Abstract
Objective To compare the effectiveness of an electrocautery strategy with ovulation induction using recombinant follicle stimulating hormone in patients with polycystic ovary syndrome.
Design Randomised controlled trial.
Setting Secondary and tertiary hospitals in the Netherlands.
Participants 168 patients with clomiphene citrate resistant polycystic ovary syndrome: 83 were allocated electrocautery and 85 were allocated recombinant follicle stimulating hormone.
Intervention Laparoscopic electrocautery of the ovaries followed by clomiphene citrate and recombinant follicle stimulating hormone if anovulation persisted, or induction of ovulation with recombinant follicle stimulating hormone.
Main outcome measure Ongoing pregnancy within 12 months.
Results. The cumulative rate of ongoing pregnancy after recombinant follicle stimulating hormone was 67%. With only electrocautery it was 34%, which increased to 49% after clomiphene citrate was given. Subsequent recombinant follicle stimulating hormone increased the rate to 67% at 12 months (rate ratio 1.01, 95% confidence interval 0.81 to 1.24). No complications occurred from electrocautery with or without clomiphene citrate. Patients allocated to electrocautery had a significantly lower risk of multiple pregnancy (0.11, 0.01 to 0.86).
Conclusion The ongoing pregnancy rate from ovulation induction with laparoscopic electrocautery followed by clomiphene citrate and recombinant follicle stimulating hormone if anovulation persisted, or recombinant follicle stimulating hormone, seems equivalent to ovulation induction with recombinant follicle stimulating hormone, but the former procedure carries a lower risk of multiple pregnancy.
Introduction
Polycystic ovary syndrome is characterised by oligomenorrhoea or amenorrhoea, infertility, hirsutism, acne, and bilaterally enlarged, cystic ovaries.1,2 The syndrome affects 4-9% of women of childbearing age.3 Infertility due to chronic anovulation is the most common reason for women seeking counselling or treatment. The drug of first choice for inducing ovulation is clomiphene citrate, taken orally, although 20% of women given clomiphene citrate fail to ovulate.4
Ovulation induction with gonadotrophins is well established in patients resistant to clomiphene citrate, but extensive monitoring is necessary because of the high sensitivity of polycystic ovaries to exogenous gonadotrophins, with the risk of multiple follicle development leading to termination of the cycle, ovarian hyperstimulation syndrome, or multiple pregnancy.5 To reduce these complications, various dose regimens have been used.6 A chronic low dose step-up regimen is probably the most efficient and safest treatment at present.7
Recently, laparoscopic electrocautery of the ovaries has been introduced as an alternative treatment for patients with clomiphene citrate resistant polycystic ovary syndrome. This involves a single procedure, which has minimal morbidity, which can lead to consecutive ovulations with minimal risks of multiple pregnancy.8 Patients may also respond to clomiphene citrate after this treatment.9,10 Disadvantages are the need for surgery under general anaesthesia, the unknown long term effects on ovarian function, and possible adhesion formation.
Patients who fail to ovulate after electrocautery of the ovaries and clomiphene citrate can still be treated with gonadotrophins, before proceeding to the costly and burdensome procedure of in vitro fertilisation and embryo transfer. Whether gonadotrophins or electrocautery should be the first treatment of choice in patients with clomiphene citrate resistant polycystic ovary syndrome is still debatable. The three comparative studies that have been published in this area have methodological flaws that weaken the conclusions.11-13
We conducted a randomised controlled trial to compare the effectiveness of an electrocautery strategy against ovulation induction with recombinant follicle stimulating hormone in women who had clomiphene citrate resistant polycystic ovary syndrome.
Methods
Our trial took place between February 1998 and October 2001 in 29 Dutch hospitals. Women were invited to participate if they had chronic anovulation (World Health Organization type II) and polycystic ovaries, diagnosed by transvaginal ultrasonography.14-16 They had also to be resistant to clomiphene citrate—that is, show persistent anovulation after taking 150 mg clomiphene citrate daily for five days. Primary exclusion criteria were other causes of infertility, including severe male factor subfertility, and age over 40 years.
Women who gave written informed consent were scheduled for diagnostic laparoscopy and chromopertubation. Secondary exclusion criteria identified during the procedure were tubal obstruction, extensive adhesions of the ovaries or fallopian tubes, and endometriosis stages III or IV according to the classification of the American Fertility Society.17 In absence of any of these, patients were randomised by computer generated block randomisation during laparoscopy, stratified for centre. They were allocated either laparoscopic electrocautery of the ovaries followed by clomiphene citrate and recombinant follicle stimulating hormone if anovulation persisted, or ovulation induction with recombinant follicle stimulating hormone.
Electrocautery strategy
The ovaries of women allocated electrocautery were cauterised with an Erbotom ICC 350 Unit (Erbe; Zaltbommel, Netherlands) immediately after randomisation. A bipolar insulated needle electrode (length 345 mm, shaft diameter 5 mm) was pressed at right angles to the surface of a follicle, and the needle (length 15 mm, diameter 0.9 mm) was inserted into the follicle and surrounding tissue. Each ovary was randomly punctured 5-10 times, depending on its size.
If patients ovulated in six subsequent cycles, no further treatment was given. They then completed the study according to protocol. If anovulation persisted for eight weeks after electrocautery or the patient became anovulatory again, treatment was started with 50 mg clomiphene citrate. If ovulation occurred, this dose was maintained for a maximum of six ovulatory cycles. If no ovulation occurred the dose was increased to a maximum of 150 mg. Patients then completed the study according to protocol after six subsequent ovulations. If they remained anovulatory, treatment with recombinant follicle stimulating hormone was started.
Recombinant follicle stimulating hormone
Patients allocated recombinant follicle stimulating hormone received 10 mg medroxyprogesterone for 10 days after randomisation to induce a withdrawal bleed. Ovulation induction was started on cycle day 3 by subcutaneous injection of 75 IU recombinant follicle stimulating hormone (follitropin alpha, Gonal-F; Serono Benelux, Netherlands) daily according to the chronic low dose step up regimen.18 If the diameter of the follicles remained < 10 mm, the dose was increased by half an ampoule (37.5 IU) on each of cycle days 16 and 23. If no follicle development (diameter > 10 mm) was seen by cycle day 30, the cycle was terminated because of poor response. Cycles were also terminated to prevent hyperstimulation or multiple pregnancy when there were more than six follicles with a diameter of 14 mm or greater or more than three follicles with a diameter of 16 mm or greater.19 If one follicle at least 18 mm in diameter and up to two follicles more than 15 mm diameter were present then ovulation was induced with 10 000 IU of human chorionic gonadotrophin (Pregnyl; Organon, Oss, Netherlands) subcutaneously or intramuscularly. Patients were treated until six subsequent cycles were achieved within 12 months. Follicle development was monitored in both treatment arms by transvaginal ultrasonography at weekly intervals, or more frequently if indicated by follicle growth.
Statistical analysis and study design
The primary end point was ongoing pregnancy within 12 months, defined as a viable pregnancy of at least 12 weeks. Secondary end points were ovulation, miscarriage, ectopic pregnancy, multiple pregnancy, and live birth. The effectiveness of the electrocautery strategy compared with recombinant follicle stimulating hormone was expressed as a relative rate ratio for pregnancy, with corresponding 95% confidence intervals. We used the log rank test to compare cumulative pregnancy rates over time.
We designed our study as a non-inferiority trial for pregnancy rates because of the anticipated benefits of electrocautery. The electrocautery strategy started with a single procedure, leading to consecutive ovulations with minimal risks of multiple follicle growth and multiple pregnancy, and was expected to have fewer adverse events. We therefore considered the strategy sufficient to show a pregnancy rate within 12 months of no lower than 5% of that achieved by ovulation induction with recombinant follicle stimulating hormone.
Assuming an ongoing pregnancy rate within 12 months of 38% after treatment with gonadotrophins, with an α of 5% and a β of 20%, and a pregnancy rate of 52% with the electrocautery strategy, we required 168 patients to exclude a difference of 5% or more to the detriment of electrocautery of the ovaries.20,21 All outcomes were analysed on an intention to treat basis.
Results
A total of 213 consecutive patients were invited to participate in the study. Thirty six were initially excluded: 27 refused, five became pregnant while awaiting laparoscopy, one had a language barrier, and three were too obese to undergo general anaesthesia. Nine further patients were excluded during diagnostic laparoscopy; one with endometriosis stage IV, five with adhesions, two with tubal occlusion, and one because electrocautery was technically not feasible.
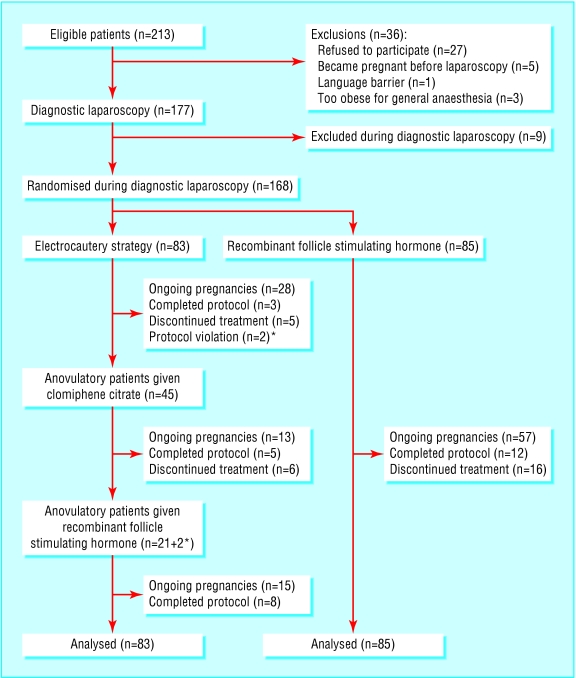
Overall, 168 patients were eligible for inclusion in our study, of whom 83 were allocated to the electrocautery strategy and 85 were allocated to recombinant follicle stimulating hormone (fig 1). Forty five patients allocated to electrocautery had persistent anovulation or recurrence of anovulatory cycles during follow up and received clomiphene citrate; 21 of these subsequently received recombinant follicle stimulating hormone, and two started recombinant follicle stimulating hormone directly after electrocautery. Table 1 lists the characteristics of the patients at baseline. The treatment groups did not differ.
Fig 1.
Flow of participants through trial. *=patients who received recombinant follicle stimulating hormone after electrocautery
Table 1.
Characteristics of women allocated to electrocautery strategy or ovulation induction with recombinant follicle stimulating hormone. Values are numbers (percentages) of women unless stated otherwise
| Characteristics | Electrocautery (n=83) | Recombinant follicle stimulating hormone (n=85) |
|---|---|---|
| Mean (SD) age (years) | 28.5 (3.7) | 28.7 (4.1) |
| Type of infertility: | ||
| Primary | 63 (76) | 64 (75) |
| Secondary | 20 (24) | 21 (25) |
| Parity: | ||
| Nulliparous | 64 (77) | 66 (78) |
| Multiparous | 19 (23) | 19 (22) |
| Mean (SD) duration of infertility (years) | 2.8 (2.2) | 2.8 (2.1) |
| Mean (SD) body mass index | 27.9 (6.3) | 27.3 (8.8) |
| Mean (SD) waist to hip ratio | 0.83 (0.09) | 0.84 (0.08) |
| Mean (SD) luteinising hormone to follicle stimulating hormone ratio | 1.99 (0.96) | 1.93 (0.90) |
| Mean (SD) testosterone (nmol/l) | 4.0 (1.7) | 3.9 (1.3) |
| Mean (SD) free androgen index | 14.0 (10.5) | 13.3 (10.2) |
| Mean (SD) volume of ovaries (ml) | 10.6 (4.5) | 11.6 (6.5) |
| Mean (SD) total motile sperm count (×106) | 108 (136) | 96 (106) |
Electrocautery versus recombinant follicle stimulating hormone
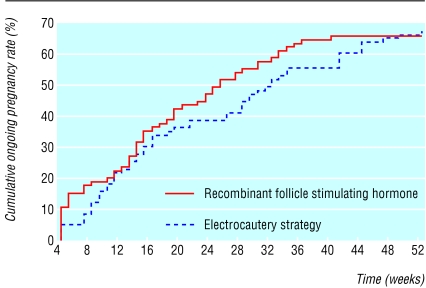
Figure 2 shows the cumulative ongoing pregnancy rates over time from electrocautery and from ovulation induction with recombinant follicle stimulating hormone. The ongoing pregnancy rate in both groups at 12 months was 67% (rate ratio 1.01, 95% confidence interval 0.81 to 1.24). Pregnancy rates in the two treatment arms over 12 months did not differ (log rank score 0.25, P = 0.62). Table 2 summarises the outcomes of pregnancy.
Fig 2.
Cumulative ongoing pregnancy rate over time from electrocautery or ovulation induction with recombinant follicle stimulating hormone
Table 2.
Pregnancy outcomes at 12 months in 83 women allocated to electrocautery strategy and 85 allocated to ovulation induction with recombinant follicle stimulating hormone. Values are numbers (percentages) of women
| Treatment regimen | No of women | Pregnant | No of miscarriages* | No of multiple pregnancies | Ongoing pregnancy | No of premature deliveries | Live births |
|---|---|---|---|---|---|---|---|
| Electrocautery strategy: | |||||||
| Electrocautery | 83 (100) | 31 (37) | 3 | — | 28 (34) | — | 28 (34) |
| Electrocautery and clomiphene citrate | 45 (54) | 14 (31) | 1 | — | 13 (29) | — | 13 (29) |
| Electrocautery, clomiphene citrate, and recombinant follicle stimulating hormone | 23 (28) | 18 (78) | 3† | 1 | 15 (65) | 3 | 12 (52) |
| Electrocautery strategy: total | 83 | 63 (76) | 7 | 1 | 56 (67) | 3 | 53 (64) |
| Recombinant follicle stimulating hormone | 85 | 64 (75) | 7 | 9 | 57 (67) | 6 | 51 (60) |
<12 weeks.
Includes one ectopic pregnancy.
Electrocautery strategy
In the 83 patients allocated to the electrocautery strategy, 61% (228 of 375) of the cycles were ovulatory. After electrocautery only, 70% (127 of 182) of cycles were ovulatory. In the subgroup that subsequently received clomiphene citrate, 45% (69/152) of cycles were ovulatory, and in the subgroup that subsequently received recombinant follicle stimulating hormone, 78% (32 of 41) of cycles were ovulatory.
Nine cycles were terminated because of poor response (five cycles), risk of ovarian hyperstimulation syndrome (two), risk of multiple pregnancy (one), and other (one). The mean duration of stimulation was 18.2 (SD 7.3) days and for use of recombinant follicle stimulating hormone was 2057 (1556) IU.
Of the 56 (67%) ongoing pregnancies in the electrocautery group, one resulted in quintuplets in a patient also given recombinant follicle stimulating hormone, and successful embryo reduction led to the live birth of twins. Neither electrocautery alone nor subsequent treatment with clomiphene citrate resulted in multiple pregnancy.
Recombinant follicle stimulating hormone
Of the 85 patients allocated recombinant follicle stimulating hormone, 69% (188 of 272) of the cycles were ovulatory. Reasons for termination of the 80 cycles were poor response (34 cycles), risk of ovarian hyperstimulation syndrome (24), risk of multiple pregnancy (13), and other (9). For each patient the mean duration of stimulation was 18.6 (6.8 SD) days and for use of recombinant follicle stimulating hormone was 1957 (975 SD) IU.
Of the 57 ongoing pregnancies in the women allocated recombinant follicle stimulating hormone, eight were twin pregnancies and one was a triplet pregnancy. Neonatal death occurred in one of the twin pregnancies at 26 weeks' gestation. The triplet pregnancy ended with premature delivery at 22 weeks.
Safety
No patient had perioperative complications or ovarian hyperstimulation syndrome. Ovulation induction with recombinant follicle stimulating hormone resulted in significantly more multiple pregnancies than with the electrocautery strategy (rate ratio 0.11, 0.01 to 0.88).
Discussion
An electrocautery strategy was as effective as recombinant follicle stimulating hormone alone for inducing ovulation in patients with clomiphene citrate resistant polycystic ovary syndrome. Although the ongoing pregnancy rate after six months was lower after electrocautery alone than with recombinant follicle stimulating hormone, this difference was abolished after administration of clomiphene citrate and recombinant follicle stimulating hormone when anovulation persisted, leading to cumulative ongoing pregnancy rates of 67% in both groups. We cannot, however, exclude small differences, as our power calculation was based on lower expected pregnancy rates after recombinant follicle stimulating hormone and after the electrocautery strategy than were observed in both study arms. In retrospect, we believe that our power calculation was informative and justifiable, but the confidence intervals are wide and do not exclude the 5% difference.
Three published trials and two abstracts compared surgical treatment with gonadotrophins in patients with clomiphene citrate resistant polycystic ovary syndrome.11-13,22,23 These under-powered studies found no differences in rates for ovulation, pregnancy, and miscarriage.
The strength of our design is that patients received further treatment if they did not respond to electrocautery, with obvious clinical benefits from these additional interventions. The cumulative ongoing pregnancy rate after electrocautery and clomiphene citrate was 49%, eliminating the need for recombinant follicle stimulating hormone. The ongoing pregnancy rate in women who were treated with recombinant follicle stimulating hormone was 67%, avoiding the need for in vitro fertilisation and embryo transfer.
Bipolar electrocautery allows control of the energy source and has an autostop function and resulted in discrete, reproducible punctures, with low risk of adhesion formation. The high pregnancy rate after the addition of clomiphene citrate and recombinant follicle stimulating hormone suggests that postoperative adhesion formation is not an important problem. Disadvantages of the electrocautery strategy are the potential risks from surgery carried out under general anaesthesia. Because ovulation induction with gonadotrophins is a non-invasive procedure it does represent a safer alternative.
Recombinant follicle stimulating hormone was given in a chronic low dose step up regimen because of its efficacy and safety compared with other regimens.20 The high percentage of ongoing pregnancies in this treatment arm could be explained by strict adherence of the gynaecologists to the protocol, because close monitoring of patients is mandatory and strict criteria for terminating cycles are defined.
Our results provide a scientific basis for counselling patients with clomiphene citrate resistant polycystic ovary syndrome, particularly as many fail to respond to treatment. The generalisability of our results is likely to be widened by the multicentre approach. We have shown that both the electrocautery strategy and recombinant follicle stimulating hormone are effective at ovulation inducing, with comparable cumulative pregnancy rates at 12 months. No cases of ovarian hyperstimulation syndrome occurred and miscarriage rates were comparable between treatment arms.
The major difference between the two strategies is that multiple pregnancies can largely be prevented by treating women with electrocautery and clomiphene citrate before recombinant follicle stimulating hormone. Although there is a need to minimise the frequency of multiple pregnancies, so far there has been little effort to issue guidelines or regulations.24 Our study may be a first step towards reducing multiple pregnancies while maintaining good pregnancy rates.
What is already known on this topic
Polycystic ovary syndrome is the most common ovulatory disorder
Patients with polycystic ovary syndrome resistant to clomiphene citrate are treated with recombinant follicle stimulating hormone or laparoscopic electrocautery of the ovaries
What this study adds
An electrocautery strategy and recombinant follicle stimulating hormone are both effective at inducing ovulation
Multiple pregnancies can largely be avoided by electrocautery and clomiphene citrate before recombinant follicle stimulating hormone
We thank the following for inclusion and treatment of patients in this study: MC Armeanu (Kennemer Gasthuis, lokatie DEO, Haarlem); RE Bernardus (Ziekenhuis Gooi Noord, Blaricum); HE Bobeck (Rode Kruis Ziekenhuis, Beverwijk); RSGM Bots, B Roozenburg (Sint Elisabeth Ziekenhuis, Tilburg); DDM Braat (Universitair Medisch Centrum, St. Radboud, Nijmegen); J Dawson (Gelre Ziekenhuizen, lokatie Juliana, Apeldoorn); HJHM van Dessel, TJG Griffioen (TweeSteden Ziekenhuis, Tilburg); B Dijkman, GLM Lips, JA Schrikx (Boven-IJ Ziekenhis, Amsterdam); JPR Doornbos (Zaans Medisch Centrum De Heel, Zaandam); CJH Dargel, PA van Dop, BC Schoot (Catharina Ziekenhuis, Eindhoven); GAJ Dunselman (Academisch Ziekenhuis Maastricht, Maastricht); MH Emanuel, K Wamsteker (Spaarne Ziekenhuis, Haarlem); PH Engelen (Slotervaart Ziekenhuis, Amsterdam); DA Gietelink (Amphia Ziekenhuis, lokatie Langendijk, Breda); CJCM Hamilton (Jeroen Bosch Ziekenhuis, lokatie Groot Ziekengasthuis, 's Hertogenbosch); MJ Heineman (Academisch Ziekenhuis Groningen, Groningen); DJ Hemrika, PA Flierman (Onze Lieve Vrouwe Gasthuis, Amsterdam); PWH Houben (Gemini Ziekenhuis, Den Helder); YM van Kasteren (Medisch Centrum Alkmaar, Alkmaar); WM Killian (Ziekenhuis Amstelveen, Amstelveen); MD Kloosterman (Ziekenhuis Rijnstate, Arnhem); RA Leerentveld (Isala Klinieken, lokatie Sophia, Zwolle); P Paaymans (Streekziekenhuis Midden-Twente, Hengelo); CNM Renckens (Westfries Gasthuis, Hoorn); RAK Samlal (Ziekenhuis Gelderse Vallei, Ede); F Scheele (Sint Lucas Andreas Ziekenhuis, lokatie Lucas, Amsterdam); WJ van der Velde (Waterlandziekenhuis, Purmerend); MAHM Wiegerinck, E Moret (Máxima Medisch Centrum, Veldhoven). We also thank M M Denyn and V I Mauer for their invaluable assistance.
Contributors: EMK, PMMB, and FvdV contributed to the study design. NB, MvW, PMMB, and FvdV helped conduct the study and analyse the data. They will act as guarantors for the paper. Members of the study group helped conduct the study. All authors contributed to writing the paper.
Funding: Serono Benelux provided financial support for recombinant follicle stimulating hormone during the first eight months of the study when this drug was not funded by the health services. FvdV was supported by a grant from the Health Insurance Funds Council (OG 97/007), Amstelveen, Netherlands.
Competing interests: None declared.
Ethical approval: The study was approved by the institutional review boards of all participating hospitals.
References
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333: 853-61. [DOI] [PubMed] [Google Scholar]
- 2.Balen A, Michelmore K. What is polycystic ovary syndrome? Hum Reprod 2002;17: 2219-27. [DOI] [PubMed] [Google Scholar]
- 3.Homburg R. What is polycystic ovarian syndrome? Hum Reprod 2002;17: 2495-9. [DOI] [PubMed] [Google Scholar]
- 4.Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab 1998;83: 2361-5. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs HS, Agrawal R. Complications of ovarian stimulation. Baillierès Clin Obstet Gynaecol 1998;12: 565-79. [DOI] [PubMed] [Google Scholar]
- 6.Homburg R, Howles CM. Low-dose FSH therapy for anovulatory infertility associated with polycystic ovary syndrome: rationale, results, reflections and refinements. Hum Reprod Update 1999;5: 493-9. [DOI] [PubMed] [Google Scholar]
- 7.Christin-Maitre S, Hugues JN on behalf of the Recombinant FSH Study Group. A comparative randomized multicentric study comparing the step-up versus step-down protocol in polycystic ovary syndrome. Hum Reprod 2003;18: 1626-31. [DOI] [PubMed] [Google Scholar]
- 8.Donesky BW, Adashi EY. Surgically induced ovulation in the polycystic ovary syndrome: wedge resection revisited in the age of laparoscopy. Fertil Steril 1995;63: 439-63. [DOI] [PubMed] [Google Scholar]
- 9.Gjonnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil Steril 1984;41: 20-5. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt E, Casper RF. Endocrine changes after laparoscopic ovarian cautery in polycystic ovarian syndrome. Am J Obstet Gynecol 1987;156: 279-85. [DOI] [PubMed] [Google Scholar]
- 11.Abdel Gadir A, Mowafi RS, Alnaser HM, Alrashid AH, Alonezi OM, Shaw RW, et al. Ovarian electrocautery versus human menopausal gonadotrophins and pure follicle stimulating hormone therapy in the treatment of patients with polycystic ovarian disease. Clin Endocrinol 1990;33: 585-92. [DOI] [PubMed] [Google Scholar]
- 12.Vicino M, Loverro G, Bettocchi S, Simonetti S, Mei L, Selvaggi L. Predictive value of serum androstenedione basal levels on the choice of gonadotropin or laparoscopic ovarian electrocautery as ovulation induction in clomiphene citrate-resistant patients with polycystic ovary syndrome. Gynecol Endocrinol 2000;14: 42-9. [DOI] [PubMed] [Google Scholar]
- 13.Farquhar CM, Williamson K, Gudex G, Johnson NP, Garland J, Sadler L. A randomized controlled trial of laparoscopic ovarian diathermy versus gonadotropin therapy for women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil Steril 2002;78: 404-11. [DOI] [PubMed] [Google Scholar]
- 14.WHO manual for the standardized investigation and diagnosis of the infertile couple. Cambridge: Cambridge University Press, 1993.
- 15.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 1985;ii: 1375-9. [DOI] [PubMed] [Google Scholar]
- 16.Van Santbrink EJ, Hop WC, Fauser BC. Classification of normogonadotropic infertility: polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril 1997;67: 452-8. [DOI] [PubMed] [Google Scholar]
- 17.Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril 1985;43: 351-2. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton-Fairley D, Kiddy D, Watson H, Sagle M, Franks S. Low-dose gonadotrophin therapy for induction of ovulation in 100 women with polycystic ovary syndrome. Hum Reprod 1991;6: 1095-9. [DOI] [PubMed] [Google Scholar]
- 19.Dutch Society for Obstetrics and Gynaecology. www.nvog.nl
- 20.Bayram N, van Wely M, van der Veen F. Recombinant FSH versus urinary gonadotrophins or recombinant FSH for ovulation induction in subfertility associated with polycystic ovary syndrome. In: Cochrane Library, issue 3. Oxford: Update Software, 2001. [DOI] [PubMed]
- 21.Kaaijk EM, Beek JF, van der Veen F. Laparoscopic surgery of chronic hyperandrogenic anovulation. Lasers Surg Med 1995;16: 292-302. [DOI] [PubMed] [Google Scholar]
- 22.Vegetti W, Ragni G, Baroni E, Testa G, Marsico S, Riccaboni A, et al. Laparoscopic ovarian drilling versus low-dose pure FSH in anovulatory clomiphene-resistant patients with polycystic ovary syndrome: randomized prospective study. Hum Reprod 1998;13: S120. [Google Scholar]
- 23.Lazovic G, Milacic D, Terzic M, Spremovic S, Mitijasevic S. Medicaments or surgical therapy of PCOS. Fertil Steril 1998;70: S472. [Google Scholar]
- 24.Cohen J, Jones HW Jr. How to avoid multiple pregnancies in assistive reproductive technologies. Semin Reprod Med 2001;19: 269-78. [DOI] [PubMed] [Google Scholar]