Abstract
Bioorthogonal chemistry allows a wide variety of biomolecules to be specifically labeled and probed in living cells and whole organisms. Here we discuss the history of bioorthogonal reactions and some of the most interesting and important advances in the field.
Since the galvanic rediscovery of Gregor Mendel’s work over a century ago, the modern biosciences have made astounding advances in our mechanistic understanding of living systems. Genetics, biochemistry, molecular biology and allied fields have provided especially impressive insight into the structures and functions of DNA, RNA and proteins, leading to such recent achievements as the sequencing of the human genome. In modern cell biology, proteins can be visualized using fluorescent protein fusions and knocked down by RNA-mediated silencing. Rapid progress in the life sciences continues as new technologies such as DNA deep sequencing, genome-wide expression profiling and mass spectrometry–based proteomics transform how biology is done.
However, not all biological molecules and processes are within the easy reach of genetics or genomics. Glycans, lipids, small metabolites and myriad post-translational modifications are not encoded directly by the genome, making them challenging to study with traditional biological tools alone. Furthermore, many dynamic biological processes occur on short time scales not amenable to genetic or biochemical interrogation. Post-genomic science has set in sharp relief the need for new technologies that take aim at these molecules and processes.
The field of bioorthogonal chemistry thus emerged from a perceived technology gap that rendered many biomolecules, initially glycans1,2, invisible to available probing strategies. Though considered a relatively new sector of chemical biology, bioorthogonal chemistry seeks to solve an old problem: finding a needle in a haystack. That is, among all of the molecular diversity inherent to cells and organisms, how can one type of biomolecule be singled out for analysis?
In the 20th century, the monoclonal antibody transformed the biosciences as we had known them3. Antibodies are unrivaled in their ability to seek out a single molecular target among millions of distractions and bind with high affinity. But antibodies are not a panacea: they generally cannot enter live cells, restricting their use to the extracellular environment; they have poor tissue penetrance in animals; and they must be laboriously generated de novo for each new antigen. Thus, in addition to its aim to target new classes of biomolecules, bioorthogonal chemistry was a solution to the challenge of replicating the exquisite selectivity of antibody-antigen binding with a single covalent reaction among complementary functional groups.
The term bioorthogonal chemistry refers to chemical transformations among abiotic reactants that can proceed in living systems (for example, cells or organisms) without interfering with, or interference from, the surrounding biological milieu. Devising such reactions presents a major and largely unfamiliar challenge to chemists, as most of us were trained that such offending substances as water and air can be excluded from our reactions, competing functional groups protected, catalysts added and temperature modulated. However, to be maximally useful in biological research, bioorthogonal reactions must proceed smoothly in water at physiological pH, temperature and pressure, provide good yield and reasonable kinetics at low reagent concentrations, remain inert to abundant biological nucleophiles, electrophiles and redox-active metabolites, and produce only nontoxic (or no) side products.
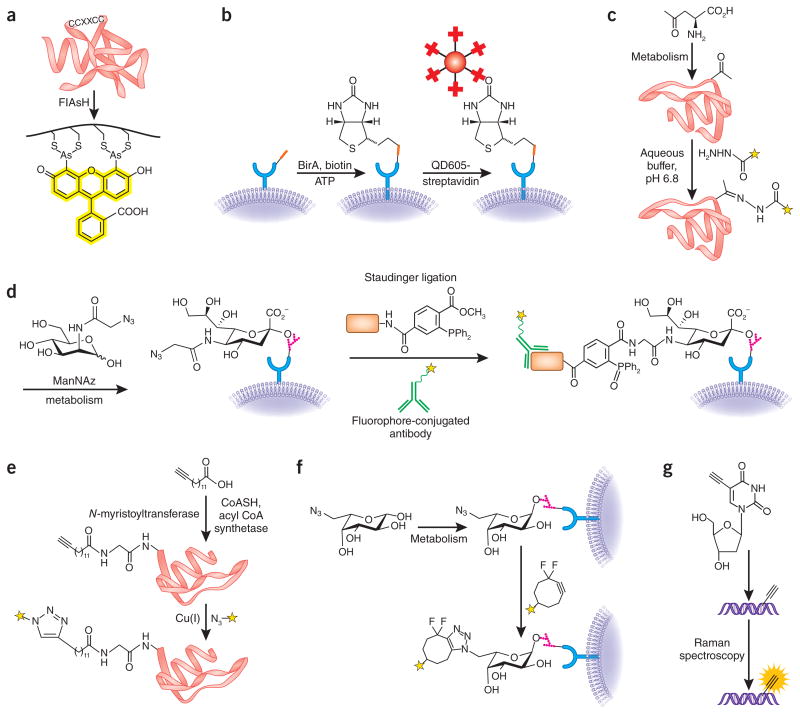
The notion that single-target selectivity can be attained by covalent reaction in live cells was validated by groundbreaking work by Roger Tsien and co-workers in 1998, using bisarsenical-functionalized fluorescent dyes4,5 (Fig. 1a). They designed these abiotic molecules to react selectively with a tetracysteine motif that is genetically engineered into the protein of interest4,5. Although the term ‘bioorthogonal chemistry’ had not yet been coined, Tsien’s work sparked the imagination of chemists, who felt empowered to attempt covalent reactions in cells with entirely abiotic reactants. Notably, Tsien’s work also modeled what is now becoming a common theme in chemical biology—tool development motivated by specific biological problems. In this case, the challenge at hand was the perturbing effects that a large fluorescent protein fusion can have on an imaging target of interest. By contrast, the tetracysteine motif was a small addition to the target protein, with its bioorthogonality derived from the unique combination of natural amino acids that was virtually absent (we later learned) from mammalian proteomes. Subsequently, other groups have exploited such genetically encoded, orthogonal peptides by using engineered enzymes to covalently modify the tag, producing a useful chemical label6,7 (Fig. 1b). For example, elegant recent work with biotin ligase in a neuronal synapse model system8 demonstrated how these techniques can shed light on aspects of biology that would be difficult to investigate with conventional techniques alone.
Figure 1.
Bioorthogonal reactions. (a) The biarsenical fluorescent dye FlAsH specifically reacts with target proteins genetically tagged with the tetracysteine motif4. (b) Small, genetically encoded peptide tags (red rectangle) can be specifically modified in or on live cells using engineered enzymes, such as the biotin ligase BirA6,7. Biotin-tagged target proteins can then be visualized by streptavidin-conjugated probes, such as quantum dots (QD605). (c) In an example of carbonyl condensations as a bioorthogonal method, unnatural, ketone-functionalized amino acids can be metabolically incorporated into proteins and then detected by reaction with a hydrazide-functionalized fluorescent probe (yellow star)11. (d) In an example of the Staudinger ligation, an azidosugar analog of N-acetylmannosamine (termed ManNAz) is used to metabolically label sialic acid residues on cell-surface glycoproteins. The azidosugar is detected by reaction with a tagged phosphine probe (orange rectangle) and visualized with an antibody to the tag2,21. (e) In an example of CuAAC chemistry, an alkynyl analog of myristic acid is metabolically processed to an acetyl coenzyme-A metabolite and then attached to protein N termini via N-myristoyltransferase17. The tagged protein can then be labeled via CuAAC using an azide probe (yellow star). (f) In an example of copper-free click chemistry, an azidosugar analog of fucose is metabolically incorporated into cell-surface glycans in live zebrafish embryos and detected by reaction with a fluorescent difluorinated cyclooctyne (yellow star)39,49. (g) DNA labeled with metabolically incorporated 5′-ethynyl-2′-deoxyuridine is visualized via direct Raman spectroscopic detection of the intact alkyne48.
More than a decade since Tsien’s first biarsenical dyes, the field of bioorthogonal chemistry has produced a range of reactions among entirely abiotic functional groups that perform beautifully amid richly functionalized biomolecules—in cells and even in live animals9. In parallel, chemists and biologists have worked together to craft creative ways to introduce bioorthogonal functional groups into targets of interest, often harnessing metabolic, enzymatic and genetic engineering tools along the way2,10–18. A common strategy is to incorporate one reaction partner into the target biomolecules in a live cell or organism (the label) and then react that label with a second, exogenously added reagent (the probe). Depending on the experimental system and goals, the reaction results in the covalent attachment of an affinity tag, imaging agent or other useful moiety to the label via reaction with the probe. But the major bottleneck in such efforts is inventing the reaction that will enable two molecules to couple with mutual exclusivity in an environment of almost hopeless complexity.
Inventing bioorthogonal reactions
A reasonable starting point for bioorthogonal reaction development is to identify functionalities not already in nature’s repertoire.
A first step in this direction came with the realization that although biomolecules are replete with nucleophiles, electrophiles are used sparingly (perhaps reflecting the realities of a world based on a nucleophilic solvent—water). Ketones and aldehydes, for example, are not found in proteins or their associated glycans and have orthogonal reactivity in the context of those biomolecules. Ketones and aldehydes condense with aminooxy or hydrazide groups—themselves also absent from biomolecules—to form stable oximes or hydrazones, respectively, albeit with an optimum pH lower than physiological pH.
Such carbonyl condensations have been used decades ago for in situ drug assembly in mammalian cells19. In the late 1990s, these reactions showed promise for labeling glycans10 and proteins11 through metabolically incorporated ketone-functionalized sugars and amino acids, in mammalian and bacterial cells (Fig. 1c).
Though absent from cell-surface molecules, ketones and aldehydes are found in many intracellular metabolites (for example, free sugars, pyruvate, pyridoxal phosphate, lipid catabolites and others) and, therefore, were not sufficiently bioorthogonal to be generally useful biological labels. A clear conclusion that emerged around the turn of the millennium is that universal bioorthogonality could only be ensured by reaching into the toolkit of reagents that were strictly man-made.
Repurposing old chemistry
The Staudinger ligation of azides and phosphines2 was the first bioorthogonal reaction among entirely abiotic reactants and, as such, established a standard for the field of bioorthogonal chemistry as we now know it. As ketones and aldehydes, the azide is a mild electrophile, but one that is considered ‘soft’ by chemists, and is therefore unreactive with nature’s predominantly ‘hard’ nucleophiles. A ‘soft’ nucleophilic partner for the azide was unveiled by Nobel Prize laureate Hermann Staudinger almost a century ago; his classic work described the reaction of triarylphosphines with azides to form, in water, amine and phosphine oxide products20.
At the time, of course, this work had no obvious connection to the still-embryonic field of experimental biology. In the late 1990s, however, Staudinger’s work caught our attention as the basis for a potential bioorthogonal reaction, owing to the complete absence of phosphines and azides from living systems, and the superior tolerance by the Staudinger reaction of bio-compatible conditions.
To harness these advantages, we modified the classic Staudinger reaction so that a transient intermediate would rearrange to form a stable amide linkage between an azide-derived nitrogen and the phosphine oxide moiety2. The modified Staudinger ligation proceeds under physiological conditions with near-perfect selectivity, providing a powerful tool for labeling azide-tagged biomolecules. In addition, the azide has the advantages of small size and low intrinsic toxicity (in alkyl azide form).
Thanks to these attributes, our group and others have metabolically labeled a wide range of biomolecules with unnatural azide-bearing components, including glycans2,12, proteins13 and nucleic acids14, in live cells and whole organisms21, and then tagged them with probes for a variety of applications (Fig. 1d). Examples of applications include proteomic analysis of glycoproteins12, profiling enzymes based on activity22, probing nucleic-acid interactions and monitoring protein turnover. The Staudinger ligation has also lent itself to several useful variations, including the development by Ronald Raines’ group of a ‘traceless’ Staudinger ligation23 and the creation of fluorogenic phosphine probes24,25, which are well-suited to biological imaging applications.
Despite its many advantages, the Staudinger ligation suffers from some drawbacks, including competing oxidation of phosphines in air and relatively slow reaction kinetics. The latter issue undermines the use of this reaction to study azide-tagged biomolecules on faster time scales. But once articulated early in the last decade, the challenge of developing bioorthogonal reactions with more rapid kinetics became a focus in multiple laboratories. Several transformative developments ensued, driven largely by the synthetic chemistry community, but with far-reaching applications in biological research.
Click chemistry goes bioorthogonal
As the field of bioorthogonal chemistry gained momentum in the early 2000s, another chemical concept was emerging for the rapid assembly of small-molecule libraries from diverse building blocks. The philosophy held that reactions meeting high standards of efficiency, selectivity, functional group tolerance and atom economy—termed ‘click chemistry’ by Nobel Prize laureate Barry Sharpless26—could accelerate the assembly of complex molecules from simple substrates.
The undisputed leader among click chemistry reactions, developed in 2002 in seminal work by Sharpless27 and Morten Meldal28, is the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) that forms a triazole-linked product. As with the Staudinger ligation, the idea for this quintessential click chemistry reaction was seeded by classic chemical literature, dating back more than a hundred years. The reaction between alkynes and azides at high temperatures to yield triazole products was first reported in the 19th century and, decades later, was described by Rolf Huisgen as a 1,3-dipolar cycloaddition29. However, catalysis by Cu(I)27,28 permitted the cycloaddition to proceed within minutes at room temperature, opening the door to applications in biological systems. Indeed, the overlapping requirements of bioorthogonal chemistry and click chemistry made applications of CuAAC to biomolecule labeling an obvious and fruitful endeavor. Crucially, alkynes, like azides and phosphines, are also nonnative to most biological systems.
CuAAC is at least 25 times faster than the Staudinger ligation in in vitro protein labeling experiments30. With such impressive kinetics, the new chemistry could be used to interrogate biological processes that were too rapid to be studied with the Staudinger ligation. Furthermore, alkynes are small and inert under physiological conditions, allowing CuAAC to be used either with azide metabolic labels and alkyne probes or vice versa. Indeed, recent examples of metabolic incorporation of alkynes into proteins15, glycans16, lipids17, nucleic acids18 and other biomolecules have underlined the versatility of CuAAC as a biomolecule-labeling chemistry (Fig. 1e).
Another underappreciated bioorthogonal application of azide-alkyne cycloaddition is the target-guided synthesis of small-molecule enzyme inhibitors, an approach pioneered by M.G. Finn, Sharpless and coworkers31. In this method, in situ uncatalyzed cycloaddition occurs between azide- and alkyne-functionalized synthons held in close proximity by noncovalent binding to a protein target of choice. As a result, high-affinity small-molecule binders can be discovered via the self-assembly of weaker-binding building blocks—a method that could not be deployed without the ‘protein-friendly’ bioorthogonal nature of the cycloaddition itself.
One indication of the impact of CuAAC on biological research is the rapid growth of commercial biomolecule-labeling kits based on azide- and alkyne-functionalized substrates and probes. These can now be found in major catalogs for applications such as protein, glycan and nucleic acid labeling with fluorophores, biotin, Flag peptides and various other tags. Phosphine reagents have also been commercialized for tagging azide-labeled biomolecules via the Staudinger ligation. The availability of such reagents through commercial sources makes them usable by biologists with no chemistry expertise, which is the ultimate goal of many chemistry-tool developers.
Copper-free click chemistry
Despite its many uses, CuAAC does have limitations in biological systems, particularly the considerable toxicity caused by the Cu(I) catalyst in a range of organisms32. Recent work has shown that this problem can be mitigated by new, biocompatible copper ligands, which promise to expand the use of CuAAC in biology33. Nevertheless, toxicity concerns have prompted the development of copper-free bioorthogonal reactions with azides.
Our group sought to exploit the cycloaddition between azides and alkynes for non-toxic bioorthogonal use by activating the alkyne through ring strain, obviating the need for any copper catalyst. The inspiration for this idea was work by Georg Wittig and Adolf Krebs in the 1960s, documenting the explosive reaction between phenyl-azide and cyclooctyne, the smallest stable cycloalkyne34. Applying principles of physical organic chemistry, we optimized this reaction by the addition of an electron-withdrawing group35 or increased ring strain and sp2-like centers to the cyclooctyne36, both of which serve to activate the alkyne, accelerating the rate. Geert-Jan Boons and co-workers independently achieved substantial rate enhancement through additional ring strain as well37.
These optimized cyclooctynes react with azides at rates on par with those of CuAAC, but, without the need for a metal catalyst, they are suitable for use in live organisms. For example, we and others have used cyclooctyne reagents to image azide-labeled glycans in cells35,37, mice38 and developing zebrafish embryos39 (Fig. 1f). Notably, no toxicity has been observed from cyclooctynes, and the reaction kinetics are sufficiently fast (within minutes) that we have been able to examine dynamic changes in glycan populations in cultured cells and live embryos during development39—a feat beyond the reach of either the Staudinger ligation or traditional CuAAC.
Nevertheless, there is room for improvement with cyclooctynes. For example, there is indirect evidence that the very hydrophobic and reactive cyclooctynes suffer from pharmacokinetic problems in mice, perhaps including sequesteration by serum proteins such as albumin and/or off-target reactions with endogenous nucleophiles such as thiols38. We have partially addressed this problem with more hydrophilic azacy-clooctynes40, but additional optimization of cyclooctynes will be necessary before they can realize their full potential in all experimental contexts.
Alternatively, in recent work, other groups have developed useful new bioorthogonal cycloaddition reaction partner pairs, including azide-oxanorbornadiene41, nitrone-cyclooctyne42 and an extraordinarily rapid tetrazine–strained alkene reaction43. Finally, Qing Lin and co-workers have developed a ‘photo-click’ reaction, in which a UV light–activated tetrazole liberates nitrogen to form a nitrile imine, which can then react with dipolarophiles such as alkenes44. Photo-click chemistry provides several potential advantages including spatiotemporal reaction control via focused UV-light irradiation, the prospect of metabolic labeling with small alkene-bearing molecules45 and the formation of a fluorescent pyrazoline product, which will undoubtedly be exploited in future biological research.
Outlook and future directions
As outlined above, bioorthogonal chemistry has already transformed the way we can study biomolecules in vitro and in living systems, despite the fact that the field is still in its early days. However, challenges remain for the future. Going forward, there is a need for additional types of bioorthogonal methods that can complement and improve on existing tools. Indeed, having a range of chemistries at our disposal will permit the dynamic, multiplex investigation of different populations of biomolecules in the same system and will expand the reach of bioorthogonal chemistry to additional molecules and processes that are not yet adequately addressed (for example, RNA and signaling post-translational modifications such as phosphorylation).
We anticipate that continued progress will be made not simply through optimization of currently available tools but through the discovery and implementation of completely new bioorthogonal reactions. As the adaptations of venerable work by Staudinger, Huisgen, Wittig and Krebs have shown, these new methods may come from mining the arcane chemical literature and transplanting known (if forgotten) reactions to the very different milieu of living systems. Indeed, promising work has been done to adapt techniques such as olefin metathesis46 and palladium-catalyzed cross-coupling47 to biological contexts.
In addition, we believe directed searches for entirely new biocompatible chemical reactivities, including those with improved bioavailability, remain an underexplored effort that may well yield fruitful new discoveries. Future technologies will likely also lead to new bioorthogonal methods, such as the recently reported direct detection of alkynes in live cells via Raman spectroscopy48 (Fig. 1g). In all these efforts, it will be important for chemists and biologists to collaborate closely, so that research can be tailored to unanswered questions and unmet experimental needs in biology, making future developments in the field not only technique-driven but also problem-driven. We suggest that the discovery of new bioorthogonal chemistries should be understood not merely as tool-making for biologists but as a bona fide intellectual challenge to chemists, who must think outside the round-bottom flask to design robust chemical reactions that can operate in, and teach us about, living systems, for decades in the future.
Acknowledgments
We thank J. Jewett, J. Prescher, E. Sletten, B. Swarts and P. Wu for helpful comments on the manuscript. Research in our laboratory is supported by grants from the US National Institutes of Health (GM066047) and Department of Defense (PC080659).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Michael Boyce, Department of Chemistry, University of California, Berkeley, California, USA.
Carolyn R Bertozzi, Email: crb@berkeley.edu, Departments of Chemistry and Molecular and Cell Biology, and Howard Hughes Medical Institute, University of California, Berkeley, California, USA.
References
- 1.Mahal LK, Yarema KJ, Bertozzi CR. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 2.Saxon E, Bertozzi CR. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 3.Winter G, Milstein C. Nature. 1991;349:293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- 4.Griffin BA, Adams SR, Tsien RY. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 5.Adams SR, et al. J Am Chem Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 6.Chen I, Howarth M, Lin W, Ting AY. Nat Methods. 2005;2:99–104. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Suarez M, et al. Nat Biotechnol. 2007;25:1483–1487. doi: 10.1038/nbt1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thyagarajan A, Ting AY. Cell. 2010;143:456–469. doi: 10.1016/j.cell.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Sletten EM, Bertozzi CR. Angew Chem. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahal LK, Bertozzi CR. Chem Biol. 1997;4:415–422. doi: 10.1016/s1074-5521(97)90193-9. [DOI] [PubMed] [Google Scholar]
- 11.Cornish VW, Hahn KM, Schultz PG. J Am Chem Soc. 1996;118:8150–8151. [Google Scholar]
- 12.Boyce M, et al. Proc Natl Acad Sci USA. 2011;108:3141–3146. doi: 10.1073/pnas.1010045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Proc Natl Acad Sci USA. 2002;99:19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sylvers LA, Wower J. Bioconjug Chem. 1993;4:411–418. doi: 10.1021/bc00024a001. [DOI] [PubMed] [Google Scholar]
- 15.Beatty KE, Xie F, Wang Q, Tirrell DA. J Am Chem Soc. 2005;127:14150–14151. doi: 10.1021/ja054643w. [DOI] [PubMed] [Google Scholar]
- 16.Hsu TL, et al. Proc Natl Acad Sci USA. 2007;104:2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heal WP, Wickramasinghe SR, Leatherbarrow RJ, Tate EW. Org Biomol Chem. 2008;6:2308–2315. doi: 10.1039/b803258k. [DOI] [PubMed] [Google Scholar]
- 18.Salic A, Mitchison TJ. Proc Natl Acad Sci USA. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rideout D. Science. 1986;233:561–563. doi: 10.1126/science.3523757. [DOI] [PubMed] [Google Scholar]
- 20.Staudinger H, Meyer J. Helv Chim Acta. 1919;2:635–646. [Google Scholar]
- 21.Prescher JA, Dube DH, Bertozzi CR. Nature. 2004;430:873–877. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 22.Ovaa H, et al. Angew Chem. 2003;42:3626–3629. doi: 10.1002/anie.200351314. [DOI] [PubMed] [Google Scholar]
- 23.Soellner MB, Nilsson BL, Raines RT. J Am Chem Soc. 2006;128:8820–8828. doi: 10.1021/ja060484k. [DOI] [PubMed] [Google Scholar]
- 24.Lemieux GA, De Graffenried CL, Bertozzi CR. J Am Chem Soc. 2003;125:4708–4709. doi: 10.1021/ja029013y. [DOI] [PubMed] [Google Scholar]
- 25.Hangauer MJ, Bertozzi CR. Angew Chem. 2008;47:2394–2397. doi: 10.1002/anie.200704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolb HC, Finn MG, Sharpless KB. Angew Chem. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 29.Huisgen R. Angew Chem Int Edn Engl. 1963;2:565–598. [Google Scholar]
- 30.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 31.Mamidyala SK, Finn MG. Chem Soc Rev. 2010;39:1252–1261. doi: 10.1039/b901969n. [DOI] [PubMed] [Google Scholar]
- 32.Link AJ, Tirrell DA. J Am Chem Soc. 2003;125:11164–11165. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- 33.Soriano Del Amo D, et al. J Am Chem Soc. 2010;132:16893–16899. doi: 10.1021/ja106553e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittig G, Krebs A. Chem Ber. 1961;94:3260–3275. [Google Scholar]
- 35.Baskin JM, et al. Proc Natl Acad Sci USA. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jewett JC, Sletten EM, Bertozzi CR. J Am Chem Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ning X, Guo J, Wolfert MA, Boons GJ. Angew Chem. 2008;120:2285–2287. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang PV, et al. Proc Natl Acad Sci USA. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sletten EM, Bertozzi CR. Org Lett. 2008;10:3097–3099. doi: 10.1021/ol801141k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Berkel SS, et al. ChemBioChem. 2007;8:1504–1508. doi: 10.1002/cbic.200700278. [DOI] [PubMed] [Google Scholar]
- 42.McKay CS, Moran J, Pezacki JP. Chem Commun. 2010;46:931–933. doi: 10.1039/b921630h. [DOI] [PubMed] [Google Scholar]
- 43.Blackman ML, Royzen M, Fox JM. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song W, Wang Y, Qu J, Madden MM, Lin Q. Angew Chem. 2008;47:2832–2835. doi: 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]
- 45.Song W, et al. ACS Chem Biol. 2010;5:875–885. doi: 10.1021/cb100193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin YA, Chalker JM, Floyd N, Bernardes GJ, Davis BG. J Am Chem Soc. 2008;130:9642–9643. doi: 10.1021/ja8026168. [DOI] [PubMed] [Google Scholar]
- 47.Kodama K, et al. ChemBioChem. 2007;8:232–238. doi: 10.1002/cbic.200600432. [DOI] [PubMed] [Google Scholar]
- 48.Yamakoshi H, et al. J Am Chem Soc. 2011;133:6102–6105. doi: 10.1021/ja108404p. [DOI] [PubMed] [Google Scholar]
- 49.Dehnert KW, et al. ACS Chem Biol. 2011;6:547–552. doi: 10.1021/cb100284d. [DOI] [PMC free article] [PubMed] [Google Scholar]