Abstract
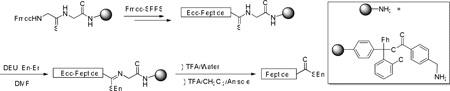
Attachment of a growing peptide chain to a glycylaminomethyl resin via a thioglycinamide bond is compatible with Fmoc-chemistry solid phase peptide synthesis. Subsequent S-alkylation of the thioamide gives a thioimide which, on treatment with aqueous trifluoroacetic acid releases the peptide from the resin in the form of a C-terminal thioester.
Introduction
Kent’s concept of native chemical ligation (NCL),1 in a which the thiol group of an N-terminal cysteine undergoes transthioesterification with a C-terminal thioester followed by an S-N shift has revolutionised the way chemists think about and approach the synthesis of large peptides, proteins and their conjugates.2 The very success of the method has spawned two major challenges, the first being diversification away from the obligate N-terminal cysteine, which has seen a number of creative responses.3 The second is the need for efficient solid phase peptide synthesis (SPPS) of the requisite C-terminal thioesters.3c This latter need has typically been addressed by Boc-chemistry because of the incompatibility of typical conditions for the cleavage of Fmoc groups with thioesters,1,4 however, as Fmoc is the preferred amine protecting group for SPPS,5 alternative methods are needed and are being pursued in numerous laboratories. Existing solutions to this problem include peptide release from the solid-phase by thiolysis of a variety of linkers,6 and variations on this theme in which cleavage from the resin affords an activated N-acyl urea that may be subsequently subjected to thiolysis,7 the development of alternative cocktails for Fmoc-cleavage,8 self-purifying systems,9 the design of a number of ingenious strategies based on O-S or N-S migration following release from the resin of various C-terminal esters and amides,3c,4a,10 and assorted other methods.11 Nevertheless, a method for direct synthesis of C-terminal thioesters by Fmoc-chemistry,5,12 with all the advantages of simplicity and practicality that it would convey, has remained elusive. Here, we present the design and implementation of such a method constructed around the use of a thioamide linker.
Results and Discussion
We envisaged a method based on the conversion of robust thioamides to thioesters, by alkylation to an intermediate thioimide followed by mild hydrolysis. Such conversions have been previously described for small molecules in the solution phase,13 and the general concept has been applied to the formation of peptidyl thioesters by the ring opening of cyclic peptide substrates containing a thioamide moiety.14,15,16 The method has also been employed as a means of preparation of polymer supported thioesters by capture of a solution-phase thioamide with a resin bound alkylating agent.17 Most pertinently, the synthesis of a series of Fmoc-protected amino acid thioesters through methylation and hydrolysis of the corresponding thioamides of the p-methylbenzydrylamine (MBHA) resin has been briefly outlined.18 The conversion of amide and peptide bonds to the corresponding thioamide19 and thiopeptidyl amines14,20 with Lawesson’s reagent21 is well known, is selective for amides in the presence of carbamates,20 and can be conducted on the polymeric supports.22 Alternatively, thiopeptide bonds may be constructed by thioacylation of amino acid derivatives with amino thioacyl derivatives of benzotriazoles.23
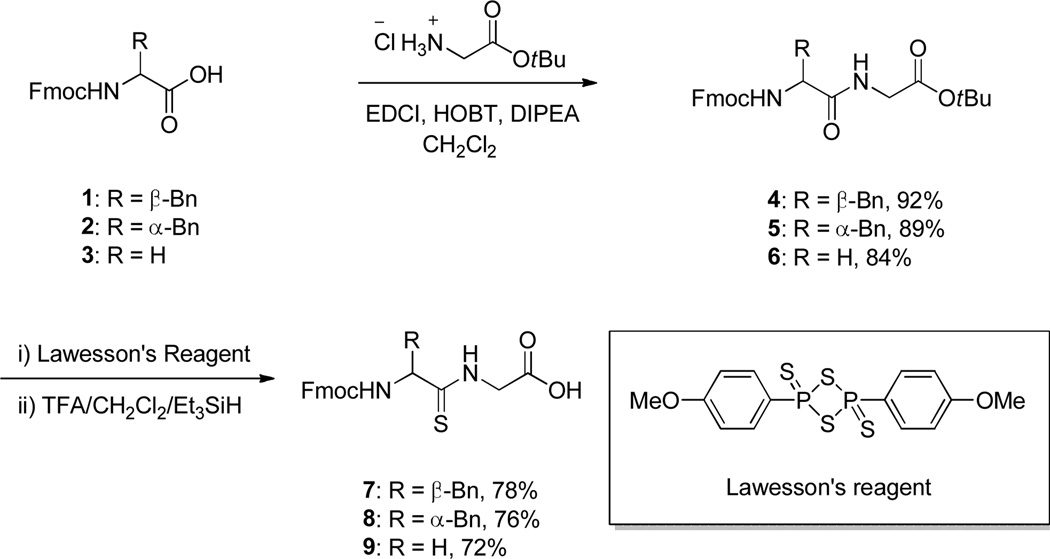
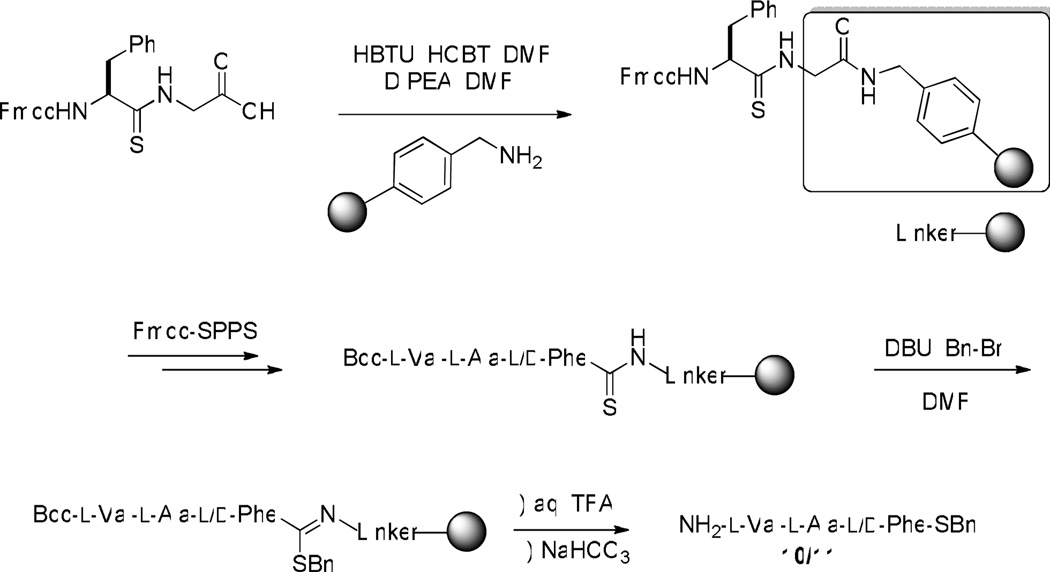
The literature precedent for the reaction of interest was conducted with thioamides based on the MBHA resin, with alkylation by methyl iodide and hydrolysis by 5% water and 10% TFA in an undisclosed solvent over 24 h, after which a range of Fmoc-protected amino acid methyl thioesters was obtained in yields ranging from 43–77%.18,24 In order to avoid lengthy hydrolysis times, and hence unnecessarily long exposure of the hydrolytically unstable thioester to such conditions, in our own work we elected to employ a short linker between the thioamide bond and the polymeric support. We selected the glycyl residue as a suitable linker between aminomethylpolystrene and the thioamide unit as it showed suitable properties both of alkylation of the appended thioamides with benzyl bromide and, more importantly, for hydrolysis of the resulting S-benzyl thioimide. Accordingly, Fmoc protected l-Phe-Gly, d-Phe-Gly, and Gly-Gly were constructed in a straightforward manner as show in Scheme 1, followed by subsequent conversion to the thioamide using Lawesson’s reagent.
Scheme 1.
Preparation of Fmoc-protected thioamides
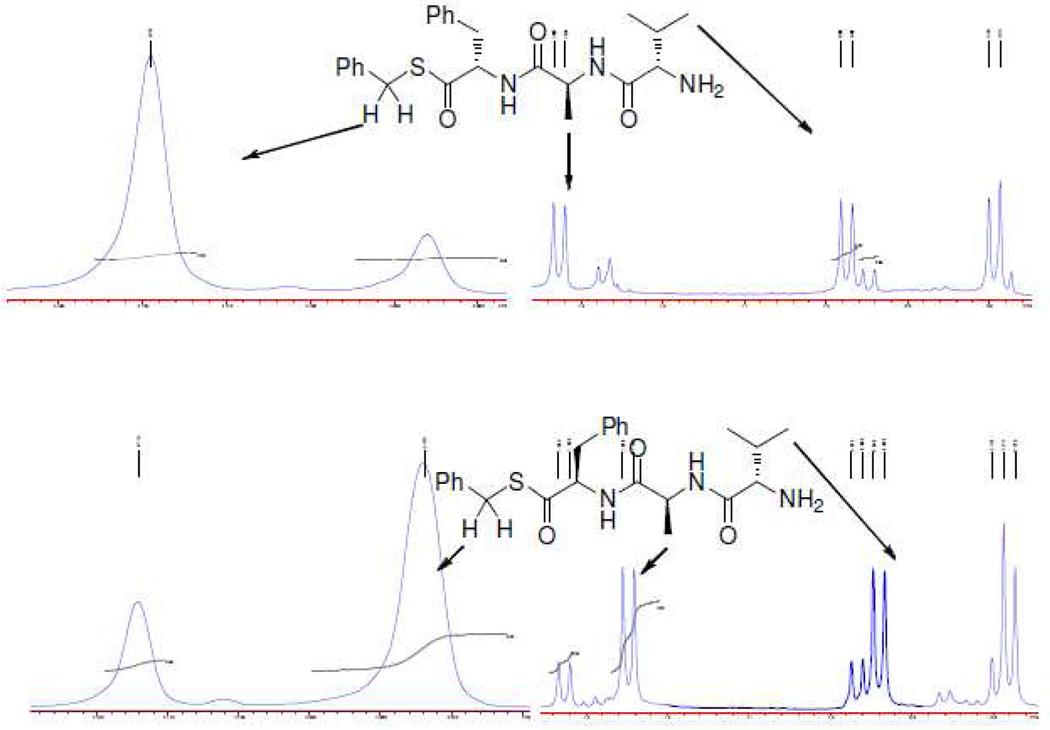
Subsequent coupling of these units to aminomethylpolystrene was achieved with HBTU and HOBT in DMF. For the case of the enantiomeric phenylalanine systems, chain extension was then achieved manually by standard Fmoc-SPPS-chemistry techniques, including cleavage of the Fmoc group with piperidine in DMF and peptide bond formation through the use of building blocks pre-activated with HBTU. In this manner two simple supported tripeptides were assembled, differing only in the configuration of the phenylalanine residue, and whose N-terminal valine carried a Boc-group. Various alkylation conditions were then assayed and these were followed by hydrolysis with aqueous TFA for 1 h, enabling isolation of two tripeptidyl S-benzyl thioesters 10 and 11 (Table 1). No thioester formation was observed after 15 h stirring with either pyridine or ethyldiiospropylamine as base, and benzyl bromide as alkylating agent, followed by exposure to aqueous TFA (Table 1, entries 1 and 2). On the other hand, DBU was found to be a suitable base for alkylation by benzyl bromide, with the yield of product increasing as the amount of base was increased (Table 1, entries 3–5). Unfortunately, NMR analysis of the two diastereomeric tripeptides showed the sequence to proceed with increasing epimerization as the amount of base was increased. (Table 1, Figure 1). Presumably, epimerization takes place at the level of the thioimidate after the alkylation has occurred, in accordance with the precedent for epimerization adjacent to thiazolines under similarly mild conditions.25 While improved methods for peptidyl thioester synthesis by the thiolysis of various activated esters with lower levels of racemization have been reported,26 the level of epimerization observed in the present experiments is comparable to that seen in most methods.
Table 1.
Optimization of Conditions for Formation of Thioesters 10/11.
| entry | base (equiv)a | benzyl bromide (equiv)a |
time (h) | yield | epimerization |
|---|---|---|---|---|---|
| 1 | Pyridine (neat) | 9.0 | 15 | 0 | - |
| 2 | DIPEA (10.0) | 9.0 | 15 | 0 | - |
| 3 | DBU (7.0) | 6.5 | 2 | 55% | 17% |
| 4 | DBU (5.0) | 4.9 | 2 | 41% | 14 % |
| 5 | DBU (10.0) | 9.0 | 2 | 72% | 22% |
With respect to peptide resin.
Figure 1.
1H-NMR spectra of H2N-l-Val-l-Ala-l-Phe-SBn (10) and its d-Phe diastereoisomer (11) in CDCl3 showing the extent of racemization (~22%) under the standard conditions.
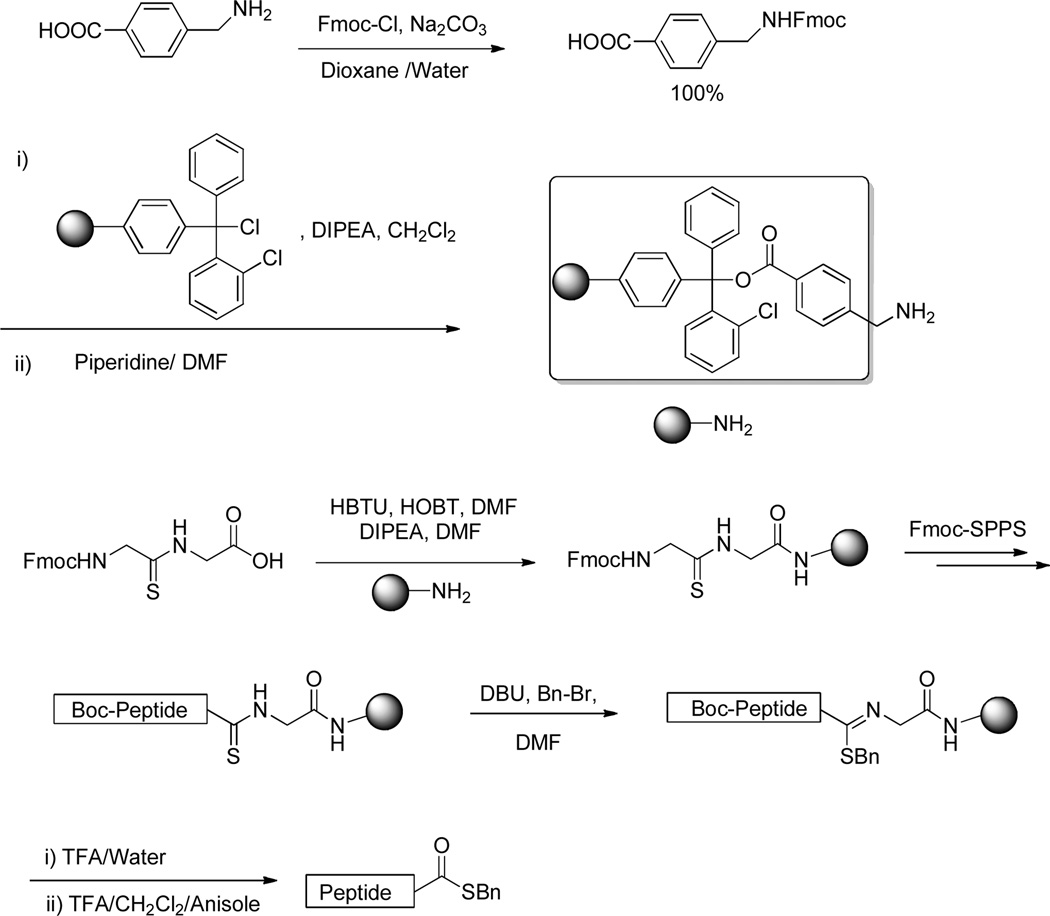
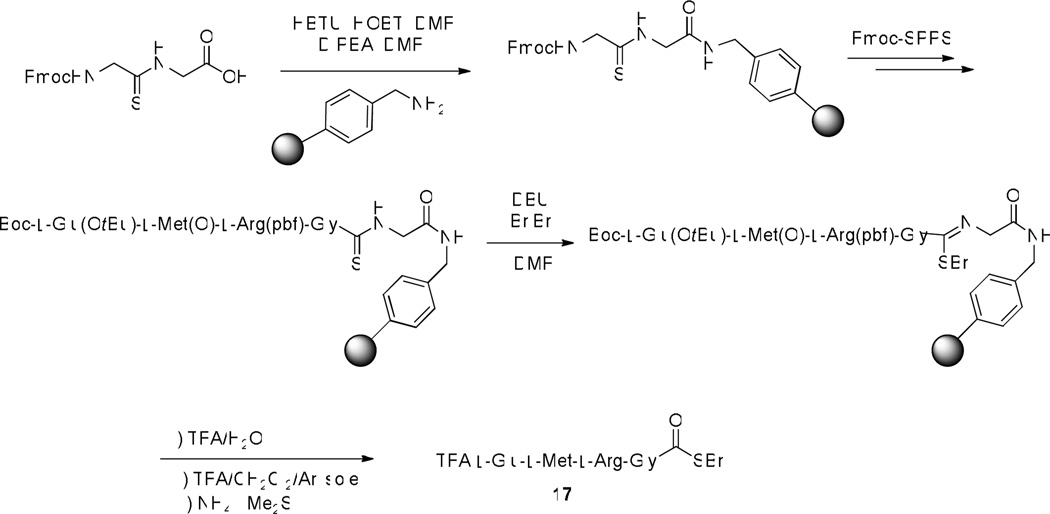
Although it is possible that optimization of the alkylation conditions will enable minimization of the epimerization, in subsequent work we have preferred to set up the chemistry so as to provide glycyl thioesters as it is a fact that NCL functions best for the formation of Gly-Cys peptide bonds.1–2 Toward this end, and with epimerization not being an issue for the glycine-based thioesters, we adopted the conditions of Table 1, entry 5 as standard for the alkylation reaction as they provided the optimal yield of thioester in a reasonable time frame. We also turned to the use of the chlorotrityl resin loaded with a 4-aminomethylbenzoyloxy linker so as to enable the ready cleavage of aliquots for monitoring of the individual reaction steps. The Fmoc-protected Gly-Gly thioamide was loaded to this resin and standard Fmoc SPPS chemistry conducted with the side chain functionality of the individual amino acids protected with TFA-labile groups. Thus, serine, threonine and tyrosine were protected as t-butyl ethers, aspartic and glutamic acids as t-butyl esters, lysine and tryptophan as Boc carbamates, asparagine, glutamine, and histidine as their N-triphenylmethyl derivatives, and arginine as the pentamethyldihydrobenzofuran-5-sulfonamide.27 Hydrolysis from the resin was achieved with aqueous TFA, which also removed any Boc groups present. However, inspection of the crude hydrolysates by mass spectrometry revealed incomplete hydrolysis of side chain protecting groups under these conditions, leading to the inclusion of a subsequent treatment with TFA and triethylsilane (Scheme 3).27a,27b Triethylsilane was not included in the initial hydrolysis step in order to avoid reduction of the thioimidate competing with the hydrolysis. Altogether, several short supported peptides (Table 2, entries 1–5) were prepared in this manner by Fmoc-chemistry SPPS using either the aminomethylpolystyrene or the chlorotrityl resin loaded with the aminomethylbenzoyloxy linker with subsequent alkylation with benzyl bromide, followed by hydrolysis with concomitant removal of all acid labile protecting groups.
Scheme 3.
Adaptation to the chlorotrityl resin
Table 2.
| entrya | resinb | deprotectionc | peptidyl thioester | yield |
|---|---|---|---|---|
| 1 | AMP | aq TFA, TFA-an | TFA.LWYVG-SBn (12) | 68% |
| 2 | AMP | aq TFA, TFA-an | TFA.AKWYVG-SBn (13) | 60% |
| 3 | AMBClT | aq TFA, TFA-an TFA | TFA.TASFSLG-SBn (14) | 57% |
| 4 | AMBClT | aq TFA, TFA-an | TFA.TFYSAYG-SBn (15) | 56% |
| 5 | AMBClT | aq TFA, TFA-an | TFA.NWRYISTFG-SBn (16) | 48% |
| 6 | AMP | aq TFA, TFA-an, NH4I-Me2S | TFA.EMRG-SBn (17) | 62% |
All amino acids except glycine have the l-configuration.
AMP, aminomethylpolystyrene; AMBCIT, aminomethylbenzoyloxy chlorotrityl.
aq TFA, aqueous trifluoroacetic acid; TFA-an, trifluoroacetic acid plus anisole
Anticipating alkylation of methionine to be problematic at the level of conversion of the thioamide to the thioimide, this amino acid was additionally protected as the sulfoxide.9b,28 Incorporation of this sulfur-containing building block then proceeded in the standard manner (Scheme 4), making use of the aminomethylpolystyrene-derived resin, and providing a tetrapeptidyl thioester 17 whose release from the resin was standard except for the additional treatment with ammonium iodide and dimethyl sulfide required to reduce the sulfoxide (Table 2, entry 6).
Scheme 4.
Synthesis of a methionine containing peptidyl thioester
Overall, we describe a method for the direct preparation of C-terminal peptidyl thioesters employing linkers based on the thioamide function, with release by S-alkylation and subsequent hydrolysis of the intermediate S-alkyl thioimidate. The method has been demonstrated in the presence of all the common amino acids, bearing suitable acid-labile side chain protection when necessary, with the exception of cysteine,29 and standard Fmoc-chemistry methods are employed for peptide chain elongation. The acidic nature of the final hydrolysis step removes the acid labile protecting groups, achieves cleavage from the resin, and delivers the thioester in the form of a trifluoroacetate salt. The method has the advantage of direct formation of the thioester in the resin cleavage step, without the requirement of an O-N or S-N shift, or of the need for a thiolysis step. With activation for release by alkylation, the chemistry presented here may be considered a variant on the safety-catch linker theme30 of Kenner31 that, unlike the adaptation of Ellman,6a leads directly to thioesters without the need for thiolysis.32,33
Experimental Section
General
Unless otherwise 1H and 13C spectra were recorded in CDCl3 solution. All solvents were dried and distilled by standard protocols. All reactions were conducted under an inert atmosphere of nitrogen unless otherwise stated. All organic extracts were dried over sodium sulfate, and concentrated under aspirator vacuum. Chromatographic purifications were carried out over silica gel. All peptide syntheses were carried out employing 1% DVB cross linked aminomethyl polystyrene resin or 2-chlorotrityl resin in a 10 mL manual synthesizer glass reaction vessel with a Teflon-lined screw cap. Reverse phase HPLC (RP-HPLC) was performed with 215 and 254 nm UV detection, using a C-18 analytical (250 × 4.6) and preparative columns (250 × 21.4). All runs used linear gradients of A in B (A: CH3CN containing 0.1% TFA and B: 5% CH3CN/H2O containing 0.1% TFA). Epimerization ratios given are those of the integrated NMR peaks. All yields refer to isolated, chromatographically homogeneous materials. V = Valine, F = Phenylalanine, A = Alanine, L = Leucine, W = Tryptophan, Y = Tyrosine, G = Glycine, K = Lysine, T = Threonine, S = Serine, N = Asparagine, R = Arginine, I = Isoleucine, D = Aspartic acid, E = Glutamic acid, HOBT = 1-hydroxybenzotriazole, EDCI = N-Ethyl-N’-(3-dimethylaminopropyl)carbodiimide hydrochloride, HBTU = O-(Benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, DIPEA = N,N’-diisopropylethylamine, DBU = diazabicyclo[5.4.0]undec-7-ene.
General Procedure 1 for the Synthesis of Fmoc Protected Peptide tert Butyl Esters
To a stirred solution of Fmoc protected amino acid (1 mmol), glycine tert butyl ester hydrochloride (1 mmol) and HOBT (1.2 mmol) in dry methylene chloride (10 mL) was added EDCI (1.2 mmol) followed by DIPEA (3 mmol) at 0 °C. After stirring of 30 min at 0 °C, the reaction mixture was warmed up to room temperature and stirred for 2 h. The solvents were removed and the product was purified by column chromatography eluting with 50% ethyl acetate in hexanes.
Fmoc-d-Phe-Gly-OtBu (5)
Prepared by the general procedure 1 with a yield of 1.15 g (89%), [α]22D +16.1 (c =1, CHCl3); IR (CHCl3) 1740, 1705, 1660 cm−1; 1H NMR (500 MHz) δ 7.76 (d, J = 8.0 Hz, 2H), 7.53 (dd, J = 8.0, 10.5 Hz, 2H), 7.41 (t, J = 7.5 Hz, 2H), 8.33–7.22 (m, 7H), 6.32 (s, 1H), 5.37 (d, J = 5.5 Hz, 1H), 4.50–4.33 (m, 3H), 4.19 (t, J = 7.0 Hz, 1H), 3.96–3.82 (m, 2H), 3.12 (s, 2H), 1.46 (s, 9H); 13C NMR (125.6 MHz) δ 172.0, 168.7, 156.2, 144.0, 141.5, 136.6, 129.5, 129.0, 128.0, 127.4, 127.3, 125.3, 120.2, 82.7, 67.3, 56.3, 47.3, 42.3, 38.7, 28.3. ESI-HRMS calcd for C30H32N2O5Na [M + Na]+, 523.2209; found, 523.2217.
Fmoc-Gly-Gly-OtBu (6)
Prepared by the general procedure 1 with a yield of 4.23 g (84%), IR (CHCl3) 1739, 1704, 1660 cm−1; 1H NMR (500 MHz) δ 7.76 (d, J = 7.5 Hz, 2H), 7.60 (d, J = 7.5 Hz, 2H), 7.40 (t, J = 7.5 Hz, 2H), 7.31 (dt, J = 7.0, 0.5 Hz, 2H), 6.65 (s, 1H), 5.73 (t, J = 5.5 Hz, 1H), 4.43 (d, J = 7.0 Hz, 2H), 4.22 (t, J = 7.0 Hz, 1H), 3.95 (t, J = 6.0 Hz, 4H), 1.47 (s, 9H); 13C NMR (125.6 MHz) δ 169.4, 169.1, 156.9, 144.0, 141.5, 128.0, 127.3, 125.3, 120.2, 82.8, 67.5, 47.3, 44.6, 42.2, 28.3. ESI-HRMS calcd for C23H26N2O5Na [M + Na]+, 433.1739; found, 433.1732.
General Procedure 2 for the Synthesis of Thioamides
A solution of Fmoc protected peptidyl tert butyl ester (4 mmol) and Lawesson’s reagent (2.4 mmol) in dry toluene (40 mL) was heated to reflux under nitrogen for 2 h. After cooling to room temperature, the toluene was removed and the crude reaction mixture was subjected to column chromatography to afford the thioamide tert butyl ester that then was dissolved in a mixture of TFA/CH2Cl2/Et3SiH (15.0/5.0/2.0 mL). After 2 h of stirring at room temperature, the solvents were removed and the concentrate was washed with 40% ethyl acetate/hexanes to afford the desired product.
Fmoc-l-thionoPhe-Gly-OH (7)
Prepared by the general procedure 2 with a yield of 1.20 g (78%), [α]24D -12.1 (c = 0.75, CH3OH); IR (CHCl3) 3305, 1720, 1705, 1247 cm−1; 1H NMR (500 MHz, CD3OD) δ 7.77 (d, J = 7.5 Hz, 2H), 7.56 (t, J = 9.0Hz, 2H), 7.37 (t, J = 7.5 Hz, 2H), 7.31–7.19 (m, 7H), 4.76 (dd, J = 5.0, 9.5 Hz, 1H), 4.35–4.10 (m, 5H), 3.36–3.31 (m, 1H), 2.94 (dd, J = 10.0, 13.0 Hz, 1H); 13C NMR (125.6 MHz, CD3OD) δ 205.3, 170.2, 156.8, 144.0, 141.3, 137.7, 129.2, 128.2, 127.6, 127.0, 126.5, 125.2, 125.1, 119.7, 71.2, 66.9, 62.8, 48.7, 47.5, 47.3, 47.1, 46.3, 41.4. ESI-HRMS calcd for C26H24N2O4SNa [M + Na]+, 483.1354; found, 483.1346.
Fmoc-d-thionoPhe-Gly-OH (8)
Prepared by the general procedure 2 with a yield of 1.15 g (76%), [α]24D +10.5 (c = 0.5, CH3OH); IR (CHCl3) 3305, 1720, 1705, 1247 cm−1; 1H NMR (500 MHz, CD3OD) δ 7.77 (d, J = 7.5 Hz, 2H), 7.56 (t, J = 9.0Hz, 2H), 7.37 (t, J = 7.5 Hz, 2H), 7.31–7.19 (m, 7H), 4.76 (dd, J = 5.0, 9.5 Hz, 1H), 4.35–4.10 (m, 5H), 3.36–3.31 (m, 1H), 2.94 (dd, J = 10.0, 13.0 Hz, 1H); 13C NMR (125.6 MHz, CD3OD) δ 205.3, 170.2, 156.8, 144.0, 141.3, 137.7, 129.2, 128.2, 127.6, 127.0, 126.5, 125.2, 125.1, 119.7, 71.2, 66.9, 62.8, 48.7, 47.5, 47.3, 47.1, 46.3, 41.4. ESI-HRMS calcd for C26H24N2O4SNa [M + Na]+, 483.1354; found, 483.1359.
Fmoc-thionoGly-Gly-OH (9)
Prepared by the general procedure 2 with a yield of 2.60 g (72%), IR (CHCl3) 3295, 1725, 1714, 1247 cm−1; 1H NMR (500 MHz, CD3OD) δ 7.75 (d, J = 7.5 Hz, 2H), 7.65 (d, J = 7.5 Hz, 2H), 7.36 (t, J = 7.5 Hz, 2H), 7.26 (t, J = 7.0 Hz, 2H), 4.38–4.34 (m, 4H), 4.20–4.19 (m, 3H); 13C NMR (125.6 MHz, CD3OD) δ 201.4, 170.5, 157.9, 144.1, 141.4, 127.7, 127.1, 125.1, 119.8, 67.1, 51.3, 48.8, 48.4, 47.4, 47.2, 46.3. ESI-HRMS calcd for C19H18N2O4SNa [M + Na]+, 393.0885; found, 393.0891.
General Procedure 3: Fmoc-SPPS of the peptidyl thioesters on the aminomethyl polystyrene resin
Derivatization of aminomethyl polystyrene resin with Fmoc-AA-Gly-OH thioamides
In a 10 mL glass reaction vessel, aminomethyl polystyrene resin (244 mg, 0.1 mmol) was swelled in DMF (4 mL) for 30 min, after which the solvent was removed by filtration. To a stirred solution of Fmoc-protected thioamide (0.4 mmol), HOBT (54 mg, 0.4 mmol) and HBTU (152 mg, 0.4 mmol) in dry DMF (3 mL) was added DIPEA (70 µL, 0.4 mmol) at room temperature. After stirring for 4 min before the so-formed solution of activated activated Fmoc-protected thioamide was added to the peptide synthesis vessel with an additional DMF (1 mL) and the resulting mixture was shaken for 2 h before the solvent was decanted and the resin was washed thoroughly using DMF (3 × 2 mL) and dichloromethane (3 × 2 mL).
Fmoc removal and iterative peptide assembly
A solution of 20% piperidine in DMF (5 mL) was added to the resin and the resulting mixture was shaken for 4 min, after which the solvents were removed by filtration. A further 5 mL of 20% piperidine in DMF was added to the resin which was then shaken for 30 min. The solvent was removed and the resin was washed with DMF (2 × 5 mL), dichloromethane (2 × 5 mL), isopropanol (2 × 5 mL) and hexane (2 × 5 mL). All couplings were carried out by adding a solution of protected amino acid (0.4 mmol), pre-activated with HOBT (0.4 mmol), DIPEA (0.4 mmol), and HBTU (0.4 mmol) in dry DMF (4.0 mL) as described above, to the resin followed by shaking at room temperature. After 2 h, the resin was washed with DMF (2 × 5 mL), dichloromethane (2 × 5 mL) and DMF (2 × 5 mL). The final amino acid was introduced with Boc protection.
Alkylation of resin bound peptidyl thioamides
The resin-bound peptidyl thioamide was treated with a solution of DBU (150 µL, 1 mmol) and benzyl bromide (107 µL, 0.9 mmol) in DMF (4 mL) and shaken at room temperature for 2 h. The resin was washed with DMF (2 × 5 mL), dichloromethane (2 × 5 mL), and DMF (2 × 5 mL) and dried to provide the resin bound thioimidate.
Cleavage from the resin with aqueous TFA
In case of tri-peptidyl thioesters (l-Val-l-Ala-d/l-Phe-SBn), the resin bound thioimidate was suspended in a 1/1 mixture of TFA/water (10 mL) and stirred magnetically for 2 h at room temperature. The resin was filtered and washed with acetonitrile (2 × 10 mL). The combined filtrates were concentrated and the concentrate was neutralized with a saturated solution of sodium bicarbonate (40 mL) followed by extraction with chloroform (3 × 50 mL). The organic layer was washed with brine and dried. Evaporation of the solvent afforded the crude peptidyl thioesters, which were subjected to chromatographic purification eluting with 20% methanol in chloroform.
Cleavage from the resin with TFA/water/anisole
The resin bound thioimidate was suspended in a 10 mL mixture of TFA/water/anisole (5.0/4.5/0.5 mL) and stirred magnetically for 2 h at room temperature. A solution of TFA/DCM/Et3SIH (7.5/2/0.5, 10 mL) was added to the reaction mixture, which was then stirred for 4 h. The resin was filtered off and washed with acetonitrile (2 × 10 mL). The combined filtrates were concentrated and the concentrate was taken up in acetonitrile/water (v/v 1:1, 5 mL) and subjected to RP-HPLC purification (10 – 100% A in B with a flow rate of 12 mL/min over 40 min and 215 nm UV detection) to afford the peptidyl thioester.
l-Val-l-Ala-l-Phe-SBn (10)
Following the general procedure 3 with a yield of 32 mg (72%). 500 MHz 1H NMR shows epimerization (ratio, l/d = 78.1/21.9). 1H NMR (500 MHz) δ 7.62 (d, J = 7.5 Hz, 1H), 7.32–7.21 (m, 8H), 7.11–7.09 (m, 2H), 7.03–7.00 (m, 1H), 4.97–4.93 (m, 1H), 4.49–4.43 (m, 1H), 4.12 (s, 1H), 3.23 (dd, J = 5.0, 14.0 Hz, 1H), 3.08 (d, J = 3.0 Hz, 1H), 2.99 (dd, J = 8.5, 14.5 Hz, 1H), 2.28–2.19 (m, 1H), 1.42–1.36 (m, 2H); 1.33 (d, J = 7.0 Hz, 3H), 0.97 (d, J = 7.0 Hz, 3H), 0.79 (d, J = 7.0 Hz, 3H); 13C NMR (125.6 MHz) δ 199.8, 175.1, 172.6, 137.1, 136.1, 129.5, 129.2, 128.9, 128.7, 127.6, 127.1, 59.7, 48.4, 38.1, 33.4, 30.7, 19.6, 17.1, 16.0. ESI-HRMS calcd for C24H32N3O3S [M + H]+, 442.2164; found, 442.2168.
l-Val-l-Ala-d-Phe-SBn (11)
Following the above described procedure with a yield of 33 mg (74%). 500 MHz 1H NMR shows epimerization (ratio, l/d = 22.5/77.5). 1H NMR (500 MHz) δ 7.76 (d, J = 7.5 Hz, 1H), 7.32–7.21 (m, 8H), 7.14 (d, J = 7.0 Hz, 2H), 7.10–7.08 (m, 1H), 4.95–4.90 (m, 1H), 4.48–4.45 (m, 1H), 4.07 (s, 1H), 3.23 (dd, J = 5.0, 14.0 Hz, 1H), 3.13 (d, J = 3.5 Hz, 1H), 2.99 (dd, J = 8.5, 14.0 Hz, 1H), 2.28–2.20 (m, 1H), 1.50–1.42 (m, 2H); 1.25 (d, J = 7.0 Hz, 3H), 0.94 (d, J = 7.0 Hz, 3H), 0.78 (d, J = 7.0 Hz, 3H); 13C NMR (125.6 MHz) δ 199.8, 175.3, 172.3, 137.1, 136.1, 129.6, 129.1, 128.9, 128.8, 128.5, 127.6, 127.3, 60.1, 48.3, 38.4, 33.6, 30.8, 19.9, 17.6, 16.2. ESI-HRMS calcd for C24H32N3O3S [M + H]+, 442.2164; found, 442.2158.
l-Leu-l-Trp-l-Tyr-l-Val-Gly-SBn (12)
Prepared by the general procedure 3 followed by the reversed phase HPLC purification (10–100% A with a flow rate of 12 mL/min over 30 min and 215 nm UV detection, retention time = 25 min) with a yield of 66 mg (68%). ESI-HRMS calcd for C40H51N6O6S [M + H]+, 743.3591; found, 743.3598. HPLC Analysis was performed with 254 nm UV detection on a C18 analytical column (4.6 × 250 mm) eluting with a gradient of 10–100% A over 30 min with a flow rate of 1.0 mL/min. Retention time = 16.91 min.
l-Ala-l-Lys-l-Trp-l-Tyr-l-Val-Gly-SBn (13)
Prepared by the general procedure 3 followed by the reversed phase HPLC purification (10–100% A with a flow rate of 12 mL/min over 40 min and 215 nm UV detection, retention time = 21 min) with a yield of 70 mg (60%). ESI-HRMS calcd for C43H57N8O7S [M + H]+, 829.4071; found, 829.4062. HPLC Analysis was performed with 254 nm UV detection on a C18 analytical column (4.6 × 250 mm) eluting with a gradient of 10–100% A over 30 min with a flow rate of 1.5 mL/min. Retention time = 11.66 min.
General Procedure 4: Fmoc-SPPS of Peptidyl Thioesters on the 2-Chlorotrityl Resin
In a 10 mL glass reaction vessel, 2-chlorotrityl chloride resin (117 mg, 0.1 mmol) was suspended in dichloromethane (5 mL), shaken for 5 min, and filtered. A solution of 4-(((((9H-fluoren-9-yl)methoxy)carbonyl)amino)methyl)benzoic acid (149 mg, 0.4 mmol) and DIPEA (174 µL, 1.0 mmol) in dichloromethane (4 mL) was added and the resulting mixture was shaken for 2 h at room temperature. After filtration, the resin was washed with DMF (2 × 5 mL). An 80:15:5 mixture of dichloromethane/MeOH/DIPEA (5 mL) was added to the resin and the resulting mixture was shaken for 0.5 h at room temperature. After filtration of solvents, the resin was again washed with DMF (2 × 5 mL). All subsequent steps of elongation of the peptide chain, alkylation of the thioamides and cleavage from the resin were conducted as described in general procedure 3.
l-Thr-l-Ala-l-Ser-l-Phe-l-Ser-l-Leu-Gly-SBn (14)
Prepared by the general procedure 4 followed by the reversed phase HPLC purification (10–100% A with a flow rate of 12 mL/min over 40 min and 215 nm UV detection, retention time = 22 min) with a yield of 51 mg (57%). ESI-HRMS calcd for C37H54N7O10S [M + H]+, 788.3653; found, 788.3661. HPLC Analysis was performed with 254 nm UV detection on a C18 analytical column (4.6 × 250 mm) eluting with a gradient of 10–90% A over 30 min with a flow rate of 1.0 mL/min. Retention time = 15.52 min.
l-Thr-l-Phe-l-Tyr-l-Ser-l-Ala-l-Tyr-Gly-SBn (15)
Prepared by the general procedure 4 followed by the reversed phase HPLC purification (10–100% A with a flow rate of 12 mL/min over 40 min and 215 nm UV detection, retention time = 26 min) with a yield of 57 mg (56%). ESI-HRMS calcd for C46H56N7O11S [M + H]+, 914.3759; found, 914.3768. HPLC Analysis was performed with 254 nm UV detection on a C18 analytical column (4.6 × 250 mm) eluting with a gradient of 10–90% A over 30 min with a flow rate of 1.0 mL/min. Retention time = 17.90 min.34
l-Asn-l-Trp-l-Arg-l-Tyr-l-Ile-l-Ser-l-Thr-l-Phe-Gly-SBn (16)
Prepared by the general procedure 4 followed by the reversed phase HPLC purification (10–100% A with a flow rate of 12 mL/min over 40 min and 215 nm UV detection, retention time = 31 min) with a yield of 76 mg (48%). ESI-HRMS calcd for C61H81N14O13S [M + H]+, 1249.5828; found, 1249.5849. HPLC Analysis was performed with 254 nm UV detection on a C18 analytical column (4.6 × 250 mm) eluting with a gradient of 10–90% A over 30 min with a flow rate of 1.0 mL/min. Retention time = 23.18 min.34
l-Glu-l-Met-l-Arg-Gly-SBn (17)
The resin bound thioimidate was prepared as described in general procedure 3. The resin bound thioimidate was suspended in a mixture of TFA/water/Anisole (6/5/1, 12 mL) and stirred magnetically for 2 h at room temperature before TFA/dichloromethane/Et3SiH (15/4/1, 20 mL) was added to the reaction mixture and stirring continued for 4 h. Ammonium iodide (25 mg, 0.17 mmol) was added to the reaction mixture followed by the addition of dimethyl sulfide (100 µL, 1.36 mmol) and stirring continued for 2 h. After that, the resin was filtered off and washed with acetonitrile (2 × 10 mL). The combined filtrates were concentrated and the concentrate was dissolved in acetonitrile/water (v/v 1:1, 5 mL) and subjected to RP-HPLC purification (10 – 100% A in B with a flow rate of 12 mL/min over 40 min and 215 nm UV detection, retention time 22 min) to afford the tetrapeptide thioester with a yield of 51 mg (62%). ESI-HRMS calcd for C25H40N7O6S2 [M + H]+, 598.2481; found, 598.2492. HPLC Analysis was performed with 254 nm UV detection on a C18 analytical column (4.6 × 250 mm) eluting with a gradient of 10–90% A over 30 min with a flow rate of 1.5 mL/min. Retention time = 10.26 min.
Supplementary Material
Scheme 2.
Synthesis of diastereomeric tripeptidyl S-benzyl thioesters
Acknowledgment
We thank Chandrasekhar Navuluri (Wayne State University) for his help and the NIH (GM 62160) for support of this work.
Footnotes
Supporting Information Available. Full experimental details and copies of the 1H and 13C NMR spectra of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hackenberger CPR, Schwarzer D. Angew. Chem. Int. Ed. 2008;47:10030–10074. doi: 10.1002/anie.200801313. [DOI] [PubMed] [Google Scholar]; (b) Kent SBH. Chem. Soc. Rev. 2009;38:338–351. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]; (c) Macmillan D. Angew. Chem. Int. Ed. 2006;45:7668–7672. doi: 10.1002/anie.200602945. [DOI] [PubMed] [Google Scholar]; (d) Becker CFW. Chemical Biology. 2009:145–157. [Google Scholar]; (e) Payne RJ, Wong C-H. Chem. Commun. 2010;46:21–43. doi: 10.1039/b913845e. [DOI] [PubMed] [Google Scholar]
- 3.a) Offer J. Biopolymers. 2010;94:530–541. doi: 10.1002/bip.21455. [DOI] [PubMed] [Google Scholar]; b) Rohde H, Seitz O. Biopolymers. 2010;94:551. doi: 10.1002/bip.21442. [DOI] [PubMed] [Google Scholar]; c) Haase C, Seitz O. Angew. Chem. Int. Ed. 2008;47:1553–1556. doi: 10.1002/anie.200704886. [DOI] [PubMed] [Google Scholar]
- 4.a) Kang J, Macmillan D. Org. Biomol. Chem. 2010;8:1993–2002. doi: 10.1039/b925075a. [DOI] [PubMed] [Google Scholar]; b) Hackeng TM, Griffin JH, Dawson PE. Proc. Natl. Acad. Sci., USA. 1999;96:10068–10073. doi: 10.1073/pnas.96.18.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan WC, White PD, editors. Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford: OUP; 2000. [Google Scholar]
- 6.(a) Shin Y, Winans KA, Backes BJ, Kent SBH, Ellman JA, Bertozzi CR. J. Am. Chem. Soc. 1999;121:11684–11689. [Google Scholar]; (b) Sewing A, Hilvert D. Angew. Chem. Int. Ed. 2001;40:3395–3396. doi: 10.1002/1521-3773(20010917)40:18<3395::aid-anie3395>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; (c) Ingenito R, Bianchi E, Fattori D, Pessi A. J. Am. Chem. Soc. 1999;121:11369–11364. [Google Scholar]; (d) Yamamoto N, Tanabe Y, Okamoto R, Dawson PE, Kajihara Y. J. Am. Chem. Soc. 2008;130:501–510. doi: 10.1021/ja072543f. [DOI] [PubMed] [Google Scholar]
- 7.(a) Blanco-Canosa JB, Dawson PE. Angew. Chem. Int. Ed. 2008;47:6851–6855. doi: 10.1002/anie.200705471. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Camarero JA, Hackel BJ, de Yoreo JJ, Mitchell AR. J. Org. Chem. 2004;69:4145–4151. doi: 10.1021/jo040140h. [DOI] [PubMed] [Google Scholar]
- 8.(a) Li X, Kawakami T, Aimoto S. Tetrahedron Lett. 1998;39:8669–8672. [Google Scholar]; (b) Hasegawa K, Sha YL, Bang JK, Kawakami T, Akaji K, Aimoto S. Lett. Pept. Sci. 2002;8:277–284. [Google Scholar]; (c) Clippingdale AB, Barrow CJ, Wade JD. J. Pept. Sci. 2000;6:225–234. doi: 10.1002/(SICI)1099-1387(200005)6:5<225::AID-PSC244>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.(a) Mende F, Seitz O. Angew. Chem. Int. Ed. 2007;46:4577–4580. doi: 10.1002/anie.200700356. [DOI] [PubMed] [Google Scholar]; (b) Mende F, Beisswenger M, Seitz O. J. Am. Chem. Soc. 2010;132:11110–11118. doi: 10.1021/ja101732a. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kawakami T, Aimoto S. Tetrahedron. 2009;65:3871–3877. [Google Scholar]; (b) Botti P, Villain M, Manganiello S, Gaertner H. Org. Lett. 2004;6:4861–4864. doi: 10.1021/ol0481028. [DOI] [PubMed] [Google Scholar]; (c) Warren JD, Miller JS, Keding SJ, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:6576–6578. doi: 10.1021/ja0491836. [DOI] [PubMed] [Google Scholar]; (d) Ollivier N, Dheur J, Mhidia R, Blanpain A, Melnyk O. Org. Lett. 2010;12:5238–5241. doi: 10.1021/ol102273u. [DOI] [PubMed] [Google Scholar]; (e) Hou W, Zhang X, Li F, Liu C-F. Org. Lett. 2011;13:386–389. doi: 10.1021/ol102735k. [DOI] [PubMed] [Google Scholar]; (f) Dheur J, Ollivier N, Melnyk O. Org. Lett. 2011;13:1560–1563. doi: 10.1021/ol2002804. [DOI] [PubMed] [Google Scholar]; (g) Dheur J, Ollivier N, Vallin A, Melnyk O. J. Org. Chem. 2011;76:3194–3202. doi: 10.1021/jo200029e. [DOI] [PubMed] [Google Scholar]; (h) George EA, Novick RP, Muir TW. J. Am. Chem. Soc. 2008;130:4914–4924. doi: 10.1021/ja711126e. [DOI] [PubMed] [Google Scholar]
- 11.(a) Alsina J, Yokum TS, Albericio F, Barany G. J. Org. Chem. 1999;64:8761–8769. doi: 10.1021/jo990629o. [DOI] [PubMed] [Google Scholar]; (b) Alsina J, Yokum TS, Albericio F, Barany G. Tetrahedron Lett. 2000;41:7277–7280. [Google Scholar]; (c) Jensen KJ, Alsina J, Songster MF, Vágner J, Albericio F, Barany G. J. Am. Chem. Soc. 1998;120:5441–5452. [Google Scholar]; (d) Brask J, Albericio F, Jensen KJ. Org. Lett. 2003;5:2951–2953. doi: 10.1021/ol0351044. [DOI] [PubMed] [Google Scholar]; (e) Shigenaga A, Sumikawa Y, Tsuda S, Sato K, Otaka A. Tetrahedron. 2010;66:3290–3296. [Google Scholar]
- 12.Mende F, Seitz O. Angew. Chem. Int. Ed. 2011;50:1232–1240. doi: 10.1002/anie.201005180. [DOI] [PubMed] [Google Scholar]
- 13.(a) Coates RM, Firsan SJ. J. Org. Chem. 1986;51:5198–5209. [Google Scholar]; (b) Masuda R, Hojo M, Ichi T, Sasano S, Kobayashi T, Kuroda C. Tetrahedron Lett. 1991;32:1195–1198. [Google Scholar]; (c) Harrowven DC, Lucas MC, Howes PD. Tetrahedron. 1999;55:1187–1196. [Google Scholar]; (d) Boeini HZ, Kashan ME. Green Chemistry. 2009;11:1987–1991. [Google Scholar]; (e) Suzuki Y, Yazaki R, Kumagai N, Shibasaki M. Angew. Chem. Int. Ed. 2009;48:5026–5029. doi: 10.1002/anie.200901588. [DOI] [PubMed] [Google Scholar]; (f) Yazaki R, Kumagai N, Shibasaki M. J. Am. Chem. Soc. 2010;132:10275–10276. doi: 10.1021/ja105141x. [DOI] [PubMed] [Google Scholar]
- 14.(a) Eberle MK, Jutzi-Eme A-M, Nuninger F. J. Org. Chem. 1994;59:7249–7258. [Google Scholar]; (b) Oberhauser B, Baumann K, Grohmann B, Sperner H. Synlett. 1999:893–896. [Google Scholar]
- 15.For alternative uses of thioamides and their activation as thioimides in peptide chemistry see: Hammond MC, Harris BZ, Lim WA, Bartlett PA. Chem. Biol. 2006;13:1247–1251. doi: 10.1016/j.chembiol.2006.11.010. Hammond MC, Bartlett PA. J. Org. Chem. 2007;72:3104–3107. doi: 10.1021/jo062664i.
- 16.For further applications of thioamides in peptides and proteins see: Frank R, Jakob M, Thunecke F, Fischer G, Schutkowski M. Angew. Chem. Int. Ed. 2000;39:1120–1122. Frank R, Schutkowski M. Chem. Commun. 1996:2509–2510. Schutkowski M, Jakob M, Landgraf G, Born I, Neubert K, Fischer G. Eur. J. Biochem. 1997;245:381–385. doi: 10.1111/j.1432-1033.1997.00381.x. Schutkowski M, Woellner S, Fischer GS. Biochemistry. 1995;34:13016–13026. doi: 10.1021/bi00040a012. Wildemann D, Drewello M, Fischer G, Schutkowski M. Chem. Commun. 1999:1809–1810.
- 17.May PJ, Bradley M, Harrowven DC, Pallin D. Tetrahedron Lett. 2000;41:1627–1630. [Google Scholar]
- 18.Pech A, Wehofsky N, Wildemann D, Schutkowski M, Bordusa F. J. Pept. Sci. 2006;12 Suppl. S:130–131. [Google Scholar]
- 19.(a) Cava MP, Levinson MI. Tetrahedron. 1985;41:5061–5087. [Google Scholar]; (b) Jesberger M, Davis TP, Barner L. Synthesis. 2003:1929–1958. [Google Scholar]; (c) Ozturk T, Ertas E, Mert O. Chem. Rev. 2007;107:5210–5278. doi: 10.1021/cr040650b. [DOI] [PubMed] [Google Scholar]
- 20.(a) Jensen OE, Lawesson S-O, Bardi R, Piazzesi AM, Toniolo C. Tetrahedron. 1985;41:5595–5606. [Google Scholar]; (b) Hammond MC, Bartlett PA. J. Org. Chem. 2007;72:3104–3107. doi: 10.1021/jo062664i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomsen I, Clausen K, Scheibye S, Lawesson S-O. Org. Synth. Coll. Vol. 1990;7:372–375. [Google Scholar]
- 22.Coats SJ, Link JS, Hlasta DJ. Org. Lett. 2003;5:721–724. doi: 10.1021/ol027493s. [DOI] [PubMed] [Google Scholar]
- 23.Shalaby MA, Grote CW, Rapoport H. J. Org. Chem. 1996;61:9045–9048. doi: 10.1021/jo961245q. [DOI] [PubMed] [Google Scholar]
- 24.The extent of epimerization in these examples ranged from “not detected” to 3% as determined by “enzymatic hydrolysis assays”. The preparation of an octapeptide (Z-KLALDLLE-SMe) in this manner was reported, but neither the yield nor the extent of epimerization was given.18
- 25.Wipf P, Fritch PC. Tetrahedron Lett. 1994;35:5397–5400. [Google Scholar]
- 26.(a) Hogenauer TJ, Wang Q, Sanki AK, Gammon AJ, Chu CHL, Kaneshiro C, Kajihara Y, Michael K. Org. Biomol. Chem. 2007;5:759–762. doi: 10.1039/b618442a. [DOI] [PubMed] [Google Scholar]; (b) Wang P, Miranda LP. Int. J. Pept. Res. Therap. 2005;11:117–123. [Google Scholar]; (c) Ficht S, Payne RJ, Guy RT, Wong C-H. Chem. Eur. J. 2008;14:3620–3629. doi: 10.1002/chem.200701978. [DOI] [PubMed] [Google Scholar]
- 27.(a) Kurosu M. In: Linker Strategies in Solid-Phase Organic Synthesis. Scott PJH, editor. Chichester: Wiley; 2009. pp. 27–76. [Google Scholar]; (b) Doherty-Kirby AL, Lajoie GA. In: Solid-Phase Synthesis: A Practical Guide. Kates SA, Albericio F, editors. Boca Raton: CRC Press; 2000. pp. 129–195. [Google Scholar]; (c) Bodanszky M. Peptide Chemistry. A Practical Textbook. 2nd ed. Berlin: Springer; 1993. [Google Scholar]; (d) Bodanszky M, Bodanszky A. The Practice of Peptide Synthesis. 2nd ed. Heidelberg: Springer-Verlag; 1994. [Google Scholar]
- 28.(a) Okamoto R, Kajihara Y. Angew. Chem. Int. Ed. 2008;47:5402–5406. doi: 10.1002/anie.200801097. [DOI] [PubMed] [Google Scholar]; (b) Okamoto R, Souma S, Kajihara Y. J. Org. Chem. 2009;74:2494–2501. doi: 10.1021/jo8026164. [DOI] [PubMed] [Google Scholar]
- 29.The compatibility of the method with the presence of N-terminal cysteine will need to be demonstrated before the method can be applied for preparation of the central segment in a triblock approach to peptide synthesis.
- 30.Lebreton S, Patek M. In: Linker Strategies in Solid-Phase Organic Synthesis. Scott PJH, editor. Chichester: Wiley; 2009. pp. 195–220. [Google Scholar]
- 31.Kenner GW, McDermott JR, Sheppard RC. J. Chem. Soc., Chem. Commun. 1971:636–637. [Google Scholar]
- 32.However, as the pKa of thioamides is ~18.45 in DMSO and much closer to that of sulfonamides (PhSO2NH2, pKa 16.1) than that of acyl sulfonamides (pKa ~2.5), it is not reasonable to consider that the thioamide linkage protects the resin-bound peptide from basic cleavage..
- 33.Bordwell FG. Acc. Chem. Res. 1988;21:456–463. [Google Scholar]
- 34.Although compounds 15 and 16 shown clean total ion counts and mass spectra, the HPLC peak shape suggests the possible presence of impurities (see supporting information).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.