Abstract
We have determined the solution structure of the C-terminal quarter of human poly(A)-binding protein (hPABP). The protein fragment contains a protein domain, PABC [for poly(A)-binding protein C-terminal domain], which is also found associated with the HECT family of ubiquitin ligases. By using peptides derived from PABP interacting protein (Paip) 1, Paip2, and eRF3, we show that PABC functions as a peptide binding domain. We use chemical shift perturbation analysis to identify the peptide binding site in PABC and the major elements involved in peptide recognition. From comparative sequence analysis of PABC-binding peptides, we formulate a preliminary PABC consensus sequence and identify human ataxin-2, the protein responsible for type 2 spinocerebellar ataxia (SCA2), as a potential PABC ligand.
Poly(A)-binding protein (PABP) is a ubiquitous and abundant cytosolic protein involved in mRNA translation. PABP binds the poly(A) tail of mRNA and mediates mRNA circularization through binding of the eIF4F translation initiation complex, which is associated with the mRNA 5′ cap structure (1). This circularization enhances translation by facilitating recycling of ribosomes (2). PABP acts as a general scaffolding protein that recruits various translation factors to the translating mRNA. In addition to the eIF4G subunit of eIF4F, protein partners of PABP include PABP-interacting proteins Paip1 and Paip2, and eukaryotic release factor eRF3 (3–5). In plants and yeast, additional partners have been identified as follows: eIF4B, Pbp1p, and Rna15p (6–8).
Structurally, PABP is composed of two parts, an N-terminal portion that contains four RNA recognition motifs (RRM) and a C-terminal portion which contains a conserved poly(A)-binding protein C-terminal domain (PABC; CTC or PABP) sequence of ≈75 residues. Because of the essential function of the RRM domains, they have received more attention than the PABC domain. Early work in yeast established that at least one RRM domain is required for binding mRNA and cell survival (9). Numerous functional and mutagenesis studies have also established the importance of the RRM domains in enhancing mRNA translation and stability. Recently, the structure of the RRM1 and RRM2 domains in a complex with poly(A) RNA was determined by x-ray crystallography (10).
In contrast, the structure and function of the C-terminal domain has remained enigmatic. Early work suggested that the C terminus is important in structuring the mRNA tail and for the cellular localization of PABP. This work was confirmed by more recent studies that describe PABP dimerization through the C-terminal domain and a requirement of the C terminus for proper nuclear shuttling (11, 12). The C-terminal half of PABP contains binding sites for eRF3 (5), Paip1 (13), Paip2 (4), Pbp1p (7), and a viral RNA polymerase (14).
Comparative sequence analysis of the C terminus of PABP reveals the phylogenetic conservation in the PABC domain (Fig. 1). PABC is highly conserved in eukaryotes and is also found associated with a subset of HECT E3 ubiquitin-protein ligases (15). The significance of this association is unknown. In humans, three different PABP proteins are known: the major form studied here and two tissue-specific or inducible forms (16, 17). The sequence similarity between these isoforms is lower than for homologous forms across species, suggesting conserved but distinct functions for the different families of PABP proteins. An unrelated nuclear poly(A)-binding protein, referred to as PAB II, is also known in humans and yeast.
Figure 1.
Amino acid sequences of PABC domains and ligands. (A) Sequence alignment of PABC domains from human PABP (h, Homo sapiens), fly PABP (d, Drosophila melanogaster), wheat PABP (w, Triticum aestivum), yeast PABP (y, Saccharomyces cerevisiae), and the rat (Rattus norvegicus) 100-kDa HECT E3 ubiquitin-protein ligase (100KD). The secondary structure and residue numbering are based on hPABP. Highly conserved residues are shown bold and underlined. (B) Sequence alignment of four PABC-binding peptides and the deduced consensus sequence. Peptides were derived from human Paip2, human Paip1, and human release factor RF3. Residues common to all four sequences are shown in red. (C) Putative PABC-sites in ataxin-2, an ataxin-2-related protein from Arabidopsis (MDC16.14), and two plant RRM-containing proteins (aF6N18.17, oRNA-BP). Numbers indicate the total number of amino acids in the protein and the first amino acid shown in the alignment.
Here, we determine the solution structure of the last 139 residues from human PABP (hPABP) and show that this sequence contains a well-folded domain of ≈74 aa. This domain, PABC, functions to bind peptides from a number of proteins known to interact with the C terminus of PABP. Analysis of chemical shift changes on peptide binding allowed us to map the peptide binding site on PABC and determine the orientation of the peptide in the binding site. Finally, we used the set of peptides and proteins known to bind to PABC domains to formulate a preliminary PABC recognition sequence.
Materials and Methods
Protein and Peptide Preparation.
Human PABP residues 498 to 636 were expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein and purified by affinity chromatography. For NMR analysis, the GST-tag was removed by PreScission protease (Amersham Pharmacia) cleavage (leaving a five residue N-terminal extension) and the protein exchanged into NMR buffer (50 mM K⋅HPO4/100 mM NaCl/1 mM NaN3, pH 6.3) at 303 K.
Human Paip2 was expressed as a GST-fusion protein and purified by affinity chromatography. For NMR, the GST moiety was removed by thrombin cleavage (leaving a four residue N-terminal extension), and Paip2 was exchanged into NMR buffer. NMR spectroscopy was carried out under the same conditions as for hPABP (498 to 636). Peptides (Fig. 1B) were synthesized by fluorenylmethoxycarbonyl (Fmoc) solid-phase peptide synthesis and purified by reverse phase chromatography on a C18 Vydac (Hesperia, CA) column. The composition and purity of the peptides were verified by ion-spray quadrupole mass spectroscopy.
Structure Determination.
NMR resonance assignments of free hPABP (498) were carried out by using standard triple-resonance techniques on a 13C,15N-labeled sample (18). Assignments of the hPABP–Paip2 complexes were based on 15N–1H-edited nuclear Overhauser effect spectroscopy (NOESY) spectra. The 15N–1H heteronuclear NOE was measured at 500 MHz on 15N-labeled hPABP (498) (19). For the structure determination, a set of 1862 NOEs were collected from homonuclear and 15N and 13C isotope-edited NOESY spectra of hPABP (498) acquired at 800 and 500 MHz. After determination of the protein fold by using manual NOE assignments (20), automatic peak NOE assignments were made by using aria (21) and the structure refined by using standard protocols in CNS v. 0.9 (22). In the final 30 structures, PABC residues 1 to 74, the backbone pairwise heavy atom rms deviation was 0.48 Å, and the all atom rms deviation was 1.16 Å. procheck showed 65.4% of residues in the most favored region of Ramachandran plot, 30.4% in additionally allowed regions, and 3.6% in generously allowed regions (23). The coordinates have been deposited in the RCSB PDB under accession number 1G9L and the NMR assignments under BMRB accession number 4915.
Peptide Titrations.
Titrations were carried out with either full-length recombinant Paip2 (residues 1–127) or a chemically synthesized peptide, Paip2 (residues 106–127), by using 15N–1H heteronuclear single quantum correlation (HSQC) spectra at 500 MHz, 303 K on a 1-mM sample of 15N-hPABP (498). The PABC residues showing the largest chemical shift changes [(Δ 1H shift)2 + (Δ 15N shift × 0.2)2]1/2 in PPM on binding of full-length Paip2 were K35 (0.9), V68 (0.63), M39 (0.59), G38 (0.5), E19 (0.46), M16 (0.43), E64 (0.41), A65 (0.41), L40 (0.38), A67 (0.36), H72 (0.32), and T37 (0.3).
Consensus Peptide Analysis.
Sequence searches of potential PABC ligands were carried out by using the preliminary PABC binding-site consensus as query (24). Potential hits were screened based on the sequence conservation of the PABC-site in related proteins, the accessibility of the PABC-site as judged from the presence of surrounding low complexity sequences, and considerations of biological relatedness. A more complete list of proteins identified is available in Table 1, which is published as supplemental data on the PNAS web site, www.pnas.org.
Results and Discussion
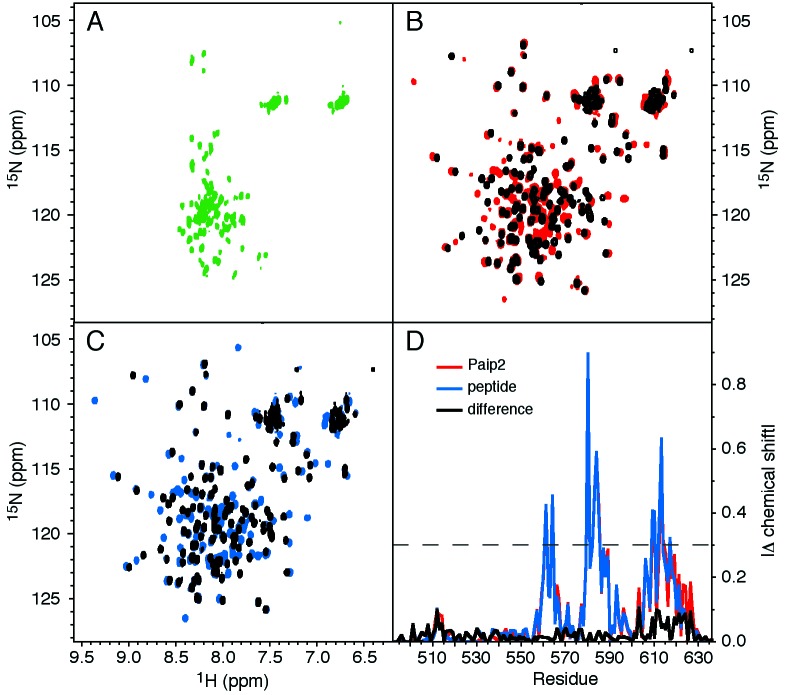
The C-terminal quarter of human PABP (residues 498–636) was prepared as an isotopically labeled, recombinant protein fragment and subjected to NMR spectroscopy. The protein gave excellent 15N–1H correlation spectra, with the dispersion of resonances typical for a protein with α-helical secondary structure and the possible presence of unfolded residues (Fig. 2B). To determine the extent of folded structure, NMR assignments were obtained, and the 15N–1H heteronuclear NOE was measured (Fig. 3A). This heteronuclear NOE is a measure of the reorientation rate of the amide nitrogen-hydrogen internuclear vector and varies between −3.6 and 0.82 (at 500 MHz) for unfolded and folded residues (19). For hPABP residues 498 to 541 and residues 623 and 636, the hNOE was negative, indicating the lack of a folded structure. Between residues 546 and 619, the hNOE was positive and generally above 0.6, indicating slow tumbling of a rigid, folded domain. This region corresponds to the PABC domain, as identified by comparative sequence analysis (Fig. 3B).
Figure 2.
Paip2 binding to PABC. (A) 15N–1H correlation (heteronuclear single quantum correlation) spectra of 15N-labeled Paip2 showing the small chemical dispersion characteristic of an unfolded protein. (B) Spectra of 15N-labeled hPABP (498 to 636) in the absence (black) and presence (red) of unlabeled Paip2. (C) Spectra of 15N-labeled hPABP in the absence (black) and presence (blue) of a peptide corresponding to residues 106–127 of Paip2. (D) Magnitude of the chemical shift changes (|Δδ|) in ppm of hPABP plotted by residue (red, intact Paip2; blue, Paip2 peptide; and black, difference between Paip2 and peptide). The dashed line indicates the cutoff for residues shown in Fig. 3D.
Figure 3.
Structure of the PABC domain and peptide-binding site. (A) Cα trace of hPABP (residues 498 to 636) colored according to residue flexibility (blue, 15N–1H heteronuclear NOE > 0.5; white, hNOE = 0; red, hNOE < −0.5). (B) Cα trace colored according to phylogenetic conservation (magenta, >80% identity; white, ≈50%; red, <20%) in a blast alignment of 40 unique PABC sequences (24). (C) Cα trace colored according the size of the amide chemical shift change (|Δδ|) on Paip2 binding (green, |Δδ| > 0.6; white, |Δδ| ≈ 0.35; red, |Δδ| < 0.1). Amide resonances for K35, V68, and M39 showed the largest changes. (D) Structure of the PABC domain. Thirty superimposed structures are shown with backbone rms deviation of 0.48 Å. Green balls represent amide groups whose chemical shifts change by more than 0.3 ppm. Ball diameter is proportional to the chemical shift change. Sidechains of the hydrophobic core of PABC are represented in light blue. (E) Secondary structure of PABC. The five helices are colored according to Fig. 1A. The conserved salt bridge between the sidechains of K35 and E42 is shown. (F) Molecular surface of PABC within 5 Å of residues with |Δδ| > 0.42. The deep hydrophobic cavity directly contacts the backbone amide of K35. Stacking by F22 could stabilize the binding of an aromatic ring in the pocket. Figures were generated with molscript (27), grasp (28), and render (29).
The solution structure of the 74-residue PABC domain was determined by NMR spectroscopy at 500 and 800 MHz. The structure is almost three-quarters α-helical, with a well defined hydrophobic core and compact globular structure. PABC consists of five α-helices arranged as an arrowhead. Helix 1 constitutes the tip, helices 2 and 4 the sides of the arrow, helix 3 is crossing, and the long C-terminal fifth helix constitutes the shaft of the arrow (Fig. 3E). Helix 2 is bent by the presence of a proline residue, P23 (residue 568 of hPABP), and is preceded by an α-helical-like loop (residues 13 to 19 in PABC). Helix 3 is distorted by the presence of branched Cα amino acids I36, T37 and of glycine G38. This destabilization is balanced by a salt bridge in helix 3 between the conserved amino acids K35 and E42.
Recently, a novel PABP interacting protein, Paip2, was identified in a Far-Western screen of a human cDNA expression library (25). To characterize Paip2 structurally, we prepared recombinant Paip2 from E. coli as a 15N-labeled GST-tag fusion protein. The 15N–1H correlation spectrum of this 127-residue protein showed the limited chemical shift dispersion characteristic of an unfolded protein (Fig. 2A). Nonetheless, recombinant Paip2 is active in binding PABP in vitro assays (25), so we attempted to detect its binding by NMR spectroscopy. On addition of unlabeled Paip2 to a 15N-labeled sample of hPABP (398), approximately half of the 15N–1H correlation peaks shifted, indicating the formation of a Paip2–PABP complex (Fig. 2A). Deletion mutagenesis of Paip2 and comparative sequence analysis had previously identified the 22 C-terminal residues of Paip2 as responsible for binding to PABP (4). Accordingly, we chemically synthesized this peptide and carried out a second titration. The peptide caused the same spectral changes as intact Paip2 (Fig. 2 C and D). Similar results were obtained with a shorter Paip2 peptide and two peptides from other PABP-binding proteins (Fig. 1B). All four peptides bound in slow-exchange, suggesting <10 μM binding affinity. These results establish that PABC is a peptide binding domain.
Based on the Paip2 titration, we identified K35, V68, and M39 of PABC as the residues displaying largest shifts on ligand binding and likely to be close to the peptide binding site (Fig. 3C). The molecular surface around the six residues most perturbed by Paip2 binding reveals the putative peptide-binding site (Fig. 3F). This nearly continuous surface wraps around the crosspoint between helices 3 and 5 and up toward helix 2. The most striking feature of the protein surface is a deep hydrophobic pocket formed between helices 2 and 3. This pocket is bounded by the amide of residue K35 and the sidechains of F22, I25, A33, and residues 34–38 of helix 3. The magnitude of the chemical shift change of K35 suggests the involvement of aromatic ring current effects. These effects could result from either the insertion of a phenylalanine (F118) from Paip2 into the hydrophobic pocket or an intramolecular rearrangement involving F22 of PABC. The presence of many residues with smaller but significant chemical shift changes suggests that some structural changes occur in much of PABC on Paip2 binding.
There is a strong correlation between the residues identified by the preceding chemical shift perturbation analysis and those that are the most conserved in the family of PABC domains. PABC contains several regions of near 100% sequence conservation. The longest stretch is KITGMLLE at position 35 to 42 in helix 3. This stretch is preceded at position 17 by LGE-LFP in helix 2 and followed at position 64 by the pair EA in helix 5. In the PABC structure, these residues are all in close proximity and appear to be involved in peptide binding. Comparison of four PABC-binding peptides reveals conserved amino acid positions over a span of 12 residues (Fig. 1B). Residues L3, N6, A7, and F10 appear to be most important for binding whereas positions 2, 5, 8, and 10 are variable. This spacing of conserved and hydrophobic residues is consistent with peptide binding in a helix-like conformation. Assuming that Paip2 follows the molecular surface of Fig. 3F, we expect it to be oriented vertically with PABC-binding site residue S1 near helix 5 and P12 near helix-like loop preceding helix 2.
By using the preliminary PABC-site consensus sequence as a key, the National Center for Biotechnology Information (NCBI) nonredundant sequence database was searched for potential PABC ligands. In addition to revealing proteins known to interact with PABP (Fig. 4), this screen identified ataxin-2, a human protein involved in a familial neurodegenerative disease and several ataxin-2-related proteins (Fig. 1C). Ataxin-2 and the related proteins show strong similarity to Pbp1p, which suggests that they may be involved in polyadenylation of mRNA (7). Ataxin-2 has also been found associated with the RRM-containing protein, A2BP1 (26). The screen also identified a number of small putative RNA binding proteins.
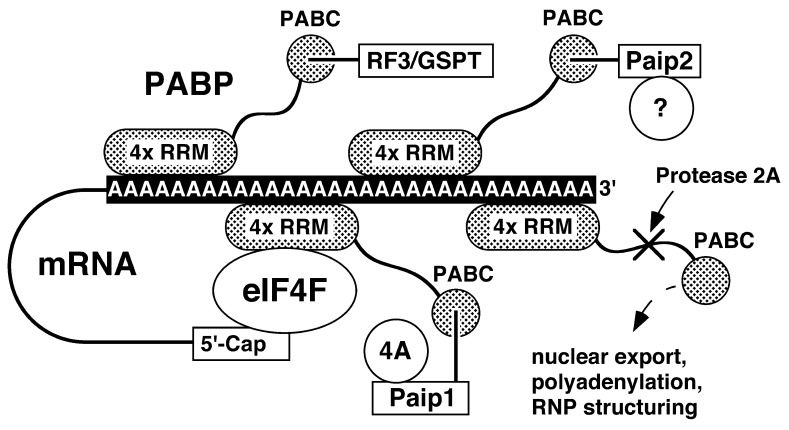
Figure 4.
Model of the interactions identified and proposed for PABC. PABP consists of an N-terminal section of four RRMs linked by a long unfolded region to PABC. Multiple PABP molecules (shown in gray) bind to the poly(A) tail via their RRM domains to form an RNA protein (RNP) complex. Cyclization of the mRNA occurs through binding of eIF4F and the mRNA 5′ cap to the RRM domains of PABP. The PABC domain of PABP binds linear peptide sequences to recruit protein factors to the mRNA RNP complex. Known binding partners include Paip1, Paip2, and eRF3 (GSPT), which themselves act as linkers to recruit eIF4A (4A) and possibly other protein factors (?) to the mRNA (3, 25). The C terminus of PABP has also been reported to be involved in PABP dimerization (11), nuclear shuttling (12), mRNA stability (30), and polyadenylation (ref. 7; dashed lines). Picornaviral protease 2 cleaves both eIF4F (not shown) and the linker region of PABP to shut off host cell protein synthesis (31, 32). A potyviral RNA-dependent RNA polymerase has also been shown to bind PABC from cucumber PABP (14).
Conclusion
The structural and functional studies described underline the role of PABP as a scaffolding protein that binds the mRNA 3′ poly(A) tail and a large number of translation factors (Fig. 4). Two types of PABP–protein interactions have been identified: those that occur via the the RRM domains and in close proximity to the mRNA poly(A) tail, and those that occur via the PABP C terminus. The large linker region between the RRM and PABC domains in PABP allows these second types of protein associations to occur farther from the mRNA strand and may relax steric or dynamic constraints in the structuring/assembly of the mRNA RNA protein (RNP) complex.
The identification of the C-terminal PABP peptide binding domain, presented here, should help clarify deletion mutagenesis studies of PABP that have often led to ambiguous interpretations about the function and importance of the C terminus. Future structural studies will define more clearly the specificity of different PABC domains and provide a detailed picture of the mechanism of peptide recognition.
Supplementary Material
Acknowledgments
We thank Nahum Sonenberg for help in the initial stages of this work and the National High Field NMR Center (NANUC) facility for assistance and access to their 800 MHz NMR spectrometer. K.G. is a Fonds de la recherche en santé du Québec Chercheur-Boursier. J.-F.T. is a Natural Sciences and Engineering Research Council (Canada) Postgraduate Scholarship-A Fellowship awardee. This work was funded by a Canadian Institutes of Health Research grant to K.G. National Research Council publication 43000.
Abbreviations
- PABC
poly(A)-binding protein C-terminal domain
- hPABP
human poly(A)- binding protein
- Paip
poly(A)-binding protein interacting protein
- RRM
RNA recognition motif
- NOESY
nuclear Overhauser effect spectroscopy
- GST
glutathione S-transferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1G9L). The NMR assignments have been deposited in the BioMagResBank, www.bmrb.wisc.edu (accession no. 4915).
See commentary on page 4288.
References
- 1.Tarun S Z, Jr, Sachs A B. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson A. In: Translational Control. Hershey J W B, Mathews M B, Sonenberg N, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. pp. 451–480. [Google Scholar]
- 3.Craig A W, Haghighat A, Yu A T, Sonenberg N. Nature (London) 1998;392:520–523. doi: 10.1038/33198. [DOI] [PubMed] [Google Scholar]
- 4.Khaleghpour K. Ph.D. thesis. Montreal: McGill University; 2000. p. 200. [Google Scholar]
- 5.Hoshino S, Imai M, Kobayashi T, Uchida N, Katada T. J Biol Chem. 1999;274:16677–16680. doi: 10.1074/jbc.274.24.16677. [DOI] [PubMed] [Google Scholar]
- 6.Le H, Tanguay R L, Balasta M L, Wei C C, Browning K S, Metz A M, Goss D J, Gallie D R. J Biol Chem. 1997;272:16247–16255. doi: 10.1074/jbc.272.26.16247. [DOI] [PubMed] [Google Scholar]
- 7.Mangus D A, Amrani N, Jacobson A. Mol Cell Biol. 1998;18:7383–7396. doi: 10.1128/mcb.18.12.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amrani N, Minet M, Le Gouar M, Lacroute F, Wyers F. Mol Cell Biol. 1997;17:3694–3701. doi: 10.1128/mcb.17.7.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs A B, Davis R W, Kornberg R D. Mol Cell Biol. 1987;7:3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deo R C, Bonanno J B, Sonenberg N, Burley S K. Cell. 1999;98:835–845. doi: 10.1016/s0092-8674(00)81517-2. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn U, Pieler T. J Mol Biol. 1996;256:20–30. doi: 10.1006/jmbi.1996.0065. [DOI] [PubMed] [Google Scholar]
- 12.Afonina E, Stauber R, Pavlakis G N. J Biol Chem. 1998;273:13015–13021. doi: 10.1074/jbc.273.21.13015. [DOI] [PubMed] [Google Scholar]
- 13.Gray N K, Coller J M, Dickson K S, Wickens M. EMBO J. 2000;19:4723–4733. doi: 10.1093/emboj/19.17.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Ullah Z, Grumet R. Virology. 2000;275:433–443. doi: 10.1006/viro.2000.0509. [DOI] [PubMed] [Google Scholar]
- 15.Callaghan M J, Russell A J, Woollatt E, Sutherland G R, Sutherland R L, Watts C K. Oncogene. 1998;17:3479–3491. doi: 10.1038/sj.onc.1202249. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Duckett C S, Lindsten T. Mol Cell Biol. 1995;15:6770–6776. doi: 10.1128/mcb.15.12.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feral C, Mattei M G, Pawlak A, Guellaen G. Hum Genet. 1999;105:347–353. doi: 10.1007/s004399900148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bax A, Grzesiek S. Acc Chem Res. 1993;26:131–138. [Google Scholar]
- 19.Peng J W, Wagner G. Methods Enzymol. 1994;239:563–596. doi: 10.1016/s0076-6879(94)39022-3. [DOI] [PubMed] [Google Scholar]
- 20.Wüthrich K. NMR of Proteins and Nucleic Acids. New York: Wiley; 1986. [Google Scholar]
- 21.Nilges M. Fold Des. 1997;2:S53–S57. doi: 10.1016/s1359-0278(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 22.Brünger A T, Adams P D, Clore G M, Gros P, Grosse-Kuntsleve R W, Jiang J-S, Kuszewski J, Nilges M, Pannu N S, Read R J. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 23.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 24.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaleghpour K, Svitkin Y V, Craig A W, DeMaria C T, Deo R C, Burley S K, Sonenberg N. Mol Cell. 2001;7:205–216. doi: 10.1016/s1097-2765(01)00168-x. [DOI] [PubMed] [Google Scholar]
- 26.Shibata H, Huynh D P, Pulst S-M. Hum Mol Genet. 2000;9:1303–1313. doi: 10.1093/hmg/9.9.1303. [DOI] [PubMed] [Google Scholar]
- 27.Kraulis P J. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 28.Nicholls A, Sharp K, Honig B. Proteins Struct Funct Genet. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 29.Merritt E A, Murphy M E P. Acta Crystallogr D Biol Crystallogr. 1994;50:869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Kiledjian M. Mol Cell Biol. 2000;20:6334–6341. doi: 10.1128/mcb.20.17.6334-6341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerekatte V, Keiper B D, Badorff C, Cai A, Knowlton K U, Rhoads R E. J Virol. 1999;73:709–717. doi: 10.1128/jvi.73.1.709-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joachims M, Van Breugel P C, Lloyd R E. J Virol. 1999;73:718–727. doi: 10.1128/jvi.73.1.718-727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.