with color Doppler which shows the eventual residual communication between atrium and appendage3 (Figure 5).
Figure 5.
(A) Catheter placed in the left atrium (arrows): trans-septal, left atrium and good left appendage positioning. (B) Periprosthetic leak (arrow) with a communication between atrium and left appendage before the definitive device deployment. (C) Definitive device repositioning; leak disappears, final device deployment.
Atrial septal defects
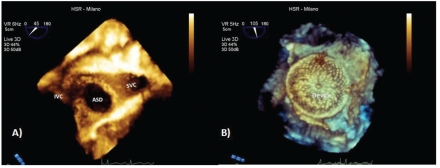
Atrial septal defects in eligible patients are commonly treated with percutaneous approaches. The feasibility of the percutaneous closure essentially depends on two main factors: i) the dimension of the defect and ii) the presence and the extension of residual rims. Two dimensional transesophageal examination is often enough to characterize the defect before the procedure and to support the intervention itself. However, a 3D en face reconstruction of the interatrial septum offers more diagnostic images showing the definition of residual edges, the shape, the position of the defect and the number of defects, than the standard 2D examination, due to the visualization of the spatial relationship with surrounding structures (Figure 6). In children, transthoracic approach is often sufficient and definitive, and the transesophageal approach is usually unnecessary. On the other side in adults, the transesophageal approach is always necessary and RT3DTEE has an additive value for the pre-procedure characterization than 2D standard examination. During the procedure, RT3DTEE allows a continuous visualization of the tip of the catheter, the device position during its expansion and final position.3–25 When the device is expanded and before its release, it's possible to evaluate with greater accuracy than 2D modality the correct position of the device and the residual shunt with color Doppler.
Figure 6.
(A) En face volume rendering from right atrium perspective. Central defect, circular in shape with complete representation of the residual rims. (B) En face volume rendering device reconstruction from the left atrium perspective that shows a good device positioning with complete defect closure. SVC: superior vena cava; IVC: inferior vena cava; ASD: atrial septal defect.
References
- 1.Pandian NG, Hsu TL, Schwartz SL, et al. Multiplane transesophageal echocardiography. Images planes, echocardiographic anatomy, and clinical experience with a prototype phased array OmniPlane probe. Echocardiography. 1992;9:649–66. doi: 10.1111/j.1540-8175.1992.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 2.Roelandt J, JR, Thomson IR, Vletter WB, et al. Multiplane tranesophageal echocardiography: latest evolution in an imaging revolution. J Am Soc Echocardiogr. 1992;5:361–7. doi: 10.1016/s0894-7317(14)80268-x. [DOI] [PubMed] [Google Scholar]
- 3.Perk G, Lang RM, Garcia-Fernandez MA, et al. Use of real time three-dimensional transesophageal echocardiography in intracardiac catheter based interventions. J Am Soc Echocardiogr. 2009;22:865–82. doi: 10.1016/j.echo.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Manda J, Kesanolla SK, Hsuing MC, et al. Comparison of real time two-dimensional with real/live three-dimensional transesophageal echocardiography in the evaluation of mitral valve prolapse and chordae rupture. Echocardiography. 2008;25:1131–7. doi: 10.1111/j.1540-8175.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 5.Eng MH, Salcedo EE, Quaife RA, Carroll JD. Implementation of real time three-dimensional transesophageal echocardiography in percutaneous mitral balloon valvuloplasty and structural heart disease interventions. Echocardiography. 2009;26:958–66. doi: 10.1111/j.1540-8175.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 6.Sugeng L, Shernan SK, Weinert L, et al. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008;21:1347–54. doi: 10.1016/j.echo.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Kronzon I, Sugeng L, Perk G, et al. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009;53:1543–7. doi: 10.1016/j.jacc.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 8.Agricola E, Oppizzi M, Pisani M, et al. Accuracy of real-time 3D echocardiography in the evaluation of functional anatomy of mitral regurgitation. Int J Cardiol. 2008;127:342–9. doi: 10.1016/j.ijcard.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 9.García-Orta R, Moreno E, Vidal M, et al. Three-dimensional versus two-dimensional transesophageal echocardiography in mitral valve repair. J Am Soc Echocardiogr. 2007;20:4–12. doi: 10.1016/j.echo.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Zamorano J, Cordeiro P, Sugeng L, et al. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004;43:2091–6. doi: 10.1016/j.jacc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Sugeng L, Shernan SK, Salgo IS, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol. 2008;52:446–9. doi: 10.1016/j.jacc.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Badano LP, Agricola E, Perez de Isla L, et al. Evaluation of the tricuspid valve morphology and function by transthoracic real-time three-dimensional echocardiography. Eur J Echocardiogr. 2009;10:477–84. doi: 10.1093/ejechocard/jep044. [DOI] [PubMed] [Google Scholar]
- 13.Zanchetta M, Maiolino P. Intracardiac echocardiography. Do we need a new ultrasonographic window? Ital Heart J. 2004;5:173–7. [PubMed] [Google Scholar]
- 14.Zanchetta M. On-line intracardiac echocardiography alone for Amplatzer Septal Occluder selection and device deployment in adult patients with atrial septal defect. Int J Cardiol. 2004;95:61–8. doi: 10.1016/j.ijcard.2003.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Kronzon I, Tunick PA, Schwinger ME, et al. Transesophageal echocardiography during percutaneous mitral valvuloplasty. J Am Soc Echocardiogr. 1989;2:380–5. doi: 10.1016/s0894-7317(89)80038-0. [DOI] [PubMed] [Google Scholar]
- 16.Mazic U, Gavora P, Masura J. The role of transesophageal echocardiography in transcatheter closure of secundum atrial septal defects by the Amplatzer septal occluder. Am Heart J. 2001;142:482–8. doi: 10.1067/mhj.2001.116770. [DOI] [PubMed] [Google Scholar]
- 17.Balzer J, Kelm M, Kühl HP. Real-time three-dimensional transoesophageal echocardiography for guidance of non-coronary interventions in the catheter laboratory. Eur J Echocardiogr. 2009;10:341–9. doi: 10.1093/ejechocard/jep006. [DOI] [PubMed] [Google Scholar]
- 18.Balzer J, Kühl H, Rassaf T, et al. Real-time transesophageal three-dimensional echocardiography for guidance of percutaneous cardiac interventions: first experience. Clin Res Cardiol. 2008;97:565–74. doi: 10.1007/s00392-008-0676-3. [DOI] [PubMed] [Google Scholar]
- 19.Grewal J, Mankad S, Freeman WK, et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr. 2009;22:34–41. doi: 10.1016/j.echo.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Jindani A, Neville EM, Venn G, Williams BT. Paraprosthetic leak: a complication of cardiac valve replacement. J Cardiovasc Surg. 1991;32:503–8. [PubMed] [Google Scholar]
- 21.Bavikati VV, Babaliaros VC, Lerakis S. Utility of three-dimensional echocardiography in percutaneous closure of paravalvular leak. Echocardiography. 2009;7:852–4. doi: 10.1111/j.1540-8175.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- 22.Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2009;7:855–8. doi: 10.1016/j.jacc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 23.Acar P, Saliba Z, Bonhoeffer P, et al. Influence of atrial septal defect anatomy in patient selection and assessment of closure with the Cardioseal device; a three-dimensional transoesophageal echocardiographic reconstruction. Eur Heart J. 2000;21:573–81. doi: 10.1053/euhj.1999.1855. [DOI] [PubMed] [Google Scholar]
- 24.Brinkman V, Kalbfleisch S, Auseon A, Pu M. Real time three-dimensional transesophageal echocardiography-guided placement of left atrial appendage occlusion device. Echocardiography. 2009;21:1362–8. doi: 10.1111/j.1540-8175.2009.00899.x. [DOI] [PubMed] [Google Scholar]
- 25.Lodato JA, Cao QL, Weinert L, et al. Feasibility of real-time three-dimensional transoesophageal echocardiography for guidance of percutaneous atrial septal defect closure. Eur J Echocardiogr. 2009;10:543–8. doi: 10.1093/ejechocard/jen337. [DOI] [PubMed] [Google Scholar]