Abstract
Malnutrition is common at hospital admission and tends to worsen during hospitalization. This controlled population study aimed to determine if serum albumin or moderate and severe nutritional depletion by Nutritional Risk Index (NRI) at hospital admission are associated with increased length of hospital stay (LOS) in patients admitted with acute decompensated heart failure (ADHF). Serum albumin levels and lymphocyte counts were retrospectively determined at hospital admission in 1740 consecutive patients admitted with primary and secondary diagnosis of ADHF. The Nutrition Risk Score (NRI) developed originally in AIDS and cancer populations was derived from the serum albumin concentration and the ratio of actual to usual weight, as follows: NRI = (1.519 × serum albumin, g/dL) + {41.7 × present weight (kg)/ideal body weight(kg)}. Patients were classified into four groups as no, mild, moderate or severe risk by NRI. Multiple logistic regressions were used to determine the association between nutritional risk category and LOS.
Three hundred and eighty-one patients (34%) were at moderate or severe nutritional risk by NRI score. This cohort had lower BMI (24 ± 5.6 kg/m2), albumin (2.8±0.5 g/dL), mean NRI (73.5±9) and lower eGFR (50±33 mL/min per 1.73 m2). NRI for this cohort, adjusted for age, was associated with LOS of 10.1 days. Using the Multiple Logistic regression module, NRI was the strongest predictor for LOS (OR 1.7, 95% CI: 1.58–1.9; P=0.005), followed by TIMI Risk Score [TRS] (OR 1.33, 95% CI: 1.03–1.71; P=0.02) and the presence of coronary artery disease (OR 2.29, 95%CI: 1.03–5.1; P=0.04). Moderate and severe NRI score was associated with higher readmission and death rates as compared to the other two groups.
Nutritional depletion as assessed by Nutritional Risk Index is associated with worse outcome in patients admitted with ADHF. Therefore; we recommend adding NRI to further risk stratify these patients.
Key words: nutrition risk and heart failure outcome.
Introduction
Malnutrition is reported to be the leading cause of disease burden in developing countries with high morbidity and mortality rates. In 1974, Butterworth et al.1 documented malnutrition and its implications in hospitalized patients. Despite great advances in medical science and diagnostic techniques, little has changed during the last three decades. Even in North America, 30–50% of hospitalized patients are described as malnourished or at a risk of malnutrition, with higher rates of malnutrition reported specifically in elderly subjects.2–4 Associations have been reported between poor nutritional status and impaired wound healing, increased post-operative complications and mortality.5–7 Given the developing awareness regarding adverse implications of malnutrition in hospitalized patient populations,8 multiple studies have been carried out to explore the association between malnutrition and various chronic diseases such as cancer,9,10 infections11,12 and chronic kidney diseases.13,14 Several studies have shown a relationship between the length of hospital stay and nutritional status, with malnutrition becoming more prevalent as the stay in hospital is prolonged.15,16 This led to further consideration of malnutrition given its contribution to the rising costs of our National Health Services and health care systems worldwide7 Thus, there is a rationale to routinely screen patients for nutritional risk and risk for complications at hospital admission.17
Numerous measures have been developed to assess the nutritional status of the hospitalized patients, among them, the Nutritional Risk Index (NRI) which was developed by the Veterans Affairs Total Parenteral Nutrition Cooperative Study Group.18 The NRI gained in popularity as it uses an objective (serum albumin and percent usual body weight) rather than subjective measurements to determine nutritional risk in hospitalized patient populations.19,20 It has been successfully modified from its original form to be used in various patient groups.20
Among chronic diseases, chronic heart failure has already been shown to be associated with a poor prognosis with shortened survival, repeated hospitalizations and sizeable costs to society,21 estimated to be about $18.8 billion per year in the United States.22 Chronic heart failure (CHF) is increasingly recognized as a chronic multi-system disease with multiple co-morbidities such as anemia, insulin resistance, autonomic dysfunction, or cardiac cachexia.23 Despite the fact that heart failure patients are more prone to experience malnutrition either due to symptomatic anorexia during an episode of ADHF,23 early satiety and ascites, pharmacological agents,24 psychological factors25 or catabolic/anabolic imbalance,26 the data regarding clinical significance of malnutrition in heart failure patients remain limited.27 Chronic heart failure patients routinely suffer from hypermetabolic states and pharmacologically induced diuresis can further compound the poor nutritional status of these patients.28,29
Our study aimed to evaluate the role of nutritional evaluation of heart failure patients by using a modified NRI tool, as well as to study the impact of malnutrition severity on hospital length of stay (LOS), readmission rates and to assess existence between malnutrition and mortality in these patients.
Materials and Methods
Subjects
We identified 1,740 consecutive patients from the Advanced Cardiac Admission Program data (ACAP-HF) who were admitted with acute decompensated heart failure (ADHF). Patients with acute myocardial infarction (<3 days duration), hemodynamically significant valvular abnormalities, hemodynamic instability, patients taking any laxatives or weight loss medication or pregnant, were excluded from the study. Serum albumin levels and lymphocyte counts were determined at hospital admission. Questionnaires providing details of the past medical history and demographics were completed by the physicians involved in management during hospitalization of these patients on admission who were kept blinded about the study. Complete records were available for 1110 patients and were included in the final analysis. Patients were followed for up to four years (mean 2.6 ± 1.0 years). The Internal Review Board (IRB) of our Institute approved this study.
Measurements
Anthropometric measures
All measurements were taken at the hospital admission. Body weight was measured to the nearest 0.1 kg (Weight Tronix, New York, NY, USA) and height to the nearest 0.5 cm using a stadiometer (Holtain; Crosswell Wales). Body Weight (BW) change was calculated as: (current BW in kilograms-ideal BW in kilograms)/ideal BW × 100. Ideal BW was calculated according to the Lorentz formula that takes into account patient’s height and sex as follows: IBW (kg) = height (cm) – 100 – {[height (cm)-150]/4}. The Body Mass Index (BMI: kilograms/meters2) was calculated as weight in kilograms divided by height (meters2).
Laboratory measures
Blood samples for serum albumin, serum brain natruretic peptide (BNP) and serum creatinine levels were drawn on admission and before initiation of intravenous diuretics or intravenous fluids if needed. Albumin was measured by immunonephelometry (normal range 3.5–5.5 g/dL). An albumin level of less than 3.5 g/dL was set as the lower limit of normal in our study.30,31 Blood was collected in tubes containing potassium EDTA (1 mg/ml blood), for the Triage BNP test. (Biosite Diagnostics Inc., San Diego, California, USA).
Nutritional risk index
The NRI18,19 was originally derived from the serum albumin concentration and the ratio of present to usual weight. Faced with the difficulty in identifying the usual body weight of heart failure patients, we alternatively used ideal body weight instead of usual body weight in the NRI formula20 as follows: NRI = (1.519 × serum albumin, g/dL) + {41.7 × present weight (kg)/ideal body weight(kg)}. From these NRI values, we defined four grades of nutrition-related risk: i) major risk (NRI<83.5); ii) moderate risk (NRI 83.5–97.5); iii) mild risk (NRI 97.5–100); iv) No risk (NRI > 100). The NRI cut-off values were determined according to weight losses of 5%, 10% or 20%. The weight loss norms of 5% and 10% have already been validated by the European Society of Parenteral and Enteral Nutrition (ESPEN) Guidelines for Nutritional Screening.32
Length of stay
Hospital LOS was the actual number of days the patients remained in the hospital, obtained from the hospital chart after patients were discharged.
Follow up
Serial prospective follow up (mean 2.7±1.0 years) was obtained in all patients by means of a physician-directed telephone interview using a standardized questionnaire. Length of stay was calculated for the index admission, and all subsequent ADHF related readmissions were also documented. If the patient died during follow up, the closest surviving relative or the patient’s physician was interviewed to determine the cause of death. Cardiac death was confirmed by review of the hospital medical records and/or death certificate.
Statistical analysis
Statistical analysis was performed using a standard statistical software package (SPSS for Windows, version 17; SPSS; Chicago, IL, USA). Continuous variables are expressed as the mean ± SD. Patient groups were compared using Student’s t-test (for normally distributed variable) or Wilcoxon’s rank-sum test (for other variables) for continuous variables and the X2 test or Fisher’s exact test for categorical variables. A P value of less than 0.05 was considered statistically significant. Multiple logistic regressions were used to determine the association between nutritional risk category and length of stay (LOS). Cumulative survival rates as a function of time after admission of ADHF were performed using Kaplan-Meier survival analysis and compared using log-rank analysis.
Study limitations
We could not perform periodic nutritional risk assessments in our patients after the initial admission assessment, which would have allowed evaluation of the progression of nutritional risk during hospital stay.
Results
The basic characteristics of our study participants (n=1110) are shown in Table 1. The patients were divided into four groups according to NRI scores identified as No Risk group (n=666) with mean age 68±14 years; 51% males, Mild Risk group (n =63) with mean age 72±14 years; 51% males, Moderate Risk group (n=213) with mean age 72±14 years; 59% males and Severe Risk group (n=168) with mean age 68±15 years; 56% males. As expected, there was a high prevalence of hypertension in the entire cohort (84%) followed by coronary heart disease (69%), diabetes mellitus (45%), hyperlipidemia (45%) and smoking (16%). In addition the BMI values were higher in the No Risk group (31±7 kg/m2) as compared to Mild Risk (25±4 kg/m2), Moderate Risk (23±5 kg/m2) or Severe Risk groups (24±7 kg/m2) with P=0.001.
Table 1. Basic demographics and clinical parameters of ADHF patients.
| Parameter (n=666) | No Risk (n=63) | Mild Risk (n=213) | Moderate Risk (n=168) | Severe Risk | P |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, yrs | 68±14 | 72±14 | 72±14 | 68±15 | 0.006 |
| Sex, % men | 318 (53) | 205 (66) | 205 (66) | 205 (66) | 0.0004 |
| Hypertension (%) | 562 (84) | 61 (96) | 172 (80) | 134 (79) | 0.08 |
| Diabetes mellitus (%) | 342 (48) | 26 (41) | 81 (38) | 50 (30) | 0.001 |
| Smoking (%) | 98 (14) | 8 (12) | 42 (19) | 24 (14) | 0.08 |
| BMI, kg/m2 | 31±7 | 25±4 | 23±5 | 24±7 | 0.001 |
| Hyperlipidemia (%) | 279 (42) | 22 (35) | 70 (33) | 61 (36) | 0.07 |
| History of heart failure | 464 (69) | 42 (66) | 149 (70) | 112 (66) | 0.55 |
| History of CAD | 193 (29) | 16 (24) | 62 (29) | 45 (27) | 0.63 |
| Albumin | 3.9±0.8 | 3.4±0.4 | 3.2±0.4 | 2.6±0.5 | 0.001 |
| Nutrition risk score (NRI) | 119±29 | 99±0.7 | 91±4 | 61±16 | 0.001 |
| Heart failure indices | |||||
| NYHA Class | 2.5±0.9 | 2.6±0.7 | 2.6±0.7 | 2.8±0.7 | 0.07 |
| Median BNP, pg/mL | 698 | 902 | 1096 | 1040 | 0.001 |
| LVEF, % | 31±18 | 31 ± 21 | 31 ± 19 | 30±19 | 0.47 |
| Kidney function | |||||
| Creatinine, mg/dL | 2.0±1.2 | 1.6±1.3 | 2.1±1.9 | 1.9±1.9 | 0.54 |
| eGFR, mL/min per 1.73 m2 | 68±45 | 53±27 | 44±29 | 58±36 | 0.001 |
BM, body mass Index; eGFR, estimated glomerular filtration rate; CAD, coronary artery disease; HF, heart failure; NRI, nutrition risk index.
Nutritional risk indices
The mean NRI score of the No Risk group was 119±29, Mild Risk group was 99±0.7, Moderate Risk group was 91±4 and Severe risk group was 61±16 (Table 1). Out of 1,110 total patients, 381 patients (34%) were at moderate or severe nutritional risk according to the NRI score. This cohort had a mean NRI score of 73±9 (P<0.001), BMI value (24 ± 5.6 kg/m2; P<0.001), albumin level (2.8±0.5 g/dL; P<0.001, Lower eGFR (50 ± 33 mL/min per 1.73 m2; P<0.001) and median BNP level (1090 pg/mL; P <0.001)) on admission.
Effects of NRI scores on length of hospital stay
Lower NRI scores were found to be strong predictors of extended LOS with HR 1.7 (95%CI: 1.58–1.9); P=0.005 (Table 2 and Figure 1). Patients with lower NRI scores allocating them in either the Moderate or the Severe Risk group were more likely to stay hospitalized for longer (Figure 2). This inverse correlation was highly significant (χ2= 36; P <0.001). The median LOS was found to be 5.8 days in the No Risk group, 7.5 days in the Mild Risk group, 10.1 days in the Moderate Risk group and 10.9 days in Severe Risk group.
Table 2. Cox proportional-hazards regression predictors for the composite endpoint of all-cause mortality and heart failure readmission.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Covariate | Exp (b) | 95% CI | P | Exp (b) | 95% CI | P |
| Age | 1.01 | 1.00–1.02 | <0.0001 | 1.01 | 1.01–1.02 | 0.02 |
| BMI | 0.96 | 0.94–0.98 | <0.0001 | 1.01 | 0.98–1.02 | 0.69 |
| Egfr | 0.99 | 0.98–0.99 | <0.0001 | 0.99 | 0.99–1.00 | 0.29 |
| Known CAD | 1.61 | 1.24–2.10 | 0.0003 | 1.48 | 1.11–1.98 | 0.006 |
| Known HF | 1.25 | 0.97–1.61 | 0.08 | 1.1 | 0.83–1.43 | 0.47 |
| NRI score | 3.03 | 2.33–3.94 | <0.0001 | 3.1 | 2.34–4.22 | <0.0001 |
| > 3 Risk factors | 1.41 | 1.11–1.81 | 0.004 | 1.37 | 1.05–1.79 | 0.017 |
BM, body mass Index; eGFR, estimated glomerular Filtration Rate; CAD, coronary artery disease; HF, heart failure; NRI, nutrition risk index.
Figure 1. Effect of nri on length of hospital stay.
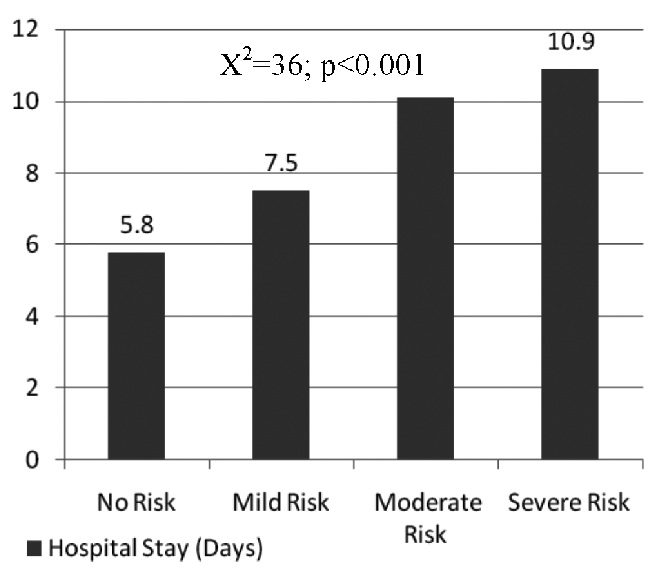
Figure 2. Effect of nri on heart failure admission.
Effect of NRI scores on heart failure readmission rate and all cause mortality
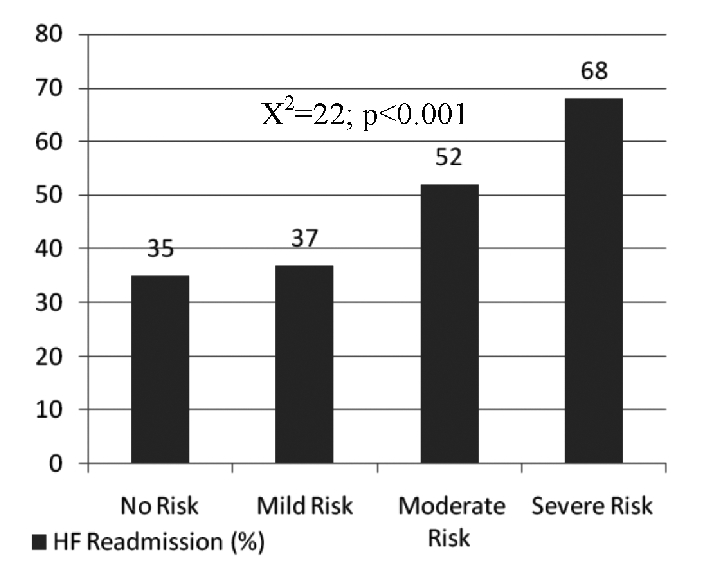
Our study showed that ADHF readmission rates were significantly higher in the patients with lower NRI scores, as seen in Figure 3. The readmission rates were 35% in the No Risk group and 37% in the Mild Risk group as compared to 52% and 68% in the Moderate Risk and the Severe Risk groups, respectively (P<0.001). Similarly, all cause mortality was higher among patients with lower NRI scores as shown in Figure 4. The all cause mortality was found to be 6% in the No Risk group and 9% in the Mild Risk groups as compared to 15% and 19% in the Moderate and Severe Risk groups, respectively (P<0.001).
Figure 3. Effect of NRI on all-cause mortality.
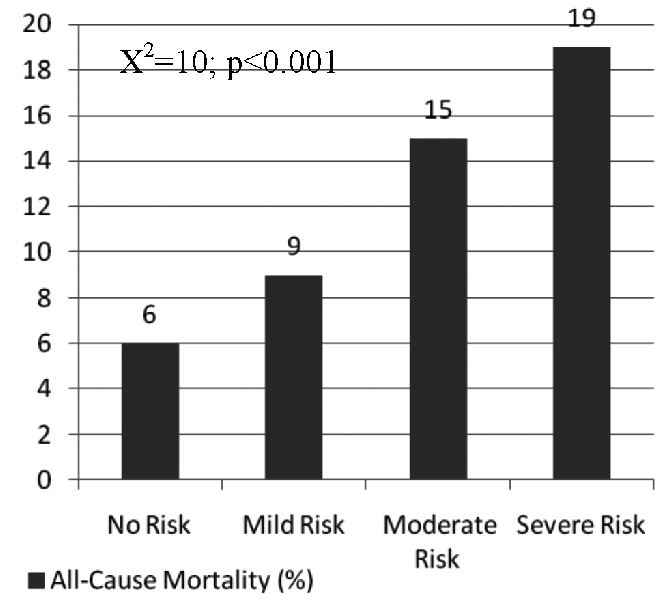
Figure 4. Event-free survival as a function of NRI score.
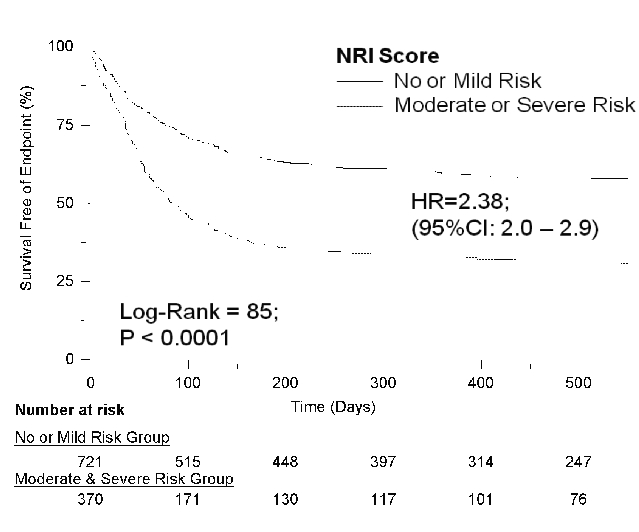
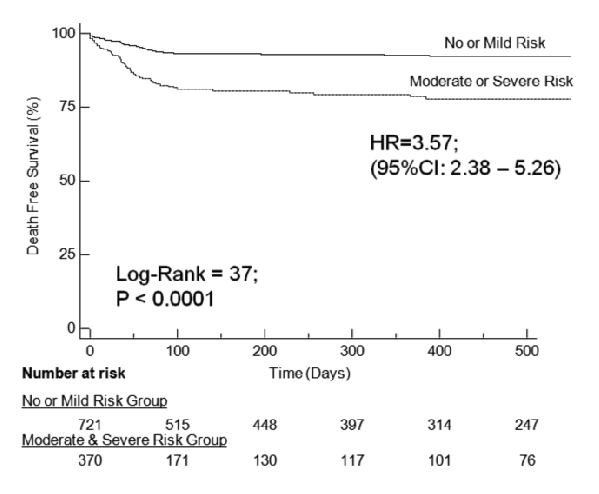
Kaplan-Meier survival analysis showed that patients with lower NRI scores and therefore in Moderate or Severe Risk groups were prone to have 6-fold higher event rate comprising either frequent readmissions or all cause mortality (HR=0.42; 95% CI 0.34–0.50 and P<0.0001) as compared to those with higher NRI scores and classified as in No or Mild Risk groups (Figure 5). Individually, Kaplan-Meier survival analysis to study free event survival as a function of NRI score showed that patients with Moderate or Severe Risk were prone to have three-fold higher mortality when compared to patients with No or Mild risk (HR=0.28; 95% CI 0.19–0.42 and P<0.0001). NRI score was found to be the most significant predictor of composite end points of all cause mortality and readmission rates with episodes of ADHF in both univariate and multivariate Cox’s hazard regression models with OR of 3.03 (95% CI: 2.33–3.94; P<0.0001) and 3.1 (95% CI: 2.34–4.22; P<0.0001), respectively. BMI was found to be a significant predictor of composite end points in univariate models with the OR=0.96 (95% CI: 0.94–0.98; P<0001) but interestingly this became insignificant when analyzed in multivariate analysis in prediction of composite end points of readmission rate and all cause mortality OR=1 (95% CI: 0.98–1.02; P = 0.69).
Figure 5. Kaplan-Meier curve depicting the event free survival of a composite end point of all cause mortality.
Association between NRI scores and BNP levels in ADHF patient
Another important finding of our study was that higher BNP levels among patients at moderate or severe nutritional risk were seen as compared to patients with no or mild nutritional risk on admission (Table 1). The median BNP levels were 698 in No Risk group, 902 in the Mild Risk group, 1096 in the Moderate Risk group and 1040 in the Severe Risk Group (P=0.001).
Discussion
This is the first study to describe a prognostic nutritional risk index (NRI), which enables quantitative determination of the risk of malnutrition related morbidity and mortality in cardiac failure patients. Our study demonstrates that nutritional depletion as assessed by NRI is associated with worse outcome in patients admitted with ADHF (Figures 4 and 5). NRI can be used as a valid expedient of nutritional status of the hospitalized chronic heart failure patients like other patient populations.20 Heart failure patients with lower NRI scores have a greater chance of prolonged hospitalization (Figure 2), higher readmission rates with episodes of ADHF (Figure 3) and higher mortality in subsequent years (Figure 4). The results of our study display the high prevalence of malnutrition in chronic heart failure patients presenting with episodes of ADHF in the United States.
The results of our study conform with the studies in the past displaying higher risk of various complications in malnourished patients as compared to non-malnourished patients.33 With the recent emerging evidence of the prevalence of cardiac cachexia in heart failure patients and the associated proposed neuro-endocrine and metabolic components involved,34 assessment of nutritional status in heart failure patients now seems more important than ever.35 There appears to be an intricate interrelationship between nutritional risk, severity of illness and clinical outcomes. The higher median values of BNP levels in moderate and severe risk malnourished patients in our study certainly point towards this, as higher BNP levels in ADHF patients have been shown to correspond to disease severity.36 In addition to its importance as a prognostic marker, another rationale to determine the nutritional status in heart failure patients admitted with an episode of ADHF is to avoid harm. Heart failure patients presenting with an additional episode of ADHF are often subjected to starvation in hospitals either due to fluid overload management or symptomatic management. If already malnourished, these patients can be at higher risks of complications, as various studies have demonstrated that significant deterioration in nutritional status occurs during hospitalization.37,38
Our study demonstrated that 34% of patients presenting with ADHF episodes were found to be at moderate or severe nutritional risk as measured by the NRI scores depicting considerable evidence towards higher prevalence of moderate to severe malnutrition among ADHF patients in the US. There are reports in literature that malnourished patients have higher morbidity and mortality than non-malnourished patients.33,39 Our study confirms that lower NRI scores are associated with high ADHF readmission rates and all cause mortality (Figure 3 and 4). It seems the patho-physiological deterioration attributed to the chronic nature of heart failure superimposed by malnutrition results in a high mortality in these patients, further emphasizing the need to measure nutritional risk severity in ADHF patients on hospital admission. Considering the high prevalence of heart failure in the US population, nutritional assessment by tools like NRI at hospital admission can certainly help in prompt identification of the additional danger at which these patients are at risk.
There is no universally accepted gold standard for defining malnutrition. Many physicians consider measurement of serum proteins such as albumin to be an adequate assessment of nutritional status. However, in hospitalized patients, albumin is influenced by multiple factors as a sufficient measure of nutritional status.40 Reduction of albumin in ADHF patients may reflect impaired metabolic conditions including hepatic dysfunction, dehydration, protein loss, renal dysfunction, infection and inflammation. Utility of measuring either serum albumin or ideal body weight alone in assessment of nutritional status in different patient populations has been questioned.41 Kyle et al.42 reported that in hospitalized patient albumin underestimates the prevalence of malnutrition if taken alone as an indicator of malnutrition. It is important to note that the development of the cachectic state in CHF is a process that can only be proven by a documented weight loss measured in a non-edematous state. This weight loss should usually be observed over a period of more than six months. The use of ideal body weight in NRI formula rather than usual body weight certainly solves this problem and gives a more comprehensive measure, which can be easily calculated in ADHF patients. Serum albumin is negatively correlated with increased extracellular fluid volume.43 Weight is also affected by hydration status, but variations in hydration status contrast strongly with variations in albumin concentrations. The use of both indicators in the NRI minimizes confounding variables such as hydration status, an important fluctuating variable observed in heart failure patients. The Nutritional Risk Index appears to capture both nutritional risk and poor clinical outcome in hospitalized patients independent of disease severity.44,45
Significant correlation was observed between severity score and anthropometric measurements. This observation conflicts with the results of Bouillane et al.20 but agrees with Buzby et al.19 The patients at moderate or severe nutritional risk by the NRI scoring system in our study were found to have lower BMI values (24±5.6 kg/m2) as compared to no or mild risk groups as has been shown in other studies.46 A noteworthy finding in our study was lower mean BMI values (23±5) in the Moderate Risk group as compared to mean BMI values (24±7) in the Severe Risk group (P=0.001).
Our study indicates that lower NRI scores on admission can provide considerable information about risks of extended hospitalization in ADHF patients. The present study agrees with previously reported results,42 that moderate or severe nutritional depletion by the NRI was significantly associated with increased LOS. Our study results also indicate that in ADHF patients the LOS increased significantly with decreasing NRI scores (Figure 2). In the past, studies have shown that serum albumin at hospital admission correlated significantly with ICU days and LOS17 but reductions in serum albumin may reflect metabolic or inflammatory conditions rather than nutritional status. There were no significant associations between low BMI and low albumin levels with increased LOS as was previously observed by Engelman et al.47 Our study demonstrates that NRI discriminates nutritional risk far better than weight loss, albumin, or BMI alone or combined in ADHF patients. Various studies have also shown that LOS was associated with NRI regardless of underlying disease or even treatment.42, 44 Associations between moderate and severe NRI scores and LOS were significant with and without adjustment for age. The left ventricular ejection fraction (LVEF %) or NYHA classes among groups were not significantly different so could not be considered confounders to this association (Table 1).
Clinical implications of nutritional risk and hospital cost
Our simulated hospital costs increased significantly with increased nutritional risk. We found hospital costs (missing data) were 5 times higher in patients with severe nutritional risk compared with patients with no risk. Actual hospital costs would most likely be greater than the estimated cost in severely malnourished patients because we did not account for costs of complications. Thus, nutritional risk contributes to increased hospital cost.
Conclusion
Nutritional depletion as assessed by Nutritional Risk Index is associated with worse outcome in patients admitted with ADHF. The NRI is a more reliable prognostic indicator of morbidity and mortality in hospitalized ADHF patients than are indices that use albumin or BMI alone. Therefore, we recommend the use of NRI to further risk stratify these patients for nutritional depletion assessment. In our study, 34% of the hospitalized ADHF patients had moderate or severe nutrition-related risk and would be suitable for nutritional supplementation. Whether this would have an effect on outcomes awaits further study.
Future direction
Proper assessments of nutritional status of heart failure patients on hospital admission can help to minimize if not prevent complications, readmissions and mortality rates. Demonstrating that treating malnutrition in chronic heart failure patients improves outcomes would be the most convincing evidence. However, evidence that aggressive treatment of malnutrition improves outcomes is surprisingly scarce and requires further exploration and research.
Acknowledgments:
we are thankful to the entire team of ACAP for their untiring efforts in the completion of this study. We are also thankful to all the physicians who helped us in data gathering of this study including the residents of St Luke’s-Roosevelt Hospital Center, New York, USA. This abstract was presented, in part, as a finalist in the Jay N. Cohn New Investigator Award: Clinical / Integrative Physiology session at the national HFSA (Heart Failure Society of America) meeting on Monday September 14, 2009 in Boston Massachusetts, USA.
References
- 1.Butterworth CE., Jr The skeleton in the hospital closet. 1974. Nutr Hosp. 2005;20:302–7. 301;discussion 297–300. [PubMed] [Google Scholar]
- 2.Edington J, Boorman J, Durrant ER, et al. Prevalence of malnutrition on admission to four hospitals in England. The Malnutrition Prevalence Group. Clin Nutr. 2000;19:191–5. doi: 10.1054/clnu.1999.0121. [DOI] [PubMed] [Google Scholar]
- 3.Bruun LI, Bosaeus I, Bergstad I, Nygaard K. Prevalence of malnutrition in surgical patients: evaluation of nutritional support and documentation. Clin Nutr. 1999;18:141–7. doi: 10.1016/s0261-5614(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 4.Constans T, Bacq Y, Bréchot JF, et al. Protein-energy malnutrition in elderly medical patients. J Am Geriatr Soc. 1992;40:263–8. doi: 10.1111/j.1532-5415.1992.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 5.Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996;12:23–9. doi: 10.1016/0899-9007(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 6.Bidlack WR. Interrelationships of food, nutrition, diet and health: the National Association of State Universities and Land Grant Colleges White Paper. J Am Coll Nutr. 1996;15:422–33. doi: 10.1080/07315724.1996.10718620. [DOI] [PubMed] [Google Scholar]
- 7.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–34. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 8.McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ. 1994;308:945–8. doi: 10.1136/bmj.308.6934.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paccagnella A, Morello M, Da Mosto MC, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010;18:837–45. doi: 10.1007/s00520-009-0717-0. [DOI] [PubMed] [Google Scholar]
- 10.August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN. 2009;33:472–500. doi: 10.1177/0148607109341804. [DOI] [PubMed] [Google Scholar]
- 11.Nassar MF, El-Batrawy SR, Nagy NM. CD95 expression in white blood cells of malnourished infants during hospitalization and catch-up growth. East Mediterr Health J. 2009;15:574–83. [PubMed] [Google Scholar]
- 12.Ivers LC, Cullen KA, Freedberg KA, et al. HIV/AIDS, undernutrition, and food insecurity. Clin Infect Dis. 2009;49:1096–102. doi: 10.1086/605573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupisti A, Saba A, D'Alessandro C, et al. Dimethylarginine levels and nutritional status in hemodialysis patients. J Nephrol. 2009;22:623–9. [PubMed] [Google Scholar]
- 14.Bellizzi V, Di Iorio BR, Brunori G, et al. Assessment of nutritional practice in italian chronic kidney disease clinics: a questionnaire-based survey. J Ren Nutr. 2010;20:82–90. doi: 10.1053/j.jrn.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Coats KG, Morgan SL, Bartolucci AA, Weinsier RL. Hospital-associated malnutrition: a reevaluation 12 years later. J Am Diet Assoc. 1993;93:27–33. doi: 10.1016/0002-8223(93)92126-i. [DOI] [PubMed] [Google Scholar]
- 16.Larsson J, Andersson M, Askelöf N, Bark T. [Malnutrition common in Swedish hospitals. Risk of complications and prolonged care increases] Nord Med. 1994;109:292–5. [PubMed] [Google Scholar]
- 17.Reilly JJ, Jr, Hull SF, Albert N, et al. Economic impact of malnutrition: a model system for hospitalized patients. JPEN. 1988;12:371–6. doi: 10.1177/0148607188012004371. [DOI] [PubMed] [Google Scholar]
- 18.Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525–32. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 19.Buzby GP, Knox LS, Crosby LO, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr. 1988;47:366–81. doi: 10.1093/ajcn/47.2.366. [DOI] [PubMed] [Google Scholar]
- 20.Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–83. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 21.Bennett SJ, Saywell RM, Jr, Zollinger TW, et al. Cost of hospitalizations for heart failure: sodium retention versus other decompensating factors. Heart Lung. 1999;28:102–9. doi: 10.1053/hl.1999.v28.a96420. [DOI] [PubMed] [Google Scholar]
- 22.Amin A. Hospitalized patients with acute decompensated heart failure: recognition, risk stratification, and treatment review. J Hosp Med. 2008;3:S16–24. doi: 10.1002/jhm.392. [DOI] [PubMed] [Google Scholar]
- 23.Mijan-de-la-Torre A. Recent insights on chronic heart failure, cachexia and nutrition. Curr Opin Clin Nutr Metab Care. 2009;12:251–7. doi: 10.1097/MCO.0b013e32832a2171. [DOI] [PubMed] [Google Scholar]
- 24.Schiffman SS. Taste and smell in disease (first of two parts) N Engl J Med. 1983;308:1275–9. doi: 10.1056/NEJM198305263082107. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsson A, Pihl E, Mårtensson J, Fridlund B. Emotions, the meaning of food and heart failure: a grounded theory study. J Adv Nurs. 2004;46:514–22. doi: 10.1111/j.1365-2648.2004.03025.x. [DOI] [PubMed] [Google Scholar]
- 26.Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85:51–66. doi: 10.1016/s0167-5273(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 27.Arutiunov GP, Kostiukevich OI, Bylova NA. [Prevalence and clinical significance of malnutrition and effectiveness of nutritional support for patients suffering from chronic heart failure] Eksp Klin Gastroenterol. 2009;2:22–33. [PubMed] [Google Scholar]
- 28.Paccagnella A, Calò MA, Caenaro G, et al. Cardiac cachexia: preoperative and postoperative nutrition management. JPEN. 1994;18:409–16. doi: 10.1177/0148607194018005409. [DOI] [PubMed] [Google Scholar]
- 29.Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–32. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 30.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–42. [PubMed] [Google Scholar]
- 31.Antonelli Incalzi R, Landi F, Pagano F, et al. Changes in nutritional status during the hospital stay: a predictor of long-term survival. Aging (Milano) 1998;10:490–6. doi: 10.1007/BF03340163. [DOI] [PubMed] [Google Scholar]
- 32.Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–21. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 33.Braunschweig C, Gomez S, Sheean PM. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. J Am Diet Assoc. 2000;100:1316–22. doi: 10.1016/S0002-8223(00)00373-4. quiz 1323–4. [DOI] [PubMed] [Google Scholar]
- 34.Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–34. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 35.Miján A, Martín E, de Mateo B. [Cardiac cachexia] Nutr Hosp. 2006;21:84–93. [PubMed] [Google Scholar]
- 36.Waldo SW, Beede J, Isakson S, et al. Pro-B-type natriuretic peptide levels in acute decompensated heart failure. J Am Coll Cardiol. 2008;51:1874–82. doi: 10.1016/j.jacc.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 37.Incalzi RA, Gemma A, Capparella O, et al. Energy intake and in-hospital starvation. A clinically relevant relationship. Arch Intern Med. 1996;156:425–9. [PubMed] [Google Scholar]
- 38.Patel MD, Martin FC. Why don't elderly hospital inpatients eat adequately? J Nutr Health Aging, 2008;12:227–31. doi: 10.1007/BF02982626. [DOI] [PubMed] [Google Scholar]
- 39.Chima CS, Barco K, Dewitt ML, et al. Relationship of nutritional status to length of stay, hospital costs, and discharge status of patients hospitalized in the medicine service. J Am Diet Assoc. 1997;97:975–8. doi: 10.1016/S0002-8223(97)00235-6. quiz 979–80. [DOI] [PubMed] [Google Scholar]
- 40.Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and patho-physiology, Part III: Albumin and disease states. JPEN. 1992;15:476–83. doi: 10.1177/0148607191015004476. [DOI] [PubMed] [Google Scholar]
- 41.Friedmann JM, Jensen GL, Smiciklas-Wright H, et al. Predicting early nonelective hospital readmission in nutritionally compromised older adults. Am J Clin Nutr. 1997;65:1714–20. doi: 10.1093/ajcn/65.6.1714. [DOI] [PubMed] [Google Scholar]
- 42.Kyle UG, Pirlich M, Schuetz T, et al. Is nutritional depletion by Nutritional Risk Index associated with increased length of hospital stay? A population-based study. JPEN. 2004;28:99–104. doi: 10.1177/014860710402800299. [DOI] [PubMed] [Google Scholar]
- 43.Jones CH, Smye SW, Newstead CG, et al. Extracellular fluid volume determined by bioelectric impedance and serum albumin in CAPD patients. Nephrol Dial Transplant. 1998;13:393–7. doi: 10.1093/oxfordjournals.ndt.a027836. [DOI] [PubMed] [Google Scholar]
- 44.Kyle UG, Genton L, Pichard C. Hospital length of stay and nutritional status. Curr Opin Clin Nutr Metab Care. 2005;8:397–402. doi: 10.1097/01.mco.0000172579.94513.db. [DOI] [PubMed] [Google Scholar]
- 45.Corish CA. Pre-operative nutritional assessment in the elderly. J Nutr Health Aging. 2001;5:49–59. [PubMed] [Google Scholar]
- 46.Corish CA, Flood P, Kennedy NP. Comparison of nutritional risk screening tools in patients on admission to hospital. J Hum Nutr Diet. 2004;17:133–9. doi: 10.1046/j.1365-277X.2003.00494.x. quiz 141–3. [DOI] [PubMed] [Google Scholar]
- 47.Engelman DT, Adams DH, Byrne JG, et al. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg. 1999;118:866–73. doi: 10.1016/s0022-5223(99)70056-5. [DOI] [PubMed] [Google Scholar]


