Abstract
Objective:
Vertical bone loss evaluations in the Nobel Biocare Replace® Select Tapered ™ implant system in the human after one-year loading time.
Materials and Methods:
This retrospective cross-sectional study was performed on 31 patients (14 men, 17 women; mean age, 60.39 years) receiving 170 implants (mean, 5.48 for each patient) of Groovy and Non-groovy designs in the Nobel Biocare Replace® Select Tapered ™ system. The marginal bone loss was measured at mesial and distal aspects of the implants on OPG x-rays after one-year follow-up. The data regarding the patient’s gender, age, history of disease, smoking, bone type at implant location, loading time of prosthesis and implant, implant design, diameter and length were recorded by the patients’ records and interview. The data were subjected to multiple linear regression and Pearson coefficient ratio regarding different factors.
Results:
The mean (standard deviation) distal, mesial and overall bone loss was 0.688 mm (0.851), 0.665 mm (0.849) and 0.935 mm (0.905), respectively in the studied implants. No significant differences were found regarding implant location, bone quality at the implant region, implant design and bone graft reception. In addition, no significant correlation was found between the occurred bone loss and implant diameter, length and number of used splints.
Conclusion:
Due to the criteria mentioned for implant success in term of bone loss values after one-year loading time, Noble Biocare Replace® Select Tapered ™ implant system is an acceptable treatment option for implant restorations in this regard.
Keywords: Dental Implants; Data Interpretation, Statistical; Radiography, Panoramic; Alveolar Bone Loss
INTRODUCTION
Dental missing impairs the patients’ facial esthetics and produces difficulty in mastication and speech. Although common dental prostheses are able to restore the patients’ esthetics and oral function, some shortcomings using them have caused patients to search for implant-supported prostheses. Decreasing treatment time and patient discomfort while achieving higher predictability and an excellent esthetic outcome are advantages of dental implant treatments. An approximate 100% success rate, lack of damage to the adjacent teeth, bone loss and unpleasant esthetics as common disadvantages of fixed and removable prostheses have drastically increased the use of implant treatments [1–6].
It has been shown that osseointegration was best achieved when the implant was placed in its position using an appropriate surgical technique with the least trauma and also avoiding overheating in the preparation procedure [6]. Implants must have primary stability and should be mechanically loaded during the healing process for 2–6 months. All the above mentioned factors could affect osseointegration [7,8].
The clinical long-term success of the implants depends on the osseointegration and the adhesion of the soft tissues and epithelium to the titanium surfaces of the implant [9,10]. As dental implants are exposed to the oral environment, they may be confronted with factors such as smoking or bacterial plaques. Furthermore, the keratinized mucosa width, the history of periodontal and systemic diseases, bone resorption and even the patients’ age and gender are all determinants of the implant success rate [11]. Osseointegrated implants are in close contact with the bone compared to natural teeth, in which PDL collagen fibers and their position design act as a barrier against bacterial leakage. Therefore, in case of failed implants, the peri-implant tissues are susceptible to infection, which makes the incidence of different complexities possible [11].
Different indices including plaque index, gingival index, bleeding, probing pocket depth, probing attachment index and bone resorption are used to assess the health of peri-implant soft tissues [12,13]. Marginal bone loss is evaluated by means of radiography and is directly associated with the long-term success of implant treatments. According to Albrektsson et al [14], marginal bone level changes in the first year after implant insertion should be less than 1–1.5 mm and the ongoing annual bone loss should be less than 0.2 mm. Using Branemark System, Adell et al [15] reported a bone loss of 1.2 mm for the first year in their 15-year study. It seems that the initial marginal bone level change occurs as an adaptation of the peri-implant bone to the occlusal load [16]. A review of 13 studies that had reported on marginal bone loss occurring around Branemark implants after the first year of insertion found a mean loss of 0.93 mm, with a range of 0.4 to 1.6 mm [17]. However, it must be noted that a more definitive conclusion about the value of bone loss around implants in time requires more scientifically designed long-term investigations over a broader age range. Investigations performed about the marginal bone changes around dental implants are important not only for the functional maintenance of the implant but also for the esthetic success of the dental implants.
The increased use of implant treatments among patients forced manufacturers to modify their implant systems and introduce new systems. The aim of the present study was to retrospectively evaluate bone loss in patients treated with the Nobel Biocare Replace Select Tapered ™ (Nobel Biocare, Gottenborg, Sweden) implant system in a private clinic in Tehran after 1 year following implant loading.
MATERIALS AND METHODS
A total of 31 patients (14 men, 17 women; mean age, 60.39 years) receiving 170 of Nobel Biocare Replace select Tapered ™ (Gottenberge, NobelBiocare, Sweden) implant systems participated in the study. The study design was approved by the research committee of Tehran Dental School. All patients were treated at a private clinic with dental implant by means of a high profile, an experienced surgeon, cooperation of an experienced surgical team and performed as recommended by the manufacturer.
The subjects had their pretreatment radiographies in their records, were free of systemic disorders effective on their peri-implant tissues, and participated in all follow-up assessments. The patients were assessed a year after abutment connection and functional loading. All patients showed acceptable periodontal status (plaque index<15).
During the follow-up examinations, the marginal bone resorption around inserted implants was determined by panoramic radiography and was recorded in specific charts. The patients’ age, gender, history of disease, smoking, bone quality, implant and prosthesis loading time, implant location in the maxilla and mandible (anterior and posterior sectors), the opposing teeth, implant length, and diameter were obtained from the patients’ records. All implants were tapered in geometry with two Groovy and Non-groovy designs. Groovy implants had microthread in their coronal part while Non-groovy implants had been instrumented with polish surface at their coronal part. All used bone grafts were demineralized bovine bone graft materials (Bio-Oss, Switzerland, Geishtlish Co).
For evaluating the influence of bone type on implant bone loss, the bone quality was recorded during the surgery with respect to the surgeon’s sense and according to lekholm and zarb index.
For the assessment of postoperative changes of the bone level from the time of implant placement to one year after implant exposure, conventional dental radiographs were taken at the time of follow-up investigations using OPG device (Planmeca CC, Proline, Finland) in standard exposures of 6mA and 66–68 kVp. The film used was AGFA (Belgium) and the material for processing was Tetenal.
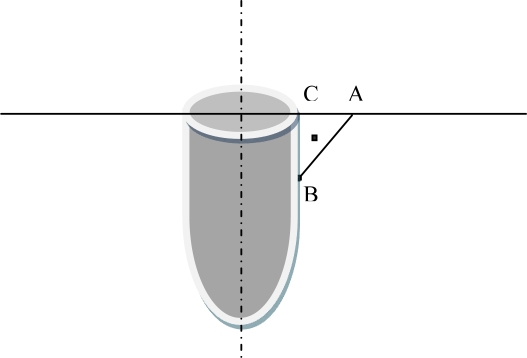
In order to measure the marginal bone loss, a line was traced from the most upper point adjacent to the implant on the crest of the alveolar bone (point A) perpendicular to the implant axis, next the lowest point located at the bone loss depth was determined as point B. By drawing, a perpendicular line from point B to the above mentioned line, point C was determined (Fig 1). The distance between B and C points was calculated by a ruler with 1.2 magnifications in millimeters for each implant. This distance was representative of the vertical bone loss aimed at the present study (Fig. 1). Furthermore, the bone loss was calculated at the mesial and distal sides of the implant and the most observed loss occurring in mesial or distal sides were considered as the final implant bone loss [18]. All measurements were performed by an instructed dental student and checked by another investigator under supervision of the main investigator of the study.
Fig 1.
Measurement of vertical bone loss
A: the most upper point adjacent to the implant on the crest of the alveolar bone. The lowest point located at the bone loss depth was determined as point B. Point C is determined by drawing a vertical line from point B to the line that passes from point A perpendicularly to the implant surface. The distance from point B to C is considered as vertical bone loss.
The mean bone loss was calculated and multiple linear regression analysis was used to assess the effect of different variables on distal, mesial, and final bone losses. Pearson coefficient was used to analyze the correlation of implant bone loss with implant diameter, length and the number of used splints. The data were analyzed by SPSS 16.0 for Windows.
RESULTS
The mean number of implants received by each patient was 5.48 (range, 1–13). Three of the patients (9.7%) were smokers and five patients (16.5%) had signs of bleeding on probing. The mean time of prostheses loading was 3.53 months (range, 2–8 months). Seven (4.1%) of the implants were placed in type I bone quality, 106 (62.4%) in type II, 50 (29.4%) in type III, and finally seven (4.1%) in type IV bone quality. Twenty (11.8%) implant sites were subjected to soft tissue grafting using Bio-Oss. Eighty-seven implants (51.2%) were placed in the mandibles and 83 (48.8%) in the maxillae. Sixty implants (35.3%) were located in the anterior areas and 110 (64.7%) in the posterior regions. In the present study, 66 (38.8%), 36 (21.2%), 26 (15.3%), 26 (15.3%), 13 (7.6%) and three (1.8%) patients had implants, natural dentitions, edentulous regions, bridges, crowns, veneers in the opposing arch respectively. One hundred and fifty seven implants (92.4%) were Groovy and 16 (7.6%) were Non-groovy. The mean number of the splints was 3.79 (range, 1–11).
The mean (standard deviation) distal bone loss was 0.688 mm (0.851) and 0.665 mm (0.849) at the mesial sides of studied implants. The mean overall bone loss was 0.935 mm and the most frequent values observed at distal and mesial sides was 0.905 mm in the studied implants.
The mean (standard error) bone loss at the distal sides of anterior and posterior implants was 0.833 mm (0.122) and 0.609 mm (0.075), respectively. These values were 0.717 mm (0.117) and 0.636 mm (0.078) at the mesial sides of anterior and posterior implants, respectively. Furthermore, the mean overall bone loss (standard error) of implants placed at anterior and posterior regions were 1.03 mm (0.128) and 0.882 mm (0.081). The distal, mesial, and overall bone loss were measured similarly for these regions too.
No significant differences were found regarding bone loss occurring at the distal and mesial sides of the mandibular and maxillary implants or the maximum bone loss, taking place at these sides between the upper and lower implants. The mean (standard error) distal bone loss of mandibular and maxillary implants were 0.759 mm (0.088) and 0.615 mm (0.097), respectively. The mean mesial bone loss of mandibular and maxillary implants was also 0.701 mm (0.088) and 0.627 mm (0.097), respectively. The mean overall bone loss was calculated as 0.966 mm (0.092) in mandibular and 0.904 mm (0.105) in maxillary upper implants. The maximum bone loss occurring at the distal and mesial sides were 0.949 mm (0.073) and 0.769 mm (0.231), respectively.
Multiple linear regression showed that the implant type (Groovy and Non-groovy) does not have any significant effect on bone loss occurring at the distal or mesial sides of implants. In addition, there was no significant difference between the overall bone loss in the two implant types. The mean (standard error) bone loss at the distal sides of Groovy and Non-groovy implants was 0.694 mm (0.068) and 0.615 mm (0.241), respectively. These values were 0.688 mm (0.069) and 0.385 mm (0.14) at the mesial sides of the implants. The maximum bone loss occurring at the distal and mesial sides were 0.949 mm (0.073) and 0.769 mm (0.231), respectively. The data suggested higher insignificant bone loss for Groovy implants, especially on the mesial sides.
The mean (standard error) distal bone loss of implants received by men and women were 0.632 mm (0.078) and 0.760 mm (0.11) compared to 0.589 mm (0.074) and 0.760 mm (0.114) mesial bone loss. The mean overall bone loss for implants placed in men and women was 0.842 mm (0.079) and 1.05 mm (0.120), respectively. Although women presented with higher values of bone loss, no statistically significant differences were observed in distal, mesial, and overall bone losses between men and women as shown by multiple linear regression.
The mean (standard error) distal, mesial and overall bone loss for implant sites with Bio-Oss grafts was 0.6 mm (0.234), 0.5 mm (0.17) and 0.85 mm (0.233), respectively compared to a distal bone loss of 0.7 mm (0.067), a mesial bone loss of 0.687 mm (0.07) and an overall bone loss of 0.947 mm (0.073) in implant sites without Bio-Oss grafts. Although slightly higher bone loss was measured for implants not receiving bone grafts, no statistically significant differences were observed between the two groups in terms of mesial, distal, and overall bone losses.
The mean (standard error) distal, mesial and overall bone losses were calculated as 0.857 mm (0.261), 1.0 mm (0.219) and 1.0 mm (0.218) for implant sites with type I bone quality, 0.632 mm (0.081), 0.623 mm (0.076) and 0.859 mm (0.083) for type II bone quality, 0.840 mm (0.129), 0.720 mm (0.137) and 1.14 mm (0.14) for type III bone quality, and 0.286 mm (0.286), 0.571 mm (0.429) and 0.571 mm (0.429) for type IV bone quality, respectively. No statistically significant differences were found between different bone qualities at implant sites regarding distal, mesial, and overall bone losses as shown by multiple linear regression analysis.
The correlation between implant lengths, diameters and splint numbers with mesial, distal and overall bone losses were assessed by Pearson correlation coefficient and the results suggested no significant correlations among them (Table 1).
Table 1.
Correlation between specific implant factors and types of bone loss.
| Factors |
Distal Bone Loss |
Mesial Bone Loss |
Overall Bone Loss |
|||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Splint Number | 0.066 | 0.390 | 0.030 | 0.680 | 0.0140 | 0.860 |
| Implant Diameter | −0.061 | 0.433 | 0.000 | 0.990 | −0.006 | 0.940 |
| Implant Length | 0.073 | 0.344 | −0.091 | 0.240 | −0.004 | 0.950 |
DISCUSSION
As in other parts of the skeleton, human jaws demonstrate specific tendencies for progressive time-dependent bone loss, including regions rehabilitated with oral implants. Therefore, as bone-anchored prostheses could be sustained in the oral environment for a long time in life, it is important to measure bone loss occurring around oral implants [19]. The measures of clinical oral implant success include immobility of individual implants, minimal bone loss, and no persistent morbidity [20]. Therefore, it is important to study bone loss, which has occurred after different loading times following implant insertion. The present study assessed mesial, distal, and overall bone loss of the Nobel Biocare Replace® System implants following one-year loading time inserted with a standard surgery protocol. Evaluation of the bony changes recorded on the mesial and distal surfaces of the selected functional implants were performed by intraoral radiographs after one year.
Our results showed a mean distal and mesial bone loss of 0.688 mm and 0.665 mm, respectively. The mean overall bone loss of Nobel Biocare Replace® System (the most values observed at both sides) was also estimated as 0.935 mm.
Different findings were presented by authors regarding implant bone loss following different loading times. Adell et al [15] reported a mean bone loss amounting to 1.5 mm in a 1-year follow-up. According to Rismanchian and Birang, the mean bone loss was 1.08 mm at loading time and 1.43 mm after 2 years following implant insertion [21]. Hobo et al [22] reported the mean bone loss of 1–1.5 mm for the first year of implant placement, whereas Worthington showed a mean maximum bone loss of 2 mm around the implant after 1-year loading time [23]. Behneke et al [24] showed a mean bone loss of 0.8 mm around ITI implants at loading time and a mean annual 0.1 mm bone loss for the following 3 years afterwards. In another study conducted by Behneke et al [25], similar findings were presented, reporting a mean bone loss of 0.7 mm from implant insertion to loading time and 0.1 mm annual bone loss. The mean bone loss of 0.9 mm was reported for implants at loading time and 1.4 mm after 2 years following implant placement in another study [4]. On the other hand, Johansson and Ekfeldt [26] showed a mean bone loss amounting to 0.4 mm at the first year and annual following 0.1 mm rate on Branemark implants. The result of the present study was similar to the findings reported by Albrektsson et al [14] for the range of 0.9–1.6 mm bone loss in the first year following implant placement. Compared with the above-mentioned reports, the mean bone loss measured in the present study was lower for some comparisons, which can be justified in term of good oral care and using standardized surgery and treatment protocols all suggesting appropriate efficacy of Nobel Biocare Replace® Implant System.
Roccuzzo et al [27] reported the mean marginal bone loss of 0.65 mm for implants after 6 weeks loading and 0.77 mm after 12 weeks loading when comparing 68 implants subjected to initial loading with 68 implants loaded by the common technique. Salvi et al [28] reported a mean crestal bone loss of 0.57 mm for implants loaded 1 week after insertion and 0.72 mm for implants loaded after 6 weeks. Furthermore, Boronat et al [18] showed the mean marginal bone loss of 0.58 mm following 1-year loading time.
Some authors studied similar brands of implant systems to achieve more generalized results diminishing the confounding effect of implant designing. In this regard, the present study was performed on tapered implants of Nober Biocare Replace® System including two Groovy and Nongroovy designs. However, no significant differences were found in the mean bone loss occurring between the two designs, which can be attributed to the similarity of the implant system used. The finding was in contrary to the results of Karoussis et al [29] who reported implant designs as a significant determinant of peri-implantitis lesions associated with bone loss.
No significant differences were found in mesial, distal, and overall bone loss regarding bone qualities at implant sites, receiving bone grafts, implant locations at anterior or posterior regions, or at maxillary or mandibular regions and the patient’s gender. Furthermore, no associations were found between mesial, distal, and overall bone loss and the implants’ diameter, length and the number of splints used. Similarly, some studies reported no significant different bone loss values between maxillary and mandibular implants [18,30,31]. Kumar et al [32] studied 1183 ITI SLA implants and observed no significant different bone loss regarding the jaw position [32]. Tamizi et al [33] showed similar bone loss values for implants placed at the maxilla and mandible. On the contrary, Penarrocha et al [34] showed more bone loss for fixtures implanted at maxilla compared to mandibular fixtures. Pham et al [35] reported more bone loss for maxillary implants than mandibular ones. The results of Wyatt and Zarb [36] were similar to the present study as no significant differences were found regarding implants placed at anterior and posterior regions; however, Boronat et al [18] showed more bone loss for posterior implants compared to the anterior ones.
No significant differences were observed between different types of bone qualities in the present study. This finding is in agreement with data previously reported by Boronat et al [18]. However, Henry et al [37] observed that the greatest peri-implant bone loss occurred in the implants located in bone types III and IV (Lekholm and Zarb classification). Tawil et al [30] reported a similarly higher bone loss for type IV bone quality (mechanically weak cancellous bone) too. These differences may be interpreted with lower frequencies of type I and IV bone qualities (7 implant sites) in the present study as compared to type II and III qualities (106 and 50 sites).
According to Tamizi et al [33], implant diameter is a significant determinant of bone loss occurring around the implant with the risk of bone loss around a 3.5 mm-diameter implant to be 5.91 folds more than a 4 mm-diameter implant. Strong et al. reported more risks of bone loss for implants with lower diameters compared to those with higher diameters [38]. However, Tawil et al [30] showed similar values of bone loss for narrow and wide implants similar to the present study. Implants studied in the present study had diameters of 3.5 mm, 4.3 mm and 5 mm with tapered designs in the Nobel Biocare Replace® System. Narrow implants with 3.5 mm or 4 mm diameters are indicated for patients faced with space limitations between the tooth and its crest, while wider implants are indicated for patients requiring additional loading or when using an implant/abutment post with higher diameter is preferred.
Differences existed on the mean bone loss values reported by different authors may be attributed to the different implant designs, the surgeon’s experience, the number of studied implants, oral hygiene status and practice in patients undergoing implant restorations, the time passed from implant reception, the bone quality at implant sites or different measures to assess implant treatments. Furthermore, the depth of implant insertion, rough surface towards the mucosa, in situ preparation, and immediate loading may have influence on the outcome.
The critical values of bone loss following one year of implantation (osseointegration period) have been proposed to be less than 1.5 mm with the mean 0.1 mm annual rate in the following years [39–41]. In In our study, all implants were tapered in one brand name, and were placed with just a highly qualified surgeon that can express a more trustable data in comparison with the other studies results which used different implant designs and/or surgeons. The mean mesial, distal, and overall bone loss of the implants were measured less than the mentioned critical value, which may be regarded as successful. In this regard, more studies are required to assess long-term bone loss or clinical parameters such as plaque, bleeding and gingival indices after implant insertion. These investigations can provide clinicians with accurate and exact knowledge about different implant systems helping them to choose the most stable and appropriate system.
CONCLUSION
The mean overall bone loss of the Nobel Biocare Replace® System implant after 1-year loading was 0.935 mm. Due to the critical values of 1.5 mm for bone loss after 1-year loading time, it can be concluded that Noble Biocare Replace® System is a suitable treatment option for implant restorations in this regard; however, more studies are required to assess long-term bone loss and other clinical parameters.
Acknowledgments
The authors wish to thank Dr. F. Nasiri for invaluable helps and comments during performance and accomplishment of the study. Also we have special thanks to Dr. R. Afzalifar and all Dr. A. Mesgarzadeh’s private office staffs for diligent collaborations.
REFERENCES
- 1.Karoussis IK, Muller S, Salvi GE, Heitz-Mayfield LJ, Bragger U, Lang NP. Association between periodontal and peri-implant conditions: a 10-year prospective study. Clin Oral Implants Res. 2004 Feb;15(1):1–7. doi: 10.1111/j.1600-0501.2004.00982.x. [DOI] [PubMed] [Google Scholar]
- 2.Anner R, Becker H, Chaushu G. The clinical effectiveness of 6 mm diameter implants. J Periodontol. 2005 Jun;76(6):1013–5. doi: 10.1902/jop.2005.76.6.1013. [DOI] [PubMed] [Google Scholar]
- 3.Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Bragger U, Hammerle CH, Lang NP. Long term implant prognosis in patients with and without a history of chronic periodontitis: a 10 year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2003 Jun;14(3):329–39. doi: 10.1034/j.1600-0501.000.00934.x. [DOI] [PubMed] [Google Scholar]
- 4.Behneke A, Behneke N, d’ Hoedt B. A 5-year longitudinal study of the clinical effectiveness of ITI solid-screw implants in the treatment of mandibular edentulism. Int J Oral Maxillofac Implants. 2002 Nov-Dec;17(6):799–810. [PubMed] [Google Scholar]
- 5.Wheeler S. Use of the Frialit-2 Implant System in private practice: a clinical report. Int J Oral Maxillofac Implants. 2003 Jul-Aug;18(4):552–5. [PubMed] [Google Scholar]
- 6.Branemark PI. Introduction to osseointegration. In: Branemark PI, Zarb GA, Albrektsson T, editors. Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence; 1985. pp. 11–76. [Google Scholar]
- 7.Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997 Jun;8(3):161–72. doi: 10.1034/j.1600-0501.1997.080302.x. [DOI] [PubMed] [Google Scholar]
- 8.Buser D, Mericske-Stern R, Dula K, Lang NP. Clinical experience with one-stage, non-submerged dental implants. Adv Dent Res. 1999 Jun;13:153–61. doi: 10.1177/08959374990130010501. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael RP, Apse P, Zarb GA, McCulloch CA. Biological, microbiological, and clinical aspects of the peri-implant mucosa. In: Albrektsson T, Zarb GA, editors. The Branemark osseointe-grated implant. Chicago, IL: Quintessence; 1989. pp. 39–78. [Google Scholar]
- 10.Listgarten MA, Lang NP, Schroeder HE, Schroeder A. Periodontal tissues and their counterparts around endosseous implants. Clin Oral Implants Res. 1991 Jul-Sep;2(3):1–19. doi: 10.1034/j.1600-0501.1991.020309.x. [DOI] [PubMed] [Google Scholar]
- 11.Goaz P, White SC. Oral radiology: principles and interpretation. St. Louis: CV Mosby Co; 1982. [Google Scholar]
- 12.Lozada JL, James RA, Boskovic M, Cordova C, Emanuelli S. Surgical repair of peri-implant defects. J Oral Implantol. 1990;16(1):42–6. [PubMed] [Google Scholar]
- 13.Bauman GR, Mills M, Rapley JW, Hallmon WH. Clinical parameters of evaluation during implant maintenance. Int J Oral Maxillofac Implants. 1992 Summer;7(2):220–7. [PubMed] [Google Scholar]
- 14.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986 Summer;1(1):11–25. [PubMed] [Google Scholar]
- 15.Adell R, Lekholm U, Rockler B, Branemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981 Dec;10(6):387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 16.Piao CM, Lee JE, Koak JY, Kim SK, Rhyu IC, Han CH, et al. Marginal bone loss around three different implant systems: radiographic evaluation after 1 year. J Oral Rehabil. 2009 Oct;36(10):748–54. doi: 10.1111/j.1365-2842.2009.01988.x. [DOI] [PubMed] [Google Scholar]
- 17.Goodacre CJ, Kan JY, Rungcharassaeng K. Clinical complications of osseointegrated implants. J Prosthet Dent. 1999 May;81(5):537–52. doi: 10.1016/s0022-3913(99)70208-8. [DOI] [PubMed] [Google Scholar]
- 18.Boronat A, Penarrocha M, Carrillo C, Marti E. Marginal bone loss in dental implants subjected to early loading (6 to 8 weeks postplacement) with a retrospective short-term follow-up. J Oral Maxillofac Surg. 2008 Feb;66(2):246–50. doi: 10.1016/j.joms.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Branemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [PubMed] [Google Scholar]
- 20.Zarb GA, Albrektsson T. Consensus report: towards optimized treatment outcomes for dental implants. J Prosthet Dent. 1998 Dec;80(6):641. doi: 10.1016/s0022-3913(98)70048-4. [DOI] [PubMed] [Google Scholar]
- 21.Rismanchian M, Biragn R. Clinical efficacy of Nysastan Screw Implant Systems in the mandibular posterior regions: A prospective 2-year study. J Islamic Dent Assoc. 2007;19(2):62–7. [Google Scholar]
- 22.Hobo S, Ishida F, Garcia LT. Osseointegration and occlusal rehabilitation. 2nd ed. Tokyo: Quintessence Pub; 1990. p. 43. [Google Scholar]
- 23.Worthington PH. Complication and failures. In: Naert I, Vansteenberghe D, Worthington P, editors. Osseointegration in Oral Rehabilitation. Chicago: Quintessence; 1985. p. 181. [Google Scholar]
- 24.Behneke A, Behneke N, d’Hoedt B, Wagner W. Hard and soft tissue reactions to ITI screw implants: 3-year longitudinal results of a prospective study. Int J Oral Maxillofac Implants. 1997 Nov-Dec;12(6):749–57. [PubMed] [Google Scholar]
- 25.Behneke A, Behneke N, d’Hoedt B. The longitudinal clinical effectiveness of ITI solid-screw implants in partially edentulous patients: a 5-year follow-up report. Int J Oral Maxillofac Implants. 2000 Sep-Oct;15(5):633–45. [PubMed] [Google Scholar]
- 26.Johansson LA, Ekfeldt A. Implant-supported fixed partial prostheses: a retrospective study. Int J Prosthodont. 2003 Mar-Apr;16(2):172–76. [PubMed] [Google Scholar]
- 27.Roccuzzo M, Bunino M, Prioglio F, Bianchi SD. Early loading of sandblasted and acid-etched (SLA) implants: a prospective split-mouth comparative study. Clin Oral Implants Res. 2001 Dec;12(6):572–8. doi: 10.1034/j.1600-0501.2001.120604.x. [DOI] [PubMed] [Google Scholar]
- 28.Salvi GE, Gallini G, Lang NP. Early loading (2 or 6 weeks) of sandblasted and acid-etched (SLA) ITI implants in the posterior mandible. Clin Oral Implants Res. 2004 Apr;15(2):142–9. doi: 10.1111/j.1600-0501.2004.01014.x. [DOI] [PubMed] [Google Scholar]
- 29.Karoussis IK, Bragger U, Salvi GE, Burgin W, Lang NP. Effect of implant design on survival and success rates of titanium oral implants: a 10-year prospective study of the ITI dental implant system. Clin Oral Implants Res. 2004 Feb;15(1):8–17. doi: 10.1111/j.1600-0501.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 30.Tawil G, Mawla M, Gottlow J. Clinical and radiographic evaluation of the 5-mm diameter regular-platform Brånemark fixture: 2- to 5-year follow-up. Clin Implant Dent Relat Res. 2002;4(1):16–26. doi: 10.1111/j.1708-8208.2002.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson GE, Lindquist LW, Jemt T. Long-term marginal periimplant bone loss in edentulous patients. Int J Prosthodont. 2000 Jul-Aug;13(4):295–302. [PubMed] [Google Scholar]
- 32.Kumar A, Jaffin R, Berman C. The effect of smoking on achieving osseointegration of surface-modified implants: a clinical report. Int J Oral Maxillofac Implants. 2002 Nov-Dec;17(6):816–9. [PubMed] [Google Scholar]
- 33.Tamizi M, Ghanavati F, Radvar M, Ghanavati F, Rahmani MA. Comparison of bone healing aroung nonsubmerged and submerged implants in Maestro system of Biohorizon technology. Shahid Beheshti Uni Dental School J. 2005;23(1):18–27. [Google Scholar]
- 34.Penarrocha M, Palomar M, Sanchís JM, Guarinos J, Balaguer J. Radiologic study of marginal bone loss around 108 dental implants and its relationship to smoking, implant location and morphology. Int J Oral Maxillofac Implants. 2004 Nov-Dec;19(6):861–7. [PubMed] [Google Scholar]
- 35.Pham AN, Fiorellini JP, Paquette D, Williams RC, Weber HP. Longitudinal radiographic study of crestal bone levels adjacent to non-submerged dental implants. J Oral Implantol. 1994;20(1):26–34. [PubMed] [Google Scholar]
- 36.Wyatt CC, Zarb GA. Treatment outcomes of patients with implant-supported fixed partial prostheses. Int J Oral Maxillofac Implants. 1998 Mar-Apr;13(2):204–11. [PubMed] [Google Scholar]
- 37.Henry PJ, Tolman DE, Bolender C. The applicability of osseointegrated implants in the treatment of partially edentulous patients: three-year results of a prospective multicenter study. Quintessence Int. 1993 Feb;24(2):123–9. [PubMed] [Google Scholar]
- 38.Strong JT, Misch CE, Bidez MW, Nalluri P. Functional surface area: thread form parameter optimization for implant body design. Compendium Contin Educ Dent. 1998;19:4–9. [Google Scholar]
- 39.Becker W, Becker BE, Israelson H, Lucchini JP, Handelsman M, Ammons W, et al. One-step surgical placement of Brånemark implants: a prospective multicenter clinical study. Int J Oral Maxillofac Implants. 1997 Jul-Aug;12(4):454–62. [PubMed] [Google Scholar]
- 40.Hänggi MP, Hänggi DC, Schoolfield JD, Meyer J, Cochran DL, Hermann JS. Crestal bone changes around titanium implants. Part I: A retrospective radiographic evaluation in humans comparing two non-submerged implant designs with different machined collar lengths. J Periodontol. 2005 May;76(5):791–802. doi: 10.1902/jop.2005.76.5.791. [DOI] [PubMed] [Google Scholar]
- 41.Astrand P, Engquist B, Anzén B, Bergendal T, Hallman M, Karlsson U, et al. A three-year follow-up report of a comparative study of ITI Dental Implants and Brånemark System implants in the treatment of the partially edentulous maxilla. Clin Implant Dent Relat Res. 2004;6(3):130–41. doi: 10.1111/j.1708-8208.2004.tb00213.x. [DOI] [PubMed] [Google Scholar]