Abstract
Objective:
The aim of this cross-sectional analytic study was to evaluate the diagnostic efficacy of panoramic-based indices of the mandible (Mental Index-MI, Mandibular Cortical Index-MCI and Panoramic Mandibular Index-PMI) and to determine their correlation with bone mineral density (BMD) of the femoral neck and lumbar vertebrae (L2-L4) in order to assess the possibility of using these parameters as indicators of osteoporosis.
Materials and Methods:
The mandibular indices of 67 women over 35 years old were measured from panoramic radiographs, and bone densitometry was performed in the femoral neck and lumbar vertebrae (L2-L4), using DXA (Dual Energy X-ray Absorptiometry) technique. The patients were divided into three categories of normal, osteopenic and osteoporotic in each skeletal region. One-way ANOVA and ROC curve analyses were applied. The results were considered statistically significant when the P-value was less than 0.05.
Results:
Comparing the mean BMD in the femoral neck in women between C1 and C3 subgroups of MCI, a significant difference was detected (P=0.04). The mean PMI in the three skeletal subgroups was not different according to the skeletal region (P>0.05). We found a significant difference in mean MI between normal and osteopenic subgroups in the femoral neck (P=0.042).
Conclusion:
Using radiomorphometric indices of the mandible (MCI-MI) may be useful in determining the skeletal status of the patients, but is not sufficient for precise evaluation.
Keywords: Osteoporosis, Femur Neck, Lumbar Vertebrae, Mandible
INTRODUCTION
Osteoporosis is a disease characterized by low bone mass and micro architectural deterioration of bone tissue [1]. Bone loss occurs with age (age-related osteoporosis) in men and women but in the latter the rate of loss increases at menopause (post-menopausal osteoporosis). This reduction in bone tissue is associated with an increased risk of fracture, pain and consequent morbidity for patients. Since the disease is preventable, diagnostic techniques are of major importance. A considerable effort has been made to identify methods of detecting individuals with osteoporosis at an early stage to limit the disease process. In much a number of studies, bone mineral density (BMD) in the mandible has been described to be strongly correlated with that in the lumbar spine and femoral neck, the most susceptible osteoporotic areas [2].
It is essential to use the panoramic radiographs for routine dental examinations especially before prosthodontic and periodontal treatments and maxillofacial surgeries. In panoramic radiographs, a number of mandibular indices have been made to do the quantitative measuring of the mandibular bone mass and trabecular architecture in order to diagnose patients with osteoporosis. Mental index (MI), panoramic mandibular index (PMI) and mandibular cortical index (MCI) are among these indices. Radiographs have shown that the decreased BMD affects the morphometric [3–5], densitometric [6] and architectural characteristics [7, 8] of the mandibular bone on radiographs. The aim of this study was to evaluate the association between various oral signs and osteoporosis in women to determine whether these radiographic measurements have accuracy in predicting BMD in order to detect the disease in the early stage and prevent the consequent side effects.
MATERIALS AND METHODS
This cross-sectional descriptive analytic study was based on 67 women in the age range of 36 to 92 years old who were referred to the Radiology Department of Mashhad Dental School. All of them had a panoramic radiography in their orders and none of the participants was known to have any prior fracture and systemic disease that would affect bone metabolism (chronic renal osteodystrophy, hyperparathyroidism, hypoparathyroidism, gastrointestinal disease, paget’s disease or rheumatoid arthritis). They were not using specific drugs or hormones (corticosteroids, cyclosporine, heparin, estrogen, progesterone, androgen and excess thyroid hormone). The data regarding age and BMI (body mass index) was obtained by questionnaire. All the panoramic radiographs were taken with PM 2002 CC apparatus (Planmeca Oy, Asentajankatu 6, 00880 Helsinki, Finland) by a single operator (AB). The position of the head was standardized as much as possible and also the exposure factors (kVp, mA) were regulated for each patient. To have similar density and contrast in all radiographs, we used both AGFA Dentus panoramic film (Heraeus Kulzer, Belgium) and Kodak screens (Kodak Lanex Regular Screen, Eastman Kodak Company, USA). Processing of the films was automatically performed by Protec processor (PROTEC medical systems, Stuttgart, Germany). For examination of the bone mineral density, DXA (Dual Energy X-ray Absorptiometry) scans were performed with Osteocore 2 (MEDILINK, France). This technique is widely accepted as the “gold standard” method of clinical bone mineral measurements [9,10]. DXA itself is subject to some minor inaccuracy in BMD measurement [6]. Precision of DXA ranges from 0.55 to 5% [11].
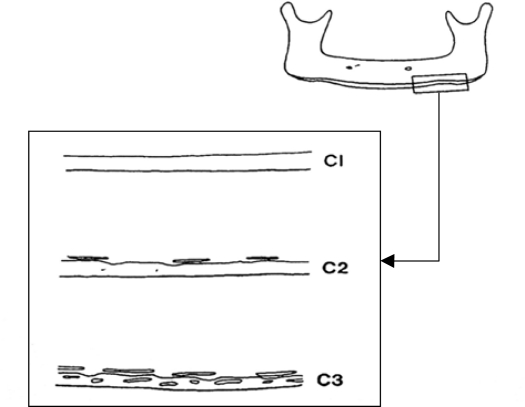
Sixty-seven panoramic radiographs were analyzed for MCI, MI and PMI. According to Jowitt et al [12], “MCI is classification of the appearance of the mandibular inferior cortex distal to the mental foramen, which includes the following criteria:
C1: The endosteal margin of the cortex is even and sharp on both sides of the mandible.
C2: The endosteal margin has semilunar defects (resorption cavities) with cortical residues one to three layers thick on one or both sides.
C3: The endosteal margin consists of thick cortical residues and is clearly porous” [13] (Fig 1).
Fig 1.
Diagram illustrating the classification of the endosteal inferior cortex on panoramic radiographs.
C1: The endosteal margin of the cortex is even and sharp on both sides of the mandible.
C2: The endosteal margin has semilunar defects (resorption cavities) with cortical residues one to three layers thick on one or both sides.
C3: The endosteal margin consists of thick cortical residues and is clearly porous.
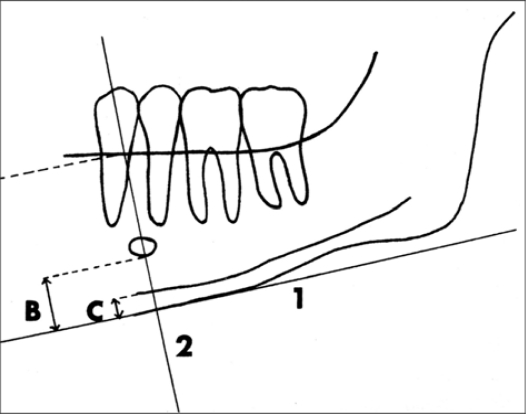
According to Devlin [14] and Karayianni [15] MI is the height of the mandibular inferior cortex. According to Wical and Swoope [16], h is the distance from the lower border of the mandible to the lower border of the mental foramen. PMI was calculated according to Benson et al’s method [17] as the ratio of MI/h. Since the mental foramen is not a region where masticatory muscles are attached, it is considered as the standard region for study in most of the surveys [17] (Fig 2).
Fig 2.
Measurements on the panoramic radiograph, made with Wical and Swoope technique. The inferior edge of the mental foramen was traced.
1: a line parallel to the long axis of the mandible and tangential to the inferior border of the mandible was drawn.
2: A line perpendicular to this tangent intersecting the inferior border of the mental foramen was constructed.
B: Distance from the lower border to the inferior edge of the mental foramen (h).
C: thickness of the mandibular cortex (MI).
All the measurements were made in millimeters (0.1 mm variation) with calipers by the same investigator (AB). The indices were measured bilaterally and the mean was recorded for each patient. When only one foramen was visible, the measurements were done only on that side [18]. The information on age and BMD statuses of the patients was blinded to the examiner in order to eliminate information bias.
Afterwards we gave the patients their radiographs and referred them to Toos Bone Densitometry Center for BMD testing. Written informed consent was obtained from the patients to carry out bone densitometry as a part of the study on osteoporosis. BMD testing was performed in the femoral neck and lumbar vertebrae (L2-L4), using DXA technique with Osteocore 2. BMD, Z-score and T-score were determined in each site. Z-score compares the individual’s data with the same age group population with the similar sex and race, but T-score compares the acquired results with the young population according to sex and race [19]. According to T-score, we classified the patients into three subgroups: normal (T-score>-1.0), osteopenic (−2.5<T-score≤-1.0) and osteoporotic (T-score≤-2.5) [20].
Calculations of means and standard deviations as well as correlations and differences were performed using SPSS 11.5 for Windows (SPSS Inc., IL). The correlation between studied variables was established using the Pearson correlation coefficient. One-way ANOVA was performed to assess a significant difference in mean MI and PMI between the various skeletal statuses in each site. The sensitivity, specificity, positive predictive values, and negative predictive values of mandibular variables in diagnosing normal and osteopenic-osteoporotic skeletal statuses were tested with dichotomous 2×2 tables. Receiver Operating Characteristic (ROC) curve analyses were used to determine the optimal cutoff thresholds of PMI and MI index for the identification of osteoporosis. A P-value less than 0.05 was considered statistically significant.
RESULTS
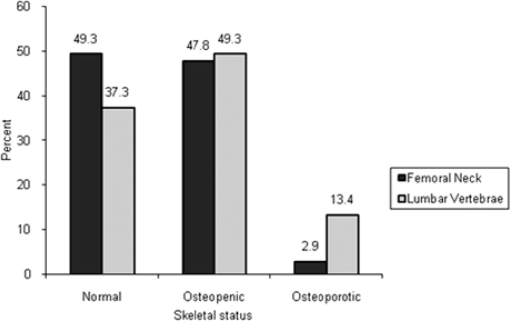
In this study 67 women with the mean age of 51.37 years (SD=9.95 years) attended. The mean for MI and PMI was 5.08 mm (SD=0.93 mm) and 0.32 (SD=0.05), respectively. The most common type of MCI in all patients was C2 (65.7%). According to the T-score we found that of the total number of 67 patients the number of normal, osteopenic and osteoporotic patients were 33, 32 and two, respectively in the femoral neck and 25, 33 and nine, respectively in the lumbar vertebrae. Both in the femoral neck and the lumbar spine the least of the patients were osteoporotic; i.e., 2.9% of the women in the femoral neck and 13.4% of them in the lumbar region were osteoporotic (Fig 3). The results showed that there was a significant difference in mean BMD in the patients classified as having C1 and C3 cortical appearance in the femoral neck (P=0.04), but the BMD values in C1-C3 groups did not differ significantly in the lumbar region (P=0.11). PMI assessments were not significantly correlated with BMD. Furthermore, the results of our study showed no significant correlation between PMI and bone densitometry variables (Table 1). The area under the ROC curve (AZ) for MI was 0.344 (95% CI, 0.214–0.475) in the lumbar vertebrae and 0.388 (95% CI, 0.249–0.526) in the femoral neck and for PMI it was 0.410 (95% CI, 0.272–0.548) in the lumbar vertebrae and 0.400 (95% CI, 0.253–0.548) in the femoral neck. Since the acquired AZ, sensitivity, and specificity were low in each cutoff threshold, we used several cutoff points to determine the sensitivity and specificity.
Fig 3.
Frequency of various skeletal statuses.
Table 1.
Correlation of PMI with BMD of the femoral neck and lumbar spine.
| Variable |
Pearson Correlation Coefficient (R) |
P-Value |
||
|---|---|---|---|---|
| femoral neck | lumbar region | femoral neck | lumbar region | |
| BMD | 0.09840 | 0.14388 | 0.4283 | 0.2454 |
| Z-Score | 0.08808 | 0.11281 | 0.4785 | 0.3634 |
| T-Score | 0.14163 | 0.16321 | 0.2529 | 0.1869 |
BMD=Bone Mineral Density
To determine the sensitivity and specificity of MCI and PMI indices, we changed the categories of the patients into two groups of normal and osteopenic-osteoporotic, who were previously classified into three subgroups of normal, osteopenic and osteoporotic, then the measurements were performed (Tables 2 and 3).
Table 2.
The ability of MCI to discriminate between normal and osteopenic-osteoporotic skeletal status.
| MCI | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy | |
|---|---|---|---|---|---|---|
| C2&C3 | Femoral neck | 79.4% | 39.3% | 57% | 65% | 60% |
| Lumbar region | 73.8% | 36.0% | 66% | 45% | 60% | |
MCI=Mandibular Cortical Index, C2&C3=Subgroups of MCI
Table 3.
The ability of PMI to discriminate between normal and osteopenic-osteoporotic skeletal statuses.
| PMI | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy | |
|---|---|---|---|---|---|---|
| ≤0.29 | Femoral neck | 20.5% | 91.0% | 70% | 53% | 55.2% |
| Lumbar region | 16.6% | 88.0% | 70% | 39% | 58.2% | |
| ≤0.32 | Femoral neck | 29.4% | 78.7% | 59% | 52% | 53.7% |
| Lumbar region | 26.1% | 76.0% | 65% | 38% | 44.7% | |
| ≤0.33 | Femoral neck | 29.4% | 66.6% | 48% | 48% | 47.7% |
| Lumbar region | 28.5% | 64.0% | 57% | 35% | 41.7% | |
| ≤0.46 | Femoral neck | 91.1% | 9.0% | 51% | 50% | 50.7% |
| Lumbar region | 95.2% | 16.0% | 65% | 67% | 65.6% | |
PMI=Panoramic Mandibular Index
According to Table 2, we may conclude that of the osteopenic-osteoporotic patients, 79.4% in the femoral neck and 73.8% in the lumbar region had C2 or C3 cortex (sensitivity). In the women with normal skeletal statuses, 39.3% in the femoral neck and 36% the lumbar region had C1 cortex (specificity). 57% and 66% of the patients with C2 or C3 cortex had osteopenia or osteoporosis in the femoral neck and the lumbar region, respectively (positive predictive value) and 65% and 45% of the patients with C1 cortex had normal skeletal statuses in the femoral neck and the lumbar region, respectively (negative predictive value). It is demonstrated in Table 3 that by increasing the PMI, sensitivity increases and specificity decreases. The maximum sensitivity and the minimum specificity are related to PMI≤0.46 and the highest positive predictive value is observed in PMI≤0.29. The maximum negative predictive value and accuracy in the femoral neck was in PMI≤0.29 and for the L2-L4 region, this value was detected in PMI≤0.46.
Our results showed a negative correlation between MI and age (P=0.016). There was also a positive correlation between MI and BMD in the femoral neck (P=0.021), T-score in the femoral neck (P=0.006), BMD in the lumbar region (P=0.013) and T- score in the L2-L4 region (P=0.008) (Table 4). One-way ANOVA was performed to assess a significant difference in mean MI between the skeletal statuses of normal, osteopenic and osteoporotic in the femoral neck and the lumbar region. A significant difference was shown by Tukey test between normal and osteopenic subgroups (P=0.042) (Table 5). According to Table 5 there was a significant difference in mean MI between various skeletal statuses of the femoral neck (P=0.046). As it is shown in Table 5, there was a significant difference in mean MI between various skeletal statuses of the lumbar region (P=0.022) and Tukey test showed a significant difference between normal and osteoporotic (P=0.017) and also osteopenic and osteoporotic subgroups (P=0.050).
Table 4.
The correlation of variables with MI in the patients.
| Variable | Pearson Correlation Coefficient (R) | P-Value |
|---|---|---|
| Age | −0.294* | 0.016 |
| BMI | 0.164 | 0.184 |
| BMD in the femoral neck | 0.282* | 0.021 |
| Z-Score in the femoral neck | 0.211 | 0.086 |
| T-Score in the femoral neck | 0.330# | 0.006 |
| BMD in the lumbar region | 0.304* | 0.013 |
| Z-Score in the lumbar region | 0.209 | 0.089 |
| T-Score in the lumbar region | 0.320# | 0.008 |
P<0.05,
P<0.01
Table 5.
Mean MI of various skeletal statuses in the femoral neck and the lumbar region
| Skeletal Statuses |
n |
Mean MI |
S.D |
95% CI |
||||
|---|---|---|---|---|---|---|---|---|
| Femoral neck | Lumbar region | Femoral neck | Lumbar region | Femoral neck | Lumbar region | Femoral neck | Lumbar region | |
| Normal | 33 | 25 | 5.37 | 5.30 | 0.95 | 0.81 | (5.03–5.71) | (4.96–5.63) |
| Osteopenic | 32 | 33 | 4.8 | 5.13 | 0.86 | 1.00 | (4.51–5.12) | (4.77–5.48) |
| Osteoporosis | 2 | 9 | 4.72 | 4.32 | 0.17 | 0.61 | (3.13–6.31) | (3.85–4.79) |
| Total | 67 | 67 | 5.08 | 5.08 | 0.93 | 0.93 | (4.85–5.31) | (4.85–5.31) |
MI=Mental Index, SD=Standard Deviation, CI=Confidence Interval
In order to obtain the sensitivity and specificity of MI we used two skeletal statuses of normal and osteopenic-osteoporotic subgroups for the further measurements (Table 6). According to Table 6, only 11.7% of the osteopenic-osteoporotic patients had MI≤3.69 mm in the femoral neck (sensitivity) but 100% of the patients with normal skeletal statuses had MI>3.69 mm in the femoral neck and the lumbar region (specificity). In addition, 100% of the patients with MI≤3.69 mm were osteopenic-osteoporotic in the femoral neck (positive predictive value) and 52% of the patients with MI>3.69 mm had normal BMD in the femoral neck (negative predictive value).
Table 6.
Ability of MI to discriminate between normal and osteopenic-osteoporotic skeletal statuses with 95% CI.
| MI (mm) | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
|---|---|---|---|---|---|
| ≤3.69 | Femoral neck | 11.7 (0.9–22.5) | 100.0 | 100.0 | 52.0 (35.2–68.8) |
| Lumbar region | 9.5 (0.6–18.3) | 100.0 | 100.0 | 40.0 (25.1–54.8) | |
| ≤4.00 | Femoral neck | 14.7(2.7–26.6) | 94.0 (86.0–100.0) | 71.0 (55.7–86.2) | 52.0 (35.2–68.8) |
| Lumbar region | 14.2 (3.6–24.7) | 96.0 (90.0–100.0) | 86.0 (75.5–96.4) | 40.0 (25.1–54.8) | |
| ≤4.50 | Femoral neck | 29.4 (14.0–44.7) | 84.8 (72.7–96.8) | 66.6 (50.7–82.4) | 53.8 (37.0–70.5) |
| Lumbar region | 26.2 (12.9–39.4) | 84.0 (72.9–95) | 73.3 (59.9–86.6) | 40.0 (25.1–54.8) | |
| ≤5.00 | Femoral neck | 64.7 (48.6–80.7) | 51.5 (34.7–68.3) | 58.0 (41.4–74.5) | 59.0 (42.4–75.5) |
| Lumbar region | 64.2 (49.7–78.7) | 56.0 (41.0–71.0) | 71.0 (57.2–84.7) | 48.0 (32.9–63.1) | |
MI=Mental Index, CI=Confidence Interval
DISCUSSION
PMI, MCI and MI are the parameters evaluated in screening osteoporosis. Some of the investigators have reported that they could be used in screening osteoporosis [3,7,13,21], but others disagree [1,22]. In this study we did not observe a significant difference between mean PMI in the various skeletal subgroups in the femoral neck and the lumbar vertebrae (L2-L4). Similarly, Watson et al [23] did not find any difference in the mean PMI between normal (0.38) and osteoporotic (0.37) females in the 54–71 years age group, which agrees with Drozdzowska et al [1], who performed BMD measurements of the hip using DXA technique. Our study reveals that PMI in women is not significantly correlated with bone density in the femoral neck and the lumbar region. Similar results were reported by Drozdzowska et al [1]. Klemetti and Kolmakow [4] also reported that the correlation between PMI and BMD of the femoral neck and spine measured in 355 British women were weak but significant (r=0.20–0.24, P=0.001), contrary to Taguchi et al [24]. One of the most commonly studied parameters of the mandibular bone in relation to osteoporosis is the porosity of mandibular cortical bone (MCI). Some of the investigators have found an association between MCI and osteoporosis [4,16,25–26]. Dalili et al [27] reported the MCI frequency of 20.9%, 71.3%, and 7.8% of C1, C2, and C3 cortices, respectively. In the present survey, these values were 29.9%, 65.7% and 4.4%, respectively. The minor difference in the data between these two studies is due to the different age group of the women, number of the patients and also the variation in nutrition.
The ability of mandible variables (MCI, PMI) to discriminate between normal and osteopenic-osteoporotic subgroups was poor to moderate. The specificity, sensitivity, positive predictive value, and negative predictive value in PMI≤0.33 in the femoral neck were 66.6%, 29.4%, 48%, and 48%, respectively and in the lumbar vertebrae were 64%, 28.5%, 57%, and 35%, respectively. In comparison with Drozdzowska et al’s study [1], all the indices, especially the specificity were lower. If the cutoff threshold increases from 0.33 to 0.46, the sensitivity increases noticeably (91.1% in the femoral neck and 95.2% in the lumbar region). Drozdzowska et al [1] reported a 31% specificity, 93% sensitivity, 54% positive predictive value and a 83% negative predictive value for MCI in the hip, but in our study these values amounted to 39.3%, 79.4%, 57% and 65%, respectively in the femoral neck and 36% 73.8%, 66% and 45%, respectively in the lumbar region. The difference may be due to racial difference and number of cases.
The current study indicates a significant difference in MI between normal and osteopenic subgroups in the femoral neck (P=0.042). Also, in the lumbar region, normal and osteoporotic (P=0.017) and osteopenic and osteoporotic subgroups (P=0.050) showed significant differences. However, Drozdzowska et al [1] noted that there was a significant difference in MI between various skeletal statuses (4.5 mm, 5.1 mm, 3.5 mm and P<0.05).
Bollen et al [18] reported that in patients with osteoporotic fractures, thickness of the mandibular inferior cortex was 0.54 mm thinner than the control group, but Mohajery and Brooks [22] found no significant difference in MI between osteoporotic and normal post-menopausal women; it should be considered that they used the cortical thickness in the mandibular angle instead of MI. We found a negative correlation between MI and age (P=0.016, r=−0.294), which is concordant with that of Ledgerton et al’s study [9]. Arifin et al [28] used a digital system for MI measurement and noted that MI and BMD had significant correlation in the lumbar region and the femoral neck (r=0.50, r=0.54, P<0.001 and P<0.001, respectively). This agrees with the significant correlation which we found (r=0.30, r=0.28, P=0.013 and P=0.021, respectively) but the lower correlation of our results may be due to the digital system, used in Arifin et al [28] study and it’s higher accuracy in measurements.
The ability of MI to discriminate between normal and osteopenic-osteoporotic groups was poor to moderate. Drozdzowska et al [1] reported the specificity, sensitivity, positive predictive and negative predictive value of 81%, 21%, 50%, and 54% for MI≤4 mm in the hip. According to Table 6, these values for MI≤4 mm in the femoral neck were 94%, 14.7%, 71% and 52%, respectively and 96%, 14.2%, 86% and 40%, respectively in the lumbar region. Taguchi et al [29] also determined the specificity, sensitivity, positive predictive and negative predictive values of 42.1%, 79.4%, 71%, and 53.3% in the lumbar vertebrae, respectively for MI≤4.5 mm, which were 84%, 26.2%, 73.3%, and 40% in the lumbar spine in our study. Since on time diagnosis of osteopenic-osteoporotic patients may prevent side effects of the disease, in comparison with high specificity, high sensitivity is more useful. Compared with MI≤4.5 mm, increasing the mandibular cortical thickness by 0.5 mm (MI=5 mm), the sensitivity increases to 64.7% in the femoral neck and 64.2% in the L2-L4 region, which has more considerable sensitivity than MI≤4.5 mm.
CONCLUSION
Contrary to PMI, which needs recording of some measurements on panoramic radiographs and also some calculations, MCI is a simple three-graded classification of the changes in the cortex with higher sensitivity in detecting osteopenic and osteoporotic patients. In addition, according to the simplicity of using the MI index, these two indices privileged PMI to discriminate the skeletal status of the patients, which of course are not adequate for precise evaluation.
Acknowledgments
This study was supported by the vice chancellor for research of Mashhad University of Medical Sciences, Dr. Jalil Tavakol Afshari, as grant No. 83082 and the authors are very thankful.
REFERENCES
- 1.Drozdzowska B, Pluskiewicz W, Tarnawska B. Panoramic-based mandibular indices in relation to mandibular bone mineral density and skeletal status assessed by dual energy X-ray absorptiometry and quantitative ultrasound. Dentomaxillofac Radiol. 2002 Nov;31(6):361–7. doi: 10.1038/sj.dmfr.4600729. [DOI] [PubMed] [Google Scholar]
- 2.Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Ohtaki M. Usefulness of panoramic radiography in the diagnosis of post-menopausal osteoporosis in women. Width and morphologhy of inferior cortex of the mandible. Dentomaxillofac Radiol. 1996 Nov;25(5):263–7. doi: 10.1259/dmfr.25.5.9161180. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi A, Tanimoto K, Suei Y, Otani K, Wada T. Oral signs as indicators of possible osteoporosis in elderly women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995 Nov;80(5):612–6. doi: 10.1016/s1079-2104(05)80158-1. [DOI] [PubMed] [Google Scholar]
- 4.Klemetti E, Kolmakow S. Morphology of the mandibular cortex on panoramic radiographs as an indicator of bone quality. Dentomaxillofac Radiol. 1997 Jan;26(1):22–5. doi: 10.1038/sj.dmfr.4600203. [DOI] [PubMed] [Google Scholar]
- 5.Kribbs PJ, Chesnut CH, 3rd, Ott SM, Kilcoyne RF. Relationships between mandibular and skeletal bone in an osteoporostic population. J Prosthet Dent. 1989 Dec;62(6):703–7. doi: 10.1016/0022-3913(89)90596-9. [DOI] [PubMed] [Google Scholar]
- 6.Horner K, Devlin H. The relationships between two indices of mandibular bone quality and bone mineral density measured by dual energy X-ray absorptiometry. Dentomaxillofac Radiol. 1998 Jan;27(1):17–21. doi: 10.1038/sj.dmfr.4600307. [DOI] [PubMed] [Google Scholar]
- 7.White SC, Rudolph DJ. Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999 Nov;88(5):628–35. doi: 10.1016/s1079-2104(99)70097-1. [DOI] [PubMed] [Google Scholar]
- 8.Law AN, Bollen AM, Chen SK. Detecting osteoporosis using dental radiographs: a comparison of four methods. J Am Dent Assoc. 1996 Dec;127(12):1734–42. doi: 10.14219/jada.archive.1996.0134. [DOI] [PubMed] [Google Scholar]
- 9.Ledgerton D, Horner K, Devlin H, Worthington H. Radiomorphometry indices of the mandible in a British female population. Dentomaxillofac Radiol. 1999 May;28(3):173–81. doi: 10.1038/sj/dmfr/4600435. [DOI] [PubMed] [Google Scholar]
- 10.Ishii K, Taghuchi A, Nakamoto T, Ohtsuka M, Sutthiprapaporn P, Tsuda M, et al. Diagnostic efficacy of alveolar bone loss of the mandible for identifying post-menopausal women with femoral osteoporosis. Dentomaxillofac Radiol. 2007 Jan;36(1):28–33. doi: 10.1259/dmfr/28366679. [DOI] [PubMed] [Google Scholar]
- 11.Adams JE. Osteoporosis and bone mineral densitometry. Curr Opin Radiol. 1992;4(6):11–20. [PubMed] [Google Scholar]
- 12.Jowitt N, MacFarlane T, Devlin H, Klemetti E, Horner K. The reproducibility of the mandibular cortical index. Dentomaxillofac Radiol. 1999 May;28(3):141–4. doi: 10.1038/sj/dmfr/4600427. [DOI] [PubMed] [Google Scholar]
- 13.Klemetti E, Klomakov S, Kroger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res. 1994 Feb;102(1):68–72. doi: 10.1111/j.1600-0722.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 14.Wical KE, Swoope CC. Studies of residual ridge resorption. Part 1. Use of panoramic radiographs for evaluation and classification of mandibular resorption. J Prosthet Dent. 1974 Jul;32(1):7–12. doi: 10.1016/0022-3913(74)90093-6. [DOI] [PubMed] [Google Scholar]
- 15.Delvin H, Horner K. Mandibular radiomorphometric indices in the diagnosis of reduced skeletal bone mineral density. Osteoporos Int. 2002 May;13(5):373–8. doi: 10.1007/s001980200042. [DOI] [PubMed] [Google Scholar]
- 16.Karayianni K, Horner K, Mitsea A, Berkas L, Mastoris M, Jacobs R, et al. Accuracy in osteoporosis diagnosis of a combination of mandibular cortical width measurement on dental panoramic radiographs and a clinical risk index (OSIRIS): The OSTEODENT project. Bone. 2007 Jan;40(1):223–9. doi: 10.1016/j.bone.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Benson BW, Prihoda TJ, Glass BJ. Variations in adult cortical bone mass as measured by a panoramic mandibular index. Oral Surg Oral Med Oral Pathol. 1991 Mar;71(3):349–56. doi: 10.1016/0030-4220(91)90314-3. [DOI] [PubMed] [Google Scholar]
- 18.Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Case-control study on self-reported osteoporotic fractures and mandibular cortical bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Oct;90(4):518–24. doi: 10.1067/moe.2000.107802. [DOI] [PubMed] [Google Scholar]
- 19.Braunwald E, Faucis AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrison’s Principle of Internal Medicine. 15th ed. New York: McGraw-Hill; 2001. p. 2226. [Google Scholar]
- 20.Kanis JA, WHO study group Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int. 1994 Nov;4(6):368–81. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 21.Pothuaud L, Lespessailles E, Harba R, Jennane R, Royant V, Eynard E, et al. Fractal analysis of trabecular bone texture on radiographs: discriminate value in postmenopausal osteoporosis. Osteoporos Int. 1998;8(5):618–25. doi: 10.1007/s001980050108. [DOI] [PubMed] [Google Scholar]
- 22.Mohajery M, Brooks SL. Oral radiographs in the detection of early signs of osteoporosis. Oral Surg Oral Med Oral Pathol. 1992 Jan;73(1):112–7. doi: 10.1016/0030-4220(92)90167-o. [DOI] [PubMed] [Google Scholar]
- 23.Watson EL, Katz RV, Adelezzi R, Gift HC, Dunn SM. The measurement of mandibular cortical bone height in osteoporotic versus non-osteoporotic post-menopausal women. Spec Care Dentist. 1995 May-Jun;15(3):124–8. doi: 10.1111/j.1754-4505.1995.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol. 1996 Jun;25(3):130–5. doi: 10.1259/dmfr.25.3.9084261. [DOI] [PubMed] [Google Scholar]
- 25.Nakamoto T, Taguchi A, Ohtsuka M, Suei Y, Fujita M, Tanimoto K, et al. Dental panoramic radiographs as a tool to detect postmenopausal women with low bone mineral density: untrained general dental practitioners, diagnostic performance. Osteoporos Int. 2003 Aug;14(8):659–64. doi: 10.1007/s00198-003-1419-y. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Ohtaki M. Usefulness of panoramic radiography in the diagnosis of postmenopausal osteoporosis in women. Width and morphology of inferior cortex of the mandible. Dentomaxillofac Radiol. 1996 Nov;25(5):263–7. doi: 10.1259/dmfr.25.5.9161180. [DOI] [PubMed] [Google Scholar]
- 27.Dalili Z, Moghadam Qujeq A. An investigation on mandibular radiometric indices in normal female population referred to maxillofacial radiology centres in Rasht. Journal of Dental Medicine. 2003;16(3):14–21. [Google Scholar]
- 28.Arifin AZ, Asano A, Taguchi A, Nakamoto T, Ohtsuka M, Tsuda M, et al. Computer aided system for measuring the mandibular cortical width on dental panoramic radiographs in identifying post-menopausal women with low bone mineral density. Osteoporos Int. 2006;17(5):753–9. doi: 10.1007/s00198-005-0045-2. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, et al. Validation of dental panoramic radiography measures for identifying post-menopausal women with spinal osteoporosis. AJR Am J Roentgenol. 2004 Dec;183(6):1755–60. doi: 10.2214/ajr.183.6.01831755. [DOI] [PubMed] [Google Scholar]