Abstract
Objective:
The aim of this study was to evaluate the bond strength between metal brackets and non-glazed ceramic with three different surface treatment methods.
Materials and Methods:
Forty-two non-glazed ceramic disks were assigned into three groups. Group I and II specimens were etched with 9.5% hydrofluoric acid. Subsequently in group I, silane and adhesive were applied and in group II, bonding agent was used only. In group III, specimens were treated with 35% phosphoric acid and then silane and adhesive were applied. Brackets were bonded with light-cured composites. The specimens were stored in water in room temperature for 24 hours and then thermocycled 500 times between 5°C and 55°C.
Results:
The difference of tensile bond strength between groups I and III was not significant (P=0.999). However, the tensile bond strength of group II was significantly lower than groups I, and III (P<0.001). The adhesive remnant index scores between the three groups had statistically significant differences (P<0.001).
Conclusion:
With the application of scotch bond multi-purpose plus adhesive, we can use phosphoric acid instead of hydrofluoric acid for bonding brackets to non-glazed ceramic restorations.
Keywords: Dental Porcelain, Orthodontic Brackets, Shear Strength
INTRODUCTION
As the demand for adult orthodontic treatment increases and the popularity of esthetic dentistry expands, orthodontists are more likely faced with the problem of placing orthodontic appliances on teeth restored with resin and porcelain fixed prostheses or veneer laminates. When esthetics is a concern, orthodontists will have to depend on the direct bonding technique. Numerous methods and materials have been suggested for bonding to ceramic restorations [1–7]. The conventional method for optimal bonding of brackets and retainer wires to ceramic surfaces is application of hydrofluoric (HF) acid for etching the ceramic surface, because phosphoric acid etching, which is used for the enamel, is ineffective in the preparation of ceramic surfaces for mechanical retention of orthodontic attachments [8–10]. However, the in vivo use of HF acid is too hazardous. Mucosal or skin contact with HF acid can cause erythema and burning associated with loss of tissue along with intense pain for several days [11,12].
The purpose of this study was to evaluate tensile bond strength between metal brackets and non-glazed ceramic surfaces with three different surface treatments to evaluate application of phosphoric acid instead of HF acid. We evaluated the non-glazed ceramic surfaces because clinicians sometimes remove the glaze layer of porcelain restorations by a low speed bur in the oral cavity and then apply phosphoric acid instead of HF. This study can show if this is an effective way to prepare porcelain.
MATERIALS AND METHODS
Forty-two non-glazed, feldspathic porcelain disks, 2.0 mm thick and 8.0 mm in diameter, were used. The disks were fabricated from super porcelain EX-3 body (Noritake, Japan) using a handmade mold. All specimens were made by one skilled ceramic technician and were checked for cracks.
The specimens were washed with water, dried with air, and then divided into three equal groups randomly. In groups I, and II; 9.5% HF acid gel (Ultradent, USA) was applied for 2 minutes, then rinsed with water and dried with oil-free air. Subsequently, in group I, a layer of silane (Scotchbond Ceramic Primer, 3M Unitek, USA) was applied and dried with light oil-free air spray. Then a layer of scotchbond multi purpose plus adhesive (3M Unitek, Monrovia, Calif., USA) was applied, thinned with air spray and cured with light (500) for 10 seconds. In group II, after etching with 9.5% HF acid, only a layer of bonding agent (Unfilled resin, 3M Unitek, USA) was used. In group III, ceramic surfaces were treated with 35% phosphoric acid (3M Unitek, USA) and then silane and adhesive were applied similar to group I.
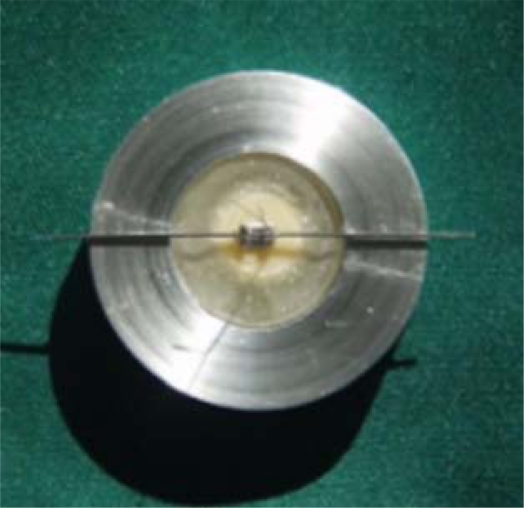
Metal brackets for maxillary central incisors (Dyna lock, Std. Edgewise 018″, 3M-Unitek, USA) were bonded to each conditioned ceramic surface with light-curing composite resin (transbond XT, 3M-Unitek, USA). The bracket was subjected to 250 grams of force. Excess composite was removed and then light cured for 40 seconds (Coltolux 75, Swiss) with an intensity of 500. The surface areas for the bases of brackets was 16.52 mm2 which were obtained bfrom the manufacturer. After bonding, all specimens were stored in a water bath in room temperature for 24 hours, then thermocycled 500 times between 5°C and 55°C with a dwelling time of 60 seconds. The transfer time between baths was eight seconds. All specimens were partially embedded in acrylic resin using a hand-made jig, especially designed for this purpose (Fig 1 and 2).
Fig 1.
Specimens were mounted in acrylic resin in a special jig.
Fig 2.
Specimens after mounting in acrylic resin.
Zwick universal testing machine (Z/100, Germany) was used to measure the tensile bond strength. The machine had an upper jaw that was mounted to a movable crosshead and a lower jaw mounted on the base. The tensile force at a crosshead speed of 0.5 mm/minute was transmitted to the bracket by a steel wire that was placed under the bracket wings. The wire was pulled upward until bond failure occurred (Fig 3).
Fig 3.
Specimen setup for testing the tensile bond strength in a universal testing machine.
The force required for debonding was recorded and the tensile bond strength in megapascal (MPa) was determined from the load at failure and the area of bracket base.
After debonding, the specimen surface was examined under magnification ×2 to determine how much residual adhesive remained on the ceramic according to the following scale: 1=all the composite remained on the ceramic, 2=more than 90% of the composite remained on the ceramic, 3=more than 10% but less than 90% remained on the ceramic, 4=less than 10% remained on the ceramic, and 5=no composite remained on the ceramic.
Descriptive statistics including the mean, standard deviation, and minimum and maximum values were calculated for the three groups. Then tensile bond strength data were subjected to one-way ANOVA and Tukey’s HSD Post Hoc tests. The nonparametric Kruskal-Wallis test was used to compare the adhesive remnant index (ARI) score for the three groups. Significance for all statistical tests was predetermined at P≤0.05.
RESULTS
Four specimens in group I, three specimens in group II, and two specimens in group III did not have a measurement of tensile bond strength because of the operator’s mistake. Table I shows the descriptive statistics of the three groups. The difference of tensile bond strength between groups I, and III was not significant (P=0.999). However, the tensile bond strength of group II was significantly lower than groups I, and III (P<0.001).
Table 1.
Descriptive statistics of the groups.
| Group | N | Mean (MPa) | Standard Deviation | Min | Max |
|---|---|---|---|---|---|
| 1 | 10 | 3.91 | 0.20 | 3.70 | 4.31 |
| 2 | 11 | 2.70 | 0.46 | 1.75 | 3.32 |
| 3 | 12 | 3.90 | 0.89 | 2.03 | 5.43 |
The ARI scores show that in group I specimens, all the composite remained on the ceramic. In group II, 63.7% of the specimens had an ARI higher than 4, meaning that less than 10% of the composite remained on the ceramic. In group III, more than half of the specimens had an ARI lower than 2 (i.e. more than 90% of the composite remained on the ceramic). The ARI scores between the three groups had statistically significant differences (P<0.001).
DISCUSSION
The direct bonding of orthodontic attachments has revolutionized and improved the clinical practice of orthodontics. More recently bonding materials have been introduced for bonding to ceramic. However, before clinical experiments, these materials should be evaluated in vitro to find which products and materials seem most valuable to include in supplementary clinical evaluation [10]. Although both shear and tensile loading modes are valid tests for studying bond strengths of orthodontic materials [13], choosing the tensile bond strength test as the more frequently used shear bond strength test needs some explanation. In order to allow the calculation of the true interfacial failure stress, the experiment should be designed so that a uniform stress distribution is created across the interface. Production of complex stress distribution in the shear bond strength test may start fracture at sites with high concentration of local stress; therefore, the adhesive characteristics of the bonded interface are not expressed [14]. The tensile bond strength test used in this study provides a specimen design with a stress distribution across the interface as near to uniform as possible.
We used long thin wires under the bracket wings, as suggested by Katone and Chan [15]. The relatively slow crosshead speed used for bond strength in this study does allow some self-adjustment of the experimental configuration during loading. Moreover, viscoelastic behavior that is absent in vivo may occur at low strain rates for the adhesive which may be an important area for future investigation [13,16].
Newman [17] stated that 14 kg/cm2 (∼1.5 MPa) was the maximum force that could be applied to a tooth by an orthodontic appliance. The tensile bond strength in our study exceeded this value greatly, but were still less than Reynold’s value [18] (50 kg/cm2∼5 MPa) recommended for in vivo success. Group I and III had the closest results to this value with a mean of 3.91 MPa and 3.90 MPa, respectively. Olsen et al [19] evaluated shear bond strength between metal brackets and enamel using scotch bond multipurpose adhesive, 37% phosphoric acid and 10% maleic acid. Their results were 13.1 MPa (SD=4.8) for phosphoric acid and 10.3 MPa (SD=3.1) for maleic acid. They did not thermocycle their specimens. Moreover, it is proved in orthodontics that shear bond strength is higher than tensile bond strength [20–22].
Thurmond et al [23] found significantly lower shear bond strength with application of phosphoric acid plus silane on ceramic than with HF acid plus silane (P<0.001), but Saygili and Sahmali [24] reported that acid etching of the surfaces with HF acid had a weak tendency to improve bond strength. Aida et al [25] showed that acid etching of porcelain with HF acid could be eliminated. Our finding showed no statistical difference between application of HF acid with scotch bond multipurpose plus adhesive and phosphoric acid with scotch bond multipurpose plus adhesive on non-glazed ceramic (P=0.999).
Major et al [26] showed that the site of failure at low bond strength tended to be at the porcelain-adhesive interface. When the bond strengths became greater, the chemical retention was equal or exceeded the mechanical retention provided by the bracket base. Therefore, increased bond strengths resulted in failure at the bracket-adhesive interface or a cohesive failure within the composite resin in a way that some composite was left on both the bracket and the porcelain surfaces. In our study, in group I, 100% of the specimens and in group III, 60% of the specimens had more than 90% composite remaining on the ceramic. However, in group II, which had a lower bond strength (2.70 MPa), about 60% of the specimens had less than 10% composite remaining on the ceramic.
The tensile bond strength to ceramic surface reported by Cochran et al [27], Kocadereli et al [28] and Harari et al [29] using the same method were higher than our results. But it should be noted that they did not thermocycle their specimens and they also used different material. Zachresson et al [9] stated their results should be interpreted cautiously.
Eustaquio et al [30] reported a tensile bond strength of bonding brackets to deglazed ceramic using conocise/scotch prime (3M) comparable with groups I and III of our study.
Overall, these differences may be due to variables such as the type of bond strength test, ceramic, adhesive, brackets, storage environment and presence or absence of thermocycling. In some researches, the specimens were stored in 37 degrees and in others; they were kept in room temperature. We used the second method [31,32].
Regarding the effect of phosphoric acid on porcelain, the results of studies are mixed and confusing. While some researches show that this acid does not have a considerable effect on the porcelain surface [8–10], others show that this effect is clinically acceptable [33–35]. Our results were in accordance with the second opinion.
The number of thermal cycle is another point of dispute between different researches. It has been 100, 150, 200 and 500 times in previous researches. We applied the biggest number in our research [1,17,20,32,36].
CONCLUSION
Our results showed that by using scotch bond multipurpose plus adhesive on non-glazed ceramic we can use phosphoric acid for treating the ceramic surface instead of HF acid gaining a statistically not different tensile bond strength and also preventing hazard for the oral tissue, skin and the respiratory system.
Table 2.
Adhesive Remnant Index (ARI) scores for the groups.
| Group |
ARI |
Total | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| 1 | Count | 10 | 0 | 0 | 0 | 0 | 10 |
| % Within Group | 100.0% | 0% | 0% | 0% | 0% | 100.0% | |
| 2 | Count | 1 | 1 | 2 | 5 | 2 | 11 |
| % Within Group | 9.1% | 9.1% | 18.2% | 45.5% | 18.2% | 100.0% | |
| 3 | Count | 5 | 2 | 4 | 1 | 0 | 12 |
| % Within Group | 41.7 | 16.7 | 33.3% | 8.3% | 0% | 100.0% | |
Acknowledgments
This investigation was supported by Dental Research Center, Tehran University of Medical Sciences, grant No. 132/4875.
REFERENCES
- 1.Smith GA, McInnes-Ledoux P, Leoux WR, Weinberg R. Orthodontic bonding to porcelain-Bond strength and refinishing. Am J Orthod Dentofacial Orthop. 1988 Sep;94(3):254–52. doi: 10.1016/0889-5406(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 2.Diaz-Arnold AM, Aquilino SA. An evaluation of the bond strengths of four organosilane materials in response to thermal stress. J Prosthet Dent. 1989 Sep;62(3):257–60. doi: 10.1016/0022-3913(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 3.Rezk-Lega F, Ogaard B. Tensile bond force of glass ionomer cements in direct bonding of orthodontic brackets: an in vitro comparative study. Am J Orthod Dentofacial Orthop. 1991 Oct;100(4):357–61. doi: 10.1016/0889-5406(91)70074-7. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa VL, Almeida MA, Chevitarese O, Keith O. Direct bonding to porcelain. Am J Orthod Dentofacial Orthop. 1995 Feb;107(2):59–64. doi: 10.1016/s0889-5406(95)70131-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen JH, Matsumura H, Atsuta M. Effect of etchant, etching period, and silane priming on bond strength to porcelain of composite resin. Oper Dent. 1998 Sep-Oct;23(5):250–7. [PubMed] [Google Scholar]
- 6.Kitayama Y, Komori A, Nakahara R. Tensile and shear bond strength of resin-reinforced glass ionomer cement to glazed porcelain. Angle Orthod. 2003 Aug;73(4):451–6. doi: 10.1043/0003-3219(2003)073<0451:TASBSO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Schmage P, Nergiz I, Herrmann W, Ozcan M. Influence of various surface-conditioning methods on the bond strength of metal brackets to ceramic surfaces. Am J Orthod Dentofacial Orthop. 2003 May;123(5):540–6. doi: 10.1067/mod.2003.S0889540602569110. [DOI] [PubMed] [Google Scholar]
- 8.Zachrisson BU. Bonding in orthodontics. In: Graber TM, VAnarsdall RL, editors. Orthodontics: current principles and practice. 3rd ed. St. Louise: Mosby; 2000. pp. 840–1. [Google Scholar]
- 9.Zachrisson YO, Zachrisson BU, Buyukyilmaz T. Surface preparation for orthodontic bonding to porcelain. Am J Orthod Dentofacial Orthop. 1996 Apr;109(4):420–30. doi: 10.1016/s0889-5406(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 10.Zachrisson BU. Orthodontic bonding to artificial tooth surfaces: clinical versus laboratory findings. Am J Orthod Dentofacial Orthop. 2000 May;117(5):592–4. doi: 10.1016/s0889-5406(00)70211-3. [DOI] [PubMed] [Google Scholar]
- 11.Jochen DG. Repair of fractured porcelain denture teeth. J Prosthet Dent. 1973 Feb;29(21):228–30. doi: 10.1016/0022-3913(73)90119-4. [DOI] [PubMed] [Google Scholar]
- 12.Moore PA, Manor RC. Hydrofluoric acid burns. J Prosthet Dent. 1982 Mar;47(3):338–9. doi: 10.1016/0022-3913(82)90165-2. [DOI] [PubMed] [Google Scholar]
- 13.Brantley WA, Eliades T. Orthodontic materials, scientific and clinical aspects. 1st ed. Stuttgart: Thieme; 2003. [Google Scholar]
- 14.Hooshmand T, van Noort R, Keshvad A. Bond durability of the resin-bonded and silane treated ceramic surface. Dent Mater. 2002 Mar;18(2):179–88. doi: 10.1016/s0109-5641(01)00047-1. [DOI] [PubMed] [Google Scholar]
- 15.Katona TR, Chen J. Engineering and experimental analysis of the tensile loads applied during strength testing of direct bonded orthodontic brackets. Am J Orthod Dentofacial Orthop. 1994 Aug;106(2):167–74. doi: 10.1016/S0889-5406(94)70035-4. [DOI] [PubMed] [Google Scholar]
- 16.Eliades T, Brantley WR. The inappropriateness of conventional orthodontic bond strength assessment protocols. Eur J Orthod. 2000 Feb;22(1):13–23. doi: 10.1093/ejo/22.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Newman GV. Epoxy adhesives for orthodontic attachments: progress report. Am J Orthod. 1965 Dec;51(12):901–12. doi: 10.1016/0002-9416(65)90203-4. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds IR. A review of direct orthodontic bonding. Br J Orthod. 1975;2:171–8. [Google Scholar]
- 19.Olsen ME, Bishara SE, Damon P, Jakobsen JR. Evaluation of scotchbond multipurpose and maleic acid as alterative methods of bonding orthodontic brackets. Am J Orthod Dentofacial Orthop. 1997 May;111(5):498–501. doi: 10.1016/s0889-5406(97)70286-5. [DOI] [PubMed] [Google Scholar]
- 20.Zelos L, Bevis RR, Keenan KM. Evaluation of the ceramic/ceramic interface. Am J Orthod Dentofacial Orthop. 1994 Jul;106(1):10–21. doi: 10.1016/S0889-5406(94)70016-8. [DOI] [PubMed] [Google Scholar]
- 21.Ghessemi-Tary B. Direct bonding to porcelain: an in vitro study. Am J Orthod. 1979 Jul;76(1):80–3. doi: 10.1016/0002-9416(79)90301-4. [DOI] [PubMed] [Google Scholar]
- 22.Merrill SW, Oesterle LJ, Harmesch CB. Ceramic bracket bonding: a comparison of shear, tensile, and torsional bond strengths of ceramic brackets. Am J Orthod Dentofacial Orthop. 1994 Sep;106(3):290–7. doi: 10.1016/S0889-5406(94)70049-4. [DOI] [PubMed] [Google Scholar]
- 23.Thurmond JW, Barkmeier WW, Wilwerding TM. Effect of porcelain surface treatments on bond strengths of composite resin bonded to porcelain. J Prosthet Dent. 1994 Oct;72(4):355–9. doi: 10.1016/0022-3913(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 24.Saygili G, Sahmali S. Effect of ceramic surface treatment on the shear bond strengths of two resin luting agents to all-ceramic materials. J Oral Rehabil. 2003 Jul;30(7):758–64. doi: 10.1046/j.1365-2842.2003.01027.x. [DOI] [PubMed] [Google Scholar]
- 25.Aida M, Hayakawa T, Mizukawa K. Adhesion of composite to porcelain with various surface conditions. J Prosthet Dent. 1995 May;73(5):464–70. doi: 10.1016/s0022-3913(05)80076-9. [DOI] [PubMed] [Google Scholar]
- 26.Major PW, Koehler JR, Manning KE. 24-hour shear bond strength of metal orthodontic brackets bonded to porcelain using various adhesion prometers. Am J Orthod Dentofacial Orthop. 1995 Sep;108(3):322–9. doi: 10.1016/s0889-5406(95)70028-5. [DOI] [PubMed] [Google Scholar]
- 27.Cochran D, O’Keefe KL, Turner DT, Powers JM. Bond strength of orthodontic composite cement to treated porcelain. Am J Orthod Dentofacial Orthop. 1997 Mar;111(3):297–300. doi: 10.1016/s0889-5406(97)70188-4. [DOI] [PubMed] [Google Scholar]
- 28.Kocadereli I, Canay S, Akca K. Tensile bond strength of ceramic orthodontic brackets bonded to porcelain surfaces. Am J Orthod Dentofacial Orthop. 2001 Jun;119(6):617–20. doi: 10.1067/mod.2001.113655. [DOI] [PubMed] [Google Scholar]
- 29.Harari D, Shapira-Davis S, Gillis I, Roman I, Redlich M. Tensile bond strength of ceramic brackets bonded to porcelain facets. Am J Orthod Dentofacial Orthop. 2003 May;123(5):551–4. doi: 10.1067/mod.2003.S0889540602569134. [DOI] [PubMed] [Google Scholar]
- 30.Eustaquio R, Garner LD, Moore BK. Comparative tensile strength of brackets bonded to porcelain with orthodontic adhesive and porcelain repair systems. Am J Orthod Dentofacial Orthop. 1988 Nov;94(5):421–5. doi: 10.1016/0889-5406(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 31.Stangel L, Nathanson D, HSU CS. Shear strength of the composite bond to etched porcelain. J Dent Res. 1987 Sep;66(9):1460–5. doi: 10.1177/00220345870660091001. [DOI] [PubMed] [Google Scholar]
- 32.Shahverdi S, Canay S, Sahin E, Bilge A. Effects of different surface treatment methods on the bond strength of composite resin to porcelain. J Oral Rehabil. 1998;25:699–705. doi: 10.1046/j.1365-2842.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 33.Chay SH, Wattanapayungkul P, Yap AU, Loh PL, Chung SM. Comparison of the bond strength of stainless steel orthodontic brackets bonded to crown porcelains. Aust Orthod J. 2005 May;21(1):19–23. [PubMed] [Google Scholar]
- 34.Larmour CJ, Bateman G, Stirrups DR. An investigation into the bonding of orthodontic attachments to porcelain. Eur J Orthod. 2006 Feb;28(1):74–7. doi: 10.1093/ejo/cji072. [DOI] [PubMed] [Google Scholar]
- 35.Pannes DD, Bailey DK, Thompson JY, Pietz DM. Orthodontic bonding to porcelain: a comparison of bonding systems. J Prosthet Dent. 2003 Jan;89(1):66–9. doi: 10.1067/mpr.2003.63. [DOI] [PubMed] [Google Scholar]
- 36.Bourke BM, Rock WP. Factors affecting the shear bond strength of orthodontic brackets to porcelain. Br J Orthod. 1999 Dec;26(4):285–90. doi: 10.1093/ortho/26.4.285. [DOI] [PubMed] [Google Scholar]