Abstract
Metformin is a dimethyl biguanide oral anti-hyperglycemic agent. Lactic acidosis due to metformin is a fatal metabolic condition that limits its use in patients in poor clinical condition, consequently reducing the number of patients who benefit from this medication. In a double blind randomized clinical trial, we investigated 200 type 2 diabetic patients after coronary artery bypass surgery in the open heart ICU of the Mazandaran Heart Center, and randomly assigned them to equal intervention and control groups. The intervention group received regular insulin infusion along with 2 metformin 500 mg tablets every twelve hours, while the control group received only intravenous insulin with 2 placebo tablets every twelve hours. Lactate level, pH, base excess, blood glucose and serum creatinine were measured over five 12 h periods, with data averaged for each period. The primary outcome in this study was high lactate levels. Comparison between the 2 groups was made by independent Student’s t-test. To compare changes in multiple measures in each group and analysis of group interaction, a repeated measurement ANOVA test was used.
There was no significant difference between the 2 groups regarding pH, base excess, or bicarbonate intake (P>0.05). No patient showed lactic acidosis in either group. Lactate levels were 23.0 vs 23.4 in the insulin-metformin and insulin only groups when the study was started, respectively. At the end of the study, those levels were 18.7 vs 18.9, respectively. In addition, the ANOVA repeated measurement test did not show a significant difference in terms of changes in the amount of lactate level between the 2 groups during the five measurement tests of the study period (P>0.05).
High-dose metformin (1,000 mg twice daily with insulin) does not cause lactic acidosis in type 2 diabetic patients after coronary artery bypass surgery.
Key words: lactic acidosis, metformin, coronary artery bypass, type 2 diabetes.
Introduction
Coronary artery bypass surgery (CABG) is the most common heart surgery. In the United States, 515,000 patients undergo coronary artery bypass surgery (CABG) each year; about 28% of them have diabetes.1
Hyperglycemia (blood glucose over 200 mg/dL) is common in patients in a poor clinical condition. This causes more infection, ischemic events, renal and neurological complications, and higher mortality.2,3 The main mechanism of increased blood glucose in this condition is insulin resistance (IR); therefore, insulin is considered to be the main drug for treatment of hyperglycemia. However, insulin accelerates the transfer of potassium and magnesium into the cells that may lead to hypokalemia and hypomagnesia.4,5 In addition, due to insulin resistance and insulin induced hypoglycemia, the use of products such as metformin, which does not cause hypoglycemia, is recommended for blood glucose control in patients with insulin resistance.4
Metformin was introduced in the United States in 1995. It is a dimethyl biguanide oral anti-hyperglycemic drug that reduces overall mortality in type 2 diabetes.6 Metformin reduces gluconeogenesis and glycogenolysis, and increases sensitivity of insulin receptors through multiple mechanisms. In addition, it reduces the level of serum lipids and lipoproteins. In particular, it reduces stroke (41%) and heart attacks (39%).7
Metformin has different mechanisms of action from other anti-hyperglycemic drugs and does not lead to a severe drop in the blood glucose level of diabetic patients.7 Between the years 1985 to 2001, 66 cases of metformin-associated lactic acidosis (MALA) were reported by the FDA and 48 cases by the Australian Adverse Drug Reactions Advisory Committee (ADRAC).8 MALA is a fatal metabolic condition that limits metformin use in patients in poor clinical condition.9 These restrictions considerably reduce the number of patients who could benefit from this advantageous drug.10 However, Salpeter et al.6 have estimated the incidence of lactic acidosis in type 2 diabetic metformin users to be less than non-metformin users (0.0043% vs 0.0054%). These results are similar to those of Stang et al. (0.002–0.009% vs 0.009%).5 This clinical trial was performed to determine whether metformin can create lactic acidosis in type 2 diabetic patients after CABG surgery. Whereas previous studies have taken a descriptive or retrospective approach with different times/ dose of metformin, to our knowledge, this is the first clinical trial studying the effect of metformin administration in such patients.
Materials and Methods
In this double blind randomized clinical trial, 200 type 2 diabetic patients were studied following CABG surgery in the open heart ICU of the Mazandaran Heart Center. Approval from the Ethics Committee of Mazandaran University of Medical Sciences and informed consent from patients were obtained. Patients were randomly and simultaneously assigned to 2 groups; 100 patients received insulin and 100 received insulin-metformin.
Inclusion criteria were: age between 35 to 75 years, type 2 diabetes, non-emergency CABG surgery, the use of cardiopulmonary pump during the operation and hemodynamic stability (MAP > 60, lack of dangerous dysrhythmia and heart rate between 50 to 100).
We excluded patients with any kind of liver disorder (SGPT and SGOT more than 75), renal dysfunction (serum creatinine more than 1.5 mg/dL in 2 consecutive tests), heart failure (ejection fraction ratio less than 30%), and the use of any contrast or history of angiography within two days before the operation. Medication would be interrupted and patients would leave the study if at any time during the study the following events were recorded: serum lactate level more than 45 mg/dL (5 mmol/L) or had values which increased more than 18 mg/dL (2 mmol/L) compared to previous levels, pH less than 7.25 or serum base excess (BE) of less than -6 mmol/L, mean arterial oxygen pressure less than 60 mmHg in two consecutive Arterial Blood Gas (ABG) tests, potassium drops of less than 3 meq/L, nausea and vomiting, use of metformin metabolism inhibitors such as amiodarone and cimetidine, use of high-dose inotrope drugs more than 3 h after extubation and need for reoperation for any reason. Patients who qualified for study entry were randomly assigned to one of these groups.
Insulin group: regular insulin infusion based on the center protocol with 2 placebo tablets (Sari pharmaceutical laboratory, Iran) every 12 h. Insulin-metformin group: regular insulin infusion based on the center protocol with 2 metformin tablets 500 mg (Aria, Iran) every 12 h.
Metformin or placebo were prescribed 3 h after extubation by oral administration and in the absence of oral tolerance through a nasogastric tube. All oral anti-hyperglycemic drugs were discontinued two days before the surgery in all patients and subsequent blood glucose levels were controlled with insulin. There was no difference in onset of intervention or patient diet before and after surgery between the 2 groups (diabetic and cardio diet based on hospital routine). Patients were placed in treatment protocols for 60 h during which blood glucose, lactate, pH and BE were measured every 2 h and creatinine was measured daily. The study was divided into 5 stages and the mean 12 h period values were calculated. Blood glucose, lactate, pH and BE were measured by advanced Blood Gas machine GEM 3000 (Instrumentation Laboratory, MA, USA) using future pulse medical company cartridge. Serum creatinine was measured by Auto Analyzer Ebra XL640 model and Pars Company Cartridge. This was a double blind study and none of the patients, physicians, nurses or laboratory staff was aware of the type of treatment received.
Primary outcome in this study was blood lactate levels. An acceptable range of arterial blood lactate level is 4.5–13.5 mg/dL. Blood glucose level, pH and BE were assessed as secondary outcomes. In this study, acidosis and hypoglycemia were considered to be complications. After collecting and classifying data, statistical analyses were carried out by SPSS 16 software. Qualitative variables were evaluated with the χ2test. After controlling the normal distribution of numerical variables by the Kolmogorov-Smirnov test, an independent student's t-test was calculated to compare the two groups. To compare changes in multiple measurements in each group and to analyze group interaction, a repeated measurement ANOVA test was used. In all statistical tests, the significance level was considered to be 0.05.
Results
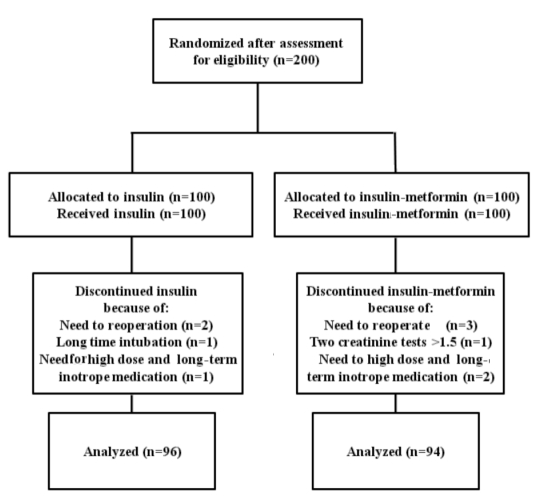
Two hundred patients were entered in this clinical trial; 100 patients in the insulin group and 100 patients in the insulin-metformin group (using an equal randomized allocation). Ten patients were excluded during the project and data from 190 patients were analyzed (Figure 1).
Figure 1.
Flow diagram of patients’ randomization, intervention and analysis.
Forty-six of 96 patients (52.1%) among the insulin group and 50 of 94 patients (46.8%) among the insulin-metformin group were men but the difference was not statistically significant (P>0.05).
Also there was no statistically significant difference in the basic and clinical characteristics between the 2 groups (Table 1).
Table 1. Pre-operative patient characteristics.
| Insulin group (n=96) | Insulin-metformin Group (n=94) | P (t-test) | |||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| Age (Y) | 60.5 | 59.2 to 61.8 | 59.8 | 58.3 to 61.2 | 0.435 |
| Weight (kg) | 66.4 | 64.4 to 68.4 | 67.8 | 65.7 to 70.0 | 0.350 |
| Height (m) | 1.62 | 1.60 to 1.64 | 1.63 | 1.61 to 1.65 | 0.367 |
| Body mass index (kg/m2) | 25.3 | 24.6 to 26.1 | 25.4 | 24.6 to 26.3 | 0.844 |
| Mean arterial pressure (mm/Hg) | 71.3 | 70.0 to 72.5 | 73.3 | 71.5 to 75.2 | 0.072 |
| Ejection fraction (%) | 46.5 | 44.7 to 48.2 | 47.1 | 45.6 to 48.5 | 0.585 |
| Intubation time (hr) | 5.47 | 5.33 to 5.62 | 5.32 | 5.18 to 5.46 | 0.150 |
| Lactate (mg/dL) | 23.4 | 25.5 to 21.4 | 23.0 | 25.0 to 21.1 | 0.793 |
| pH | 7.353 | 7.359 to 7.346 | 7.357 | 7.364 to 7.350 | 0.392 |
| Base excess (mmol/L) | −3.68 | −3.48 to -3.89 | −3.61 | −3.40 to −3.81 | 0.606 |
| Serum creatinine | 0.89 | 0.93 to 0.85 | 0.92 | 0.95 to 0.87 | 0.426 |
| Potassium (meq/L) | 4.32 | 4.38 to 4.26 | 4.37 | 4.42 to 4.32 | 0.235 |
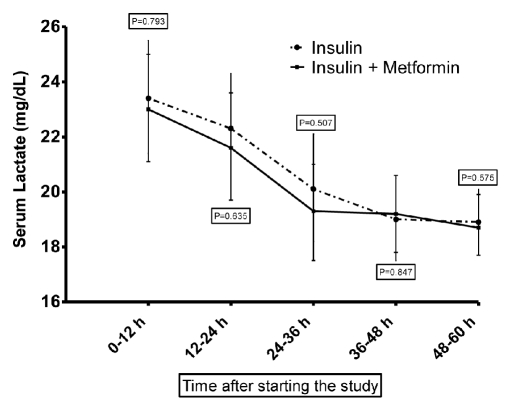
Lactic acidosis was not observed in either of the study arms. In patients receiving insulin, at baseline the serum lactate level was 23.4±10.1 (mg/dL), while at the end of study, it reached 18.9±5.9 (a 19% reduction). Similarly, an approximately 19% reduction in serum lactate level was seen in the metformin group (Figure 2).
Figure 2.
Changes in blood lactate level (mean and SEM) of patients in insulin and insulin-metformin groups during the study.
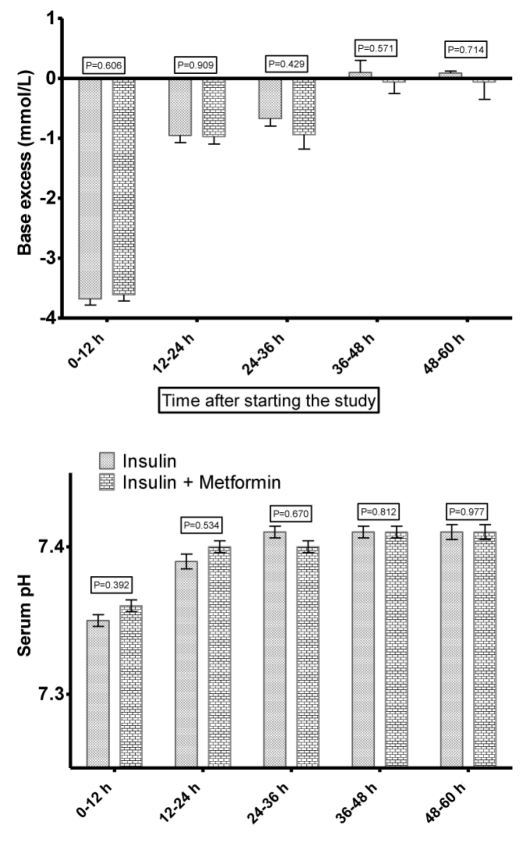
Changes in pH and BE were similar in both groups with no statistically significant difference (F(1, 187)=0.286; P=0.853 for pH and F(1, 187)=0.159; P=0.932) for BE (Figure 3).
Figure 3.
Changes in pH level of blood and the amount of base excess (mean and SEM) of patients in insulin and insulin-metformin groups during the study.
Average consumed bicarbonate during the study was 6.77 meq (95%CI: 4.07 to 9.470) in the insulin group and 6.91meq (95%CI: 3.97 to 9.86) in the insulin-metformin group. There was no significant difference between the 2 groups (t= −0.072; P=0.943).
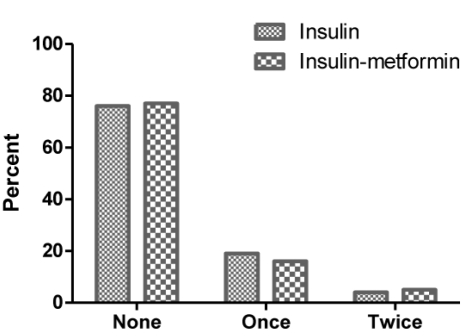
No significant difference was observed in the number of high blood lactate events (between 30–45 mg/dL) during the study in the insulin group and the insulin-metformin group (χ2=0.850; P=0.467) (Figure 4).
Figure 4.
High blood lactate (between 30 and 45 mg/dL) separately in studied groups.
Average blood glucose of patients in both groups decreased during the study. However, the reduction observed in the insulin-metformin group was more than the insulin group and this difference was statistically significant (F(1, 187) =9.873; P<0.001).
Discussion and Conclusions
The study findings showed that use of metformin (1,000 mg/BD) together with insulin in type 2 diabetic ICU patients after CABG surgery did not cause lactic acidosis. Blood lactate levels in both groups gradually decreased during the study, but we observed no significant difference between the 2 groups. There was no significant difference in blood pH between the 2 groups during the study period and mean blood pH in patients were in the normal range (7.35–7.45). No dangerous reduction or increase in blood pH occurred. Some cases of lactic acidosis caused by metformin were reported by Preiser and Vincet,11 Lustik et al.,12 Silvestre et al.,13 Ashall and Dawes,14 and Cezure et al.,15 that led to stricter limits in the use of metformin in type 2 diabetic patients. However, Lalau and Race16 and Kamber17 have stated that lactic acidosis cannot be directly attributed to metformin use. Their rationale was that many patients who developed lactic acidosis while taking metformin were those who suffered from underlying problems such as heart failure, kidney and liver diseases, and diabetes, which are known as risk factors of lactic acidosis. Duncan et al. showed that type 2 diabetic patients treated with metformin would not develop more severe acidosis. Also long-term intubation, infection and overall morbidity in the metformin group were significantly less than the non-metformin group.18 In the Duncan et al. study, serum lactate level was measured only in patients with primary diseases, while the exact dose and the last dose of metformin were not clear and metformin was continued up to cardiac surgery. Importantly, the present study showed that the use of insulin along with high-dose metformin, even immediately after cardiac surgery, does not create acidosis, but improves blood glucose control in these patients.
Our results in a larger number of patients confirmed the study by Mojtahedzadeh et al. which showed no significant relationship between plasma level of metformin, blood lactate and pH. Also blood pH was in the normal range and no pH less than 7.3 was seen.19 We showed that the use of 1,000 mg metformin twice daily plus insulin does not cause lactic acidosis in type 2 diabetic patients, while there was no difference in the amount of bicarbonate prescribed to each of the 2 groups. Importantly, no lactic acidosis occurred following high-dose metformin in patients undergoing CABG with cardiopulmonary pump who have a higher potential risk of lactic acidosis. Ansari showed that taking metformin with insulin in ICU patients did not cause acidosis, which is consistent with the results of this study.20 Therefore, based on the current published data and results of the present study, it seem that metformin administration in postoperative or critically injured patients is not absolutely contraindicated, but rather beneficial, particularly if selected for those patients with satisfactory hepatic, renal and cardiac function, and as long as these parameters are appropriately monitored.21 Lactic acidosis due to metformin is easily preventable by avoiding dehydration, especially in concomitant nephrotoxic drug use.22 This study showed that high-dose metformin along with appropriate monitoring of renal and liver function not only protects against but also reduces microvascular complications and deaths from cardiovascular disease, even after coronary artery surgery in type 2 diabetic patients.23 During the present study, after frequent monitoring of the patients in the ICU, a patient was excluded due to a creatinine level higher than 1.5 (UNIT) on 2 consecutive tests. On the other hand, blood glucose level in the metformin-insulin group was reduced more than the insulin group. This result suggests the positive effect of metformin in the control of blood glucose in type 2 diabetic patients undergoing CABG surgery, which is probably due to the metformin effect on the insulin resistance of these patients. This is consistent with the findings of previous studies.19
Use of metformin in type 2 diabetic ICU patients after CABG surgery is associated with better glucose control without theoccurrence of lactic acidosis. Consequently, it is a safe and effective drug and, so, a good choice for controlling blood glucose in order to reduce the complications and improve survival in these patients.
Acknowledgements:
this study was conducted with the financial support of the Mazandaran University of Medical Sciences. The contributions of the staff of the open heart ICU at the Mazandaran Heart Center, Sari, Iran are sincerely appreciated. Also, the authors wish to thank all the study participants for their tremendous cooperation and support.
References
- 1.Gandhi GY, Nuttal GA, Abell MD, et al. Intraoperative Hyperglycemia and Perioperative Outcomes in Cardiac Surgery Patients. Mayo Clin Prac. 2005;80:862–6. doi: 10.4065/80.7.862. [DOI] [PubMed] [Google Scholar]
- 2.Furnary PA, Gao G, Grunkemeier LG, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–21. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 3.Falciglia M, Freyberg RW, Almenoff PL, et al. Hyperglycemia-Related Mortality in Critically Ill Patients Varies with Admission Diagnosis. Critical Care Medicine. 2009;37:3001–9. doi: 10.1097/CCM.0b013e3181b083f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods HF, Cohen RD. Metformin and lactic acidosis. Diabetes Care. 1999;22:1010–1. doi: 10.2337/diacare.22.6.1010. [DOI] [PubMed] [Google Scholar]
- 5.Stang M, Wysowski DK, Bulter JD. Incidence of lactic acidosis in metformin users. Diabetes Care. 1999;22:925–7. doi: 10.2337/diacare.22.6.925. [DOI] [PubMed] [Google Scholar]
- 6.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane database syst rev. 2010;14:2967–2967. doi: 10.1002/14651858.CD002967.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabatabaei O, Heshmat R, Hejazi A, et al. The Comparison Effects of generic metformin and brand metformin in Type 2 Diabetic Patients. Iranian J Diabetes Lipid Disord. 2005;5:49–58. [Google Scholar]
- 8.Nisbet JC, Sturtevant JM, Prins JB. Metformin and serious adverse effects. Med J Austral. 2004;180:53–4. [PubMed] [Google Scholar]
- 9.Shelley R, Greyber E, Gary A, et al. Risk of Fatal and Nonfatal Lactic Acidosis With Metformin Use in Type 2 Diabetes Mellitus. Arch Intern Med. 2003;163:2594–602. doi: 10.1001/archinte.163.21.2594. [DOI] [PubMed] [Google Scholar]
- 10.Cynthia M. Hyperglycemia in Critically Ill Patients. Boca Veterinary Referral. Emergency and Critical Care Center Boca Raton. 2007:360–71. [Google Scholar]
- 11.Preiser JC, Vincent JL. Specific Therapies of Biguanide-induced Lactic Acidosis. Anesthesiology. 1998;89:267–267. doi: 10.1097/00000542-199807000-00038. [DOI] [PubMed] [Google Scholar]
- 12.Lustik SJ, Vogt A, Chhibber AK. Postoperative Lactic Acidosis in Patients Receiving Metformin. Anesthesiology. 1998;89:266–266. doi: 10.1097/00000542-199807000-00037. [DOI] [PubMed] [Google Scholar]
- 13.Silvestre J, Carvalho S, Mendes V, et al. Metformin-induced lactic acidosis: a case series. J Med Case Reports. 2007;1:1–4. doi: 10.1186/1752-1947-1-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashall V, Dawes T. Metformin and lactic acidosis. Brit J Anaesth. 2008;101:876–84. doi: 10.1093/bja/aen286. [DOI] [PubMed] [Google Scholar]
- 15.Cezur M, Celmen N, Cetinbas R, et al. A clinical case of lactic acidosis development in a diabetic patient taking metformin. Anesteziologiia I Reanimatologiia. 2009;2:74–6. [PubMed] [Google Scholar]
- 16.Lalau JD, Race JM. Metformin and lactic acidosis in diabetic humans. Diabetes Obes Metab. 2000;2:131–7. doi: 10.1046/j.1463-1326.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamber N, Davis AW, Bruce GD, Davis EMT. Metformin and lactic acidosis in an Australian community setting: the Fremantle Diabetes Study. Med J Austral. 2008;188:446–9. doi: 10.5694/j.1326-5377.2008.tb01713.x. [DOI] [PubMed] [Google Scholar]
- 18.Duncan AI, Koch CG, Meng Xu, et al. Recent Metformin Ingestion Does Not Increase In-Hospital Morbidity or Mortality After Cardiac Surgery. Anesth Analg. 2007;104:42–50. doi: 10.1213/01.ane.0000242532.42656.e7. [DOI] [PubMed] [Google Scholar]
- 19.Mojtahedzadeh M, Rouini MR, Kajbaf F, et al. Advantage of adjunct metformin and insulin therapy in the management of Critically ill patients. Arch Med Sci. 2008;4:174–81. [Google Scholar]
- 20.Ansari G, Mojtahedzadeh M, Kajbaf F, et al. How does blood glucose control with metformin influence intensive insulin protocols? Evidence for involvement of oxidative stress and inflammatory cytokines. Advances in Therapy. 2008;25:681–702. doi: 10.1007/s12325-008-0075-1. [DOI] [PubMed] [Google Scholar]
- 21.Schure P, De Gooijer A, Van Zanten ARH. Unexpected survival from severe metformin-associated lactic acidosis. Medicine. 2003;2:1–6. [PubMed] [Google Scholar]
- 22.Fitzgerald E, Mathieu S, Ball A. Metformin associated lactic acidosis. BMJ. 2009;339 doi: 10.1136/bmj.b3660. [DOI] [PubMed] [Google Scholar]
- 23.Seidowsky A, Nseir S, Houdret N, Fourrier F. Metformin-associated lactic acidosis: a Prognostic and therapeutic study. Crit Care Med. 2009;37:2191–6. doi: 10.1097/CCM.0b013e3181a02490. [DOI] [PubMed] [Google Scholar]