Abstract
Our study evaluates the long-term effect of microalbuminuria on mortality among patients with acute myocardial infarction. We followed 151 patients from 1996 to 2007 to investigate if microalbuminuria is a risk factor in coronary heart disease. All patients admitted with acute myocardial infarction in 1996 were included. At baseline, we recorded urinary albumin/creatinine concentration ratio, body mass index, blood pressure, left ventricle ejection fraction by echocardiography, smoking status, medication, diabetes, age, and gender. Deaths were traced in 2007 by means of the Danish Personal Identification Register. Microalbuminuria, defined as a urinary albumin/creatinine concentration ratio above 0.65 mg/mmoL, occurred in 50% of the patients and was associated with increased all-cause mortality. Thus, 68% of the patients with microalbuminuria versus 48% of the patients without microalbuminuria had died during the 10 years of follow-up (P=0.04). The crude hazard ratio for death associated with microalbuminuria was 1.78 (CI: 1.18–2.68) (P=0.006), whereas the gender- and age-adjusted hazard ratio was 1.71 (CI: 1.03–2.83) (P=0.04). We concluded that microalbuminuria in hospitalized patients with acute myocardial infarction is prognostic for increased long-term mortality. We recommend measurement of microalbuminuria to be included as a baseline risk factor in patients with acute myocardial infarction and in future trials in patients with coronary heart disease.
Key words: acute myocardial infarction, microalbuminuria, risk factors, atherosclerosis, cardiovascular disease.
Introduction
A subclinical elevation of urinary albumin excretion, that is, microalbuminuria, has been associated with an increased risk of cardiac morbidity and mortality in reported population studies.1–5 Microalbuminuria was first introduced as a risk factor for chronic renal failure among patients with diabetes,6,7 and later was found to reflect systemic vascular damage.8,9 Furthermore, microalbuminuria was correlated with left ventricular wall thickness independent of blood pressure.10 Previously we reported an increased prevalence of microalbuminuria in patients with acute myocardial infarction.10 In the present report we have analyzed 10-year follow-up results in the previously studied cohort in order to further evaluate the association between the risk of death in patients with acute myocardial infarction and microalbuminuria.
Materials and Methods
In 1996, 250 Caucasian individuals were admitted to Hvidovre University Hospital, Department of Cardiology, with acute myocardial infarction. The diagnosis was based on the presence of chest pain, electrocardiographic alterations, and significant elevations of coronary enzymes. Patients who died during admission were excluded as were patients who underwent acute percutaneous coronary intervention or coronary artery bypass grafting, as such interventions would probably overrule any prognostic effect of microalbuminuria. In addition, patients with known renal or urinary tract disease were excluded. In total, 151 of the eligible patients could be included in the primary study,10 all of whom gave informed consent. The study was in accordance with the Helsinki II Declaration and approved by the local ethics committee.
On the day of discharge (about one week after admission), the patients provided an early morning urine specimen. Urinary albumin concentration was measured using an enzymelinked immunosorbent assay (ELISA).11 Urinary creatinine concentration was measured using an enzymatic colorimetric method. The albumin/creatinine concentration ratio was taken as an index of the albumin excretion rate in urine.12 In accordance with previous studies, microalbuminuria was defined as a urinary albumin/creatinine concentration ratio above 0.65 mg/mmoL.2 The left ventricle ejection fraction was estimated as a percentage by the wall motion index using the nine-segment model multiplied by 30.13 A Vingmed echocardiograph model CFM 750 (Norway) was used. Blood pressures were measured using a standard mercury sphygmomanometer and an appropriately sized cuff. The body mass index was calculated as weight divided by height squared (kg/m2). Information about smoking status, presence of diabetes, and medication at discharge were obtained from patient records. In 2007, the patients were traced by means of the Danish Personal Identification Register. Data are given as means, geometric means, or proportions with 95% confidence intervals. Differences in mean values between the groups were tested using the t-test for unpaired comparisons. The effect of microalbuminuria and other baseline variables on mortality was analyzed by the Cox proportional hazards regression analysis and expressed as a hazard ratio. All analyses were performed with the SPSS 14.0 computer package. A value of P<0.05 was considered significant.
Results
The baseline characteristics of patients with acute myocardial infarction with or without microalbuminuria are given in Table 1. The patients with microalbuminuria were older and had a lower body mass index. There were no statistically significant differences found in gender, systolic or diastolic blood pressure, left ventricle ejection fraction, smoking status, or diabetes. After a 10-year follow-up, 52% of the patients with normoalbuminuria were still alive, whereas only 32% of the patients with microalbuminuria were (Figure 1). The hazard ratio for death associated with microalbuminuria was 1.78 (1.18–2.68) (P=0.006). Adjusted for age and gender, microalbuminuria was associated with mortality with a hazard ratio of 1.71 (1.03–2.83) (P=0.04). As shown in Table 2, microalbuminuria was associated with a similar risk of death as a left ventricle ejection fraction below 40%. Conversely, diabetes, hypertension, smoking, or male gender had no impact on mortality, and high body mass index was associated with enhanced survival.
Table 1. Baseline characteristics in 151 patients with acute myocardial infarction with or without microalbuminuria (urine albumin/creatinine concentration ratio >0.65 mg/mmoL).
| Normoalbuminuria | Microalbuminuria | p | |
|---|---|---|---|
| (n=76) | (n=75) | ||
| Age (years) | 65 (63–68) | 73 (70–76) | <0.001 |
| Men† (%) | 70 (60–80) | 60 (49–71) | 0.24 |
| Systolic blood pressure (mmHg) | 129 (125–133) | 131 (127–135) | 0.51 |
| Diastolic blood pressure (mmHg) | 78 (75–81) | 76 (74–78) | 0.42 |
| Left ventricle ejection fraction‡ (%) | 50 (20–60) | 47 (20–60) | 0.19 |
| Body mass index (kg/m2) | 26.7 (25.7–27.7) | 24.6 (23.8–25.4) | 0.002 |
| Smokers† (%) | 42 (31–53) | 44 (33–55) | 1.00 |
| Diabetes patients† (%) | 11 (4–18) | 13 (5–21) | 0.63 |
| Urine albumin/creatinine* (mg/mmoL) | 0.35 (0.30–0.40) | 2.31 (1.73–3.08) | <0.001 |
Data are means
geometric means or
proportions with 95% confidence intervals. Left ventricle ejection fraction is shown by
medians with interquartile ranges.
Figure 1.
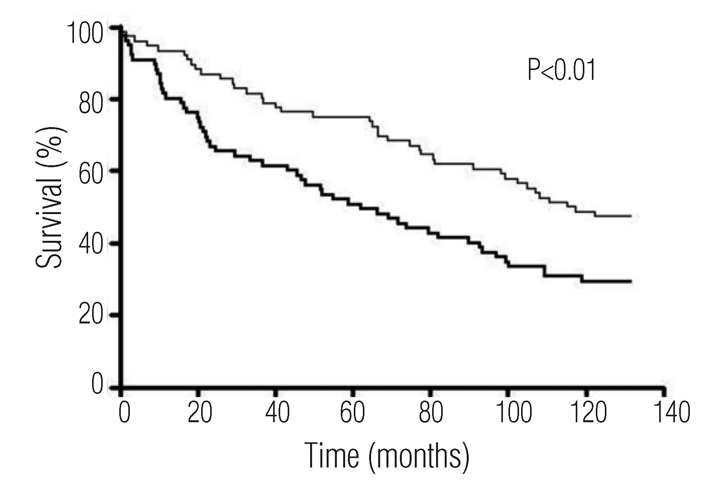
Unadjusted survival curves for patients with acute myocardial infarction and microalbuminuria (bold) or normoalbuminuria (thin). Relative risk of death associated with microalbuminuria versus normoalbuminuria = 1.78 (95 % CI, 1.18–2.68); P<0.01.
Table 2. Relative risks of ten-year mortality associated with risk factors measured during baseline admission in 151 patients with acute myocardial infarction.
| Baseline variable | Relative risk (hazard ratio) | P |
|---|---|---|
| Age >65 years | 3.06 (1.82–5.12) | <0.001 |
| Left ventricle ejection fraction <40% | 1.80 (1.12–2.89) | 0.02 |
| Microalbuminuria | 1.78 (1.18–2.68) | 0.006 |
| Microalbuminuria* | 1.71 (1.03–2.83) | 0.04 |
| Diabetes | 1.22 (0.66–2.23) | 0.52 |
| Hypertension | 0.89 (0.55–1.44) | 0.64 |
| Smoking | 0.87 (0.58–1.32) | 0.51 |
| Male | 0.86 (0.57–1.31) | 0.49 |
| Obesity | 0.63 (0.42–0.96) | 0.03 |
Microalbuminuria, urine albumin/creatinine concentration ratio >0.65 mg/mmoL; hypertension, systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg; obesity, body mass index >25 kg/m2.
Adjusted for age and sex. Relative risks are shown with 95% confidence intervals in parentheses.
Discussion
Microalbuminuria is an established predictor of coronary heart disease in diabetic patients as well as in non-diabetic healthy persons.2–4,11,14–19 In this study we confirm that microalbuminuria is strongly associated with an increased hazard of mortality in patients with acute myocardial infarction. Even though the study population consisted of 151 patients only, we were able to show that microalbuminuria significantly increases the risk of death, independently of age and gender. Thus, microalbuminuria is a very strong and robust risk indicator among patients with acute myocardial infarction. This observation confirms and extends previous observations by our group.20
The finding in our present study could likely be explained by more extensive vascular disease, for example, atherosclerosis, in patients with microalbuminuria. This is supported by other studies in which the severity of carotid atherosclerosis, measured ultrasonographically, was correlated with urinary albumin excretion.21–23 Moreover, the link between microalbuminuria and atherosclerosis is confirmed by the fact that half of the patients included in our study had microalbuminuria, as defined by a urinary albumin excretion above the upper 10% range in the background population.2 In our study we used spot urines, not timed urine collections. However, calculation of the albumin/creatinine ratio yields an acceptable measure of the urinary albumin excretion rate in terms of specificity and sensitivity when screening for microalbuminuria.12
The definition of microalbuminuria in diabetology was based originally on the level of urinary albumin excretion above which the risk of chronic renal failure, but not of atherosclerotic cardiovascular disease, was increased;24 that is, an albumin excretion rate above 30 mg/day or an albumin/creatinine ratio above 2 mg/mmoL. In addition, it has become evident that the risk of atherosclerotic cardiovascular disease is increased at even lower levels of urinary albumin excretion in diabetic as well as non-diabetic subjects.17,25–27 We used our previous definition of microalbuminuria, for example, a urinary albumin/ creatinine ratio above 0.65 mg/mmoL, which in non-diabetic subjects independently increases the risk of atherosclerotic cardiovascular disease without additional gain in risk with increasing values.2,28
We concluded that microalbuminuria is associated with impaired survival in patients hospitalized with myocardial infarction. Microalbuminuria may be useful for risk stratification in these patients and, moreover, should be included as a baseline variable in intervention trials.
References
- 1.Kuusisto J, Mykkanen L, Pyorala K, et al. Hyperinsulinemic microalbuminuria. A new risk indicator for coronary heart disease. Circulation. 1995;91:831–7. doi: 10.1161/01.cir.91.3.831. [DOI] [PubMed] [Google Scholar]
- 2.Borch-Johnsen K, Feldt-Rasmussen B, Strandgaard S, et al. Urinary albumin excretion. An independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol. 1999;19:1992–7. doi: 10.1161/01.atv.19.8.1992. [DOI] [PubMed] [Google Scholar]
- 3.Jager A, Kostense PJ, Ruhe HG, Heine RJ, et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:617–24. doi: 10.1161/01.atv.19.3.617. [DOI] [PubMed] [Google Scholar]
- 4.Jensen JS, Feldt-Rasmussen B, Strandgaard S, et al. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35:898–903. doi: 10.1161/01.hyp.35.4.898. [DOI] [PubMed] [Google Scholar]
- 5.Gall MA, Borch-Johnsen K, Hougaard P, et al. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44:1303–9. doi: 10.2337/diab.44.11.1303. [DOI] [PubMed] [Google Scholar]
- 6.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–60. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 7.Viberti GC, Hill RD, Jarrett RJ, et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–2. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 8.Feldt-Rasmussen B. Increased transcapillary escape rate of albumin in type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1986;29:282–6. doi: 10.1007/BF00452063. [DOI] [PubMed] [Google Scholar]
- 9.Nannipieri M, Rizzo L, Rapuano A, et al. Increased transcapillary escape rate of albumin in microalbuminuric type II diabetic patients. Diabetes Care. 1995;18:1–9. doi: 10.2337/diacare.18.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Taskiran M, Feldt-Rasmussen B, Jensen GB, et al. Urinary albumin excretion in hospitalized patients with acute myocardial infarction. Prevalence of microalbuminuria and correlation to left ventricle wall thickness. Scand Cardiovasc J. 1998;32:163–6. doi: 10.1080/14017439850140139. [DOI] [PubMed] [Google Scholar]
- 11.Feldt-Rasmussen B, Dinesen B, Deckert M. Enzyme immunoassay: an improved determination of urinary albumin in diabetics with incipient nephropathy. Scand J Clin Lab Invest. 1985;45:539–44. doi: 10.3109/00365518509155256. [DOI] [PubMed] [Google Scholar]
- 12.Jensen JS, Clausen P, Borch-Johnsen K, et al. Detecting microalbuminuria by urinary albumin/creatinine concentration ratio. Nephrol Dial Transplant. 1997;12:6–9. [PubMed] [Google Scholar]
- 13.Heger JJ, Weyman AE, Wann LS, et al. Cross-sectional echocardiographic analysis of the extent of left ventricular asynergy in acute myocardial infarction. Circulation. 1980;61:1113–8. doi: 10.1161/01.cir.61.6.1113. [DOI] [PubMed] [Google Scholar]
- 14.Sukhija R, Aronow WS, Kakar P, et al. Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitus. Am J Cardiol. 2006;98:279–81. doi: 10.1016/j.amjcard.2006.01.098. [DOI] [PubMed] [Google Scholar]
- 15.Culleton BF, Larson MG, Parfrey PS, et al. Proteinuria as a risk factor for cardiovascular disease and mortality in older people: a prospective study. Am J Med. 2000;109:1–8. doi: 10.1016/s0002-9343(00)00444-7. [DOI] [PubMed] [Google Scholar]
- 16.Hillege HL, Fidler V, Diercks GF, et al. Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 17.Damsgaard EM, Froland A, Jorgensen OD, et al. Microalbuminuria as predictor of increased mortality in elderly people. Br Med J. 1990;300:297–300. doi: 10.1136/bmj.300.6720.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yudkin JS, Forrest RD, Jackson CA. Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet. 1988;2:530–3. doi: 10.1016/s0140-6736(88)92657-8. [DOI] [PubMed] [Google Scholar]
- 19.Romundstad S, Holmen J, Kvenild K, et al. Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: a 4.4-year follow-up study. The nord-trondelag health study (HUNT), Norway. Am J Kidney Dis. 2003;42:466–73. doi: 10.1016/s0272-6386(03)00742-x. [DOI] [PubMed] [Google Scholar]
- 20.Klausen KP, Scharling H, Jensen JS. Very low level of microalbuminuria is associated with increased risk of death in subjects with cardiovascular or cerebrovascular diseases. J Intern Med. 2006;260:231–7. doi: 10.1111/j.1365-2796.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 21.Agewall S, Wikstrand J, Ljungman S, et al. Urinary albumin excretion is associated with the intima-media thickness of the carotid artery in hypertensive males with non-insulin-dependent diabetes mellitus. J Hypertens. 1995;13:463–9. [PubMed] [Google Scholar]
- 22.Bigazzi R, Bianchi S, Nenci R, et al. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens. 1995;9:827–33. [PubMed] [Google Scholar]
- 23.Mykkanen L, Zaccaro DJ, O'Leary DH, et al. Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects. The Insulin Resistance Atherosclerosis Study (IRAS) Stroke. 1997;28:1710–6. doi: 10.1161/01.str.28.9.1710. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen CE, Chachati A, Christensen CK, et al. Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest. 1985;9:85–95. doi: 10.3109/08860228509088195. [DOI] [PubMed] [Google Scholar]
- 25.MacLeod JM, Lutale J, Marshall SM. Albumin excretion and vascular deaths in NIDDM. Diabetologia. 1995;38:610–6. doi: 10.1007/BF00400732. [DOI] [PubMed] [Google Scholar]
- 26.Gorgels WJ, van der GY, Hjemdahl P, et al. Urinary excretions of high molecular weight beta-thromboglobulin and albumin are independently associated with coronary heart disease in women, a nested case-control study of middle-aged women in the Diagnostisch Onderzoek Mammacarcinoom (DOM) Cohort, Utrecht, Netherlands. Am J Epidemiol. 1995;142:1157–64. doi: 10.1093/oxfordjournals.aje.a117574. [DOI] [PubMed] [Google Scholar]
- 27.Deckert T, Yokoyama H, Mathiesen E, et al. Cohort study of predictive value of urinary albumin excretion for atherosclerotic vascular disease in patients with insulin dependent diabetes. Br Med J. 1996;312:871–4. doi: 10.1136/bmj.312.7035.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–5. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]


