Abstract
Background
Tacrolimus (FK506) is an effective immunosuppressant for human heart transplantation, but information about its effects on cardiac allograft and nonallograft kidney and liver histopathologic study is limited.
Methods
We therefore reviewed 1145 endomyocardial biopsy specimens and eight autopsy results from 80 heart transplant recipients who received tacrolimus as baseline immunosuppression. These were compared with 619 endomyocardial biopsy specimens and four autopsy results from 51 patients treated with cyclosporine-based immunosuppression with lympholytic induction (CLI) by use of rabbit anti-thymocyte globulin. Twenty-one histologic features including the International Society for Heart and Lung Transplantation histopathologic grade were retrospectively assessed without knowledge of the treatment regimen. The lymphocyte growth index on biopsy specimens obtained from these patients was also compared.
Results
In general, there were no qualitative differences in the histopathologic appearance of various allograft syndromes between tacrolimus- and CLI-treated patients. Thus histopathologic criteria used to diagnose various graft syndromes are applicable under tacrolimus immunosuppression. However, early (between 10 and 30 days) after transplantation, biopsy specimens from patients treated with tacrolimus showed a significantly higher percentage of inflamed fragments (p = 0.02), the inflammation tended to be more severe (p = 0.09), and the rejection grade tended to be slightly higher (p = 0.08). In contrast, during the late transplantation period (275 to 548 days), biopsy specimens from patients treated with CLI showed a significantly higher percentage of inflamed fragments (p = 0.03), more severe inflammation (p = 0.03), higher rejection grades (p = 0.01), and a higher frequency of Quilty lesions (p = 0.05). Although overall freedom from any grade 3A or higher rejection was greater in the CLI-treated arm, tacrolimus was successfully used to treat refractory rejection in three patients from the CLI-treated arm. Concern has been raised in the literature about the possibility of tacrolimus being a direct hepatotoxin and an accelerant of allograft obliterative arteriopathy. However, no evidence to support either of these contentions was detected in this patient population. In contrast, tacrolimus is clearly nephrotoxic, although similar to cyclosporine in this regard.
Conclusions
Tacrolimus is an effective immunosuppressive drug for heart transplantation. The cardiac allograft histopathologic study of patients treated with tacrolimus immunosuppression does not significantly differ from those given conventional, cyclosporine-based triple therapy with lympholytic induction.
Tacrolimus is an effective immunosuppressant that controls allograft rejection in a variety of experimental animal models.1-7 Excellent results have also been reported in early and randomized clinical trials of liver transplantation.8-14 Tacrolimus has also been shown to have therapeutic advantages in clinical kidney,15-18 heart,19 and lung20 transplantation. In general, tacrolimus-treated recipients experience a lower incidence of and less severe acute rejection, less requirement of corticosteroids, and less chronic rejection; in addition, tacrolimus can effectively control rejection refractory to other forms of treatment. However, little information is available about the effect of tacrolimus on the histopathologic study of human cardiac allograft rejection and possible adverse affects on other vital organs such as the kidney or liver of these recipients.21
We therefore reviewed the histopathologic features and the associated clinical events of 80 adult cardiac allograft recipients who received tacrolimus/steroids as the baseline immunosuppressive agents. This group of patients was compared with a group of 51 patients who were treated with cyclosporine-based immunosuppression with lympholytic induction (CLI), which consisted of cyclosporine, azathioprine, and steroids including induction with antithymocyte globulin. This was not a randomized trial: the two regimens were not directly comparable (i.e., double versus triple therapy with antilymphocyte induction), and it was not our intent to compare the overall efficacy of tacrolimus to cyclosporine as an immunosuppressant. Rather, the purpose of this study was to compare the cardiac histopathologic findings in these two groups of patients and to determine whether there were specific hepatic or renal toxic manifestations attributable to tacrolimus.
MATERIAL AND METHODS
Patient Enrollment and Evaluation
All adult cardiac allograft recipients who underwent transplantation at the University of Pittsburgh Medical Center between October 8, 1989, and October 16, 1993, because of irreversible end-stage heart failure (New York Heart Association class IV) were included in this study. Between October 8, 1989, and June 22,1992, 76/85 (89.4%) of these patients chose the option of tacrolimus immunosuppression, after appropriate informed consent was obtained. The patients are a subset of those whose clinical course under tacrolimus was previously reported. 19 This treatment regimen initially consisted of tacrolimus and corticosteroids, and later in the course, azathioprine was added in selected patients to reduce nephrotoxicity or to control rejection. The remaining nine patients who underwent heart replacement during this time were given CLI, because of lack of informed consent (n = 3), unavailability of tacrolimus at the time of transplantation (n = 2), elevated creatinine (n = 1), or lack of insurance coverage (n = 1). In two cases the reasons were not clear. The population profile, cause(s) of heart failure, and allograft number at the time of entry into the study are listed in Table I.
TABLE I.
Pretransplantation characteristics of the patient populations
| Tacrolimus (N = 30) |
CLI (N = 51) |
|
|---|---|---|
| Age | ||
| Mean ± standard deviation | 48.8 ± 10 | 49.4 ± 9.6 |
| Range | 20-64 | 20-65 |
| Sex (male:female) | 64:16 | 45:06 |
| Original disease, no. | ||
| lschemic cardiomyopathy | 45 | 35 |
| Idiopathic dilated cardio- myopathy |
19 | 9 |
| Viral myocarditis | 7 | 0 |
| Hypersensitivity myocarditis | 1 | 0 |
| Valvular disease | 2 | 0 |
| Congenital heart disease | 2 | 2 |
| Amyloidosis (familial type) | 1 | 0 |
| Rheumatic heart disease | 1 | 1 |
| Other | 2 | 4 |
| Allograft in place at initiation of study |
||
| First | 75 | 50 |
| Second | 5 | 1 |
| Causes of primary allo- graft failure |
||
| Chronic rejection | 4 | 0 |
| Acute rejection | 1 | 0 |
| Atherosclerotic coro- nary artery disease* |
0 | 1 |
The histologic appearance of the lesions was more typical of atherosclerosis than of chronic obliterative arteriopathy associated with heart rejection.
Tacrolimus was no longer available to those who underwent heart transplantation between June 27, 1992, and June 16, 1993. Forty-two (91.3%) of the 46 patients who undelwent transplantation during this time were routinely given CLI, which consisted of induction with rabbit anti-thymocyte globulin, followed by cyclosporine, azathioprine, and corticosteroids. The remaining four patients received tacrolimus because they underwent heart retransplantation (n = 1), received a heart in combination with an extracardiac organ allograft (n = 2), or were treated under the pediatric protocol (n = 1).
The operative procedure, details of the baseline immunosuppression regimens, patient management, and follow-up have been described elsewhere.19-22-24 Pretransplantation cross-match and panel reactive antibodies (PRA) status before transplantation were determined with standard methods. All allografts were ABO blood group identical.
All patients underwent endomyocardial biopsy once a week for the first 4 weeks after transplantation, one biopsy per month for the next 3 months, one biopsy every other month for the following 6 months, and then, unless there was a clinical indication to biopsy, routinely every 6 months thereafter. Moderate or severe acute rejection (grade 3A or higher) were treated with pulse steroids (methylprednisolone 1 gr/day intravenously for 3 consecutive days) in both groups. Depending on the clinical circumstances, rejection episodes histopathologically graded as 1B or 2, according to the International Society for Heart and Lung Transplantation criteria25 were treated slightly different in the two arms. In the tacrolimus group, when treated, these patients usually received augmentation of the tacrolimus alone, whereas patients treated with CLI were generally treated with corticosteroids. If a repeat endomyocardial biopsy performed witbin 2 weeks after treatment for grade 3A or higher revealed persistent rejection, the patient was treated with a course of OKT3 (5 mg/day intravenously for 14 days or 10 mg/day for 5 days). If the rejection persisted or worsened, the patient was given OKT3 (as above) or rabbit antithymocyte globulin (1.5 mg/kg/day administered intramuscularly for 5 days).
The graft and patient status and posttransplantation clinical course were obtained from a retrospective review of the patient’s records and discussion with the clinical transplant physicians. The closure of the study was on August 31, 1994, and complete follow-up was available until that date.
Histopathologic Studies
A total of 1145 allograft endomyocardial biopsy specimens and eight autopsy results were available for review from patients in the tacrolimus arm. In the control group there were 619 allograft endomyocardial biopsy specimens and four autopsy results. All tissue sections were reviewed by one of the authors (A.T.) without knowledge of the immunosuppressive regimen, time after transplantation, or any additional treatment. A subset of at least 10% randomly chosen biopsy specimens and all of the autopsy results were also reviewed by two of the other authors (O.P. arid A.J.D.). Slides for which there was any disagreement were reviewed together, and a consensus diagnosis was reached. Specific histopathologic features examined included the number of fragments; adequacy of the specimen; the number of inflamed fragments; the location (perivascular, interstitial, endocardial), distribution (focal, multifocal, diffuse), density (mild, moderate, severe), and cell type of inflammation (lymphoblasts, small lymphocytes, plasma cells, eosinophils, neutrophils, macropbages), if present; myocyte necrosis and dropout; interstitial edema, hemorrhage, and neutrophilia; arterial or venular inflammation; endocardial and interstitial fibrosis; endocardial infiltrates (Quilty effect); the presence or absence of granulation tissue and previous biopsy sites; and infectious organisms or posttransplantation lymphoproliferative disorders. Endocardial infiltrates, or Quilty lesions, were subclassified as endocardial (type A) or infiltrative (type B), according to accepted criteria.25,26 If a patient had development of at least one biopsy specimen with an infiltrative Quilty effect, they were listed under the Quilty type B category. Finally, all of the biopsy specimens were retrospectively graded according to the International Society for Heart and Lung Transplantation classification scheme for acute rejection (AR).25 In addition, the grade of acute rejection diagnosed at the time of surgical pathology sign-out was recorded and will be referred to as the “sign-out grade.”
For comparison and statistical analysis, the time after transplantation was arbitrarily separated into two intervals: early (corresponding to biopsy specimens taken between 10 and 30 days after transplantation) and late (corresponding to biopsy specimens obtained between 9 and 18 months after transplantation). The early period was chosen because it encompasses the peak period of alloreactivity and rejection. The later period represents a more “quiescent” time. For the comparison of histologic study results early versus late, only patients who had biopsy specimens obtained during both time periods were included.
Lymphocyte Growth Assays
For lymphocyte functional analysis, three separate sterile fragments of endomyocardium were collected, divided into smaller portions, and cultured in the presence of medium containing recombinant human interleukin-2 (Sandoz Pharmaceuticals, Basel, Switzerland) as previously described.27 The cultures were observed daily on an inverted phase microscope, and a lymphocyte growth index was calculated on the basis of the number of fragments showing out-growth of lymphocytes in culture. For statistical analysis, the results for this assay were separated into three groups: those showing no growth; those in which growth was observed in 50% or less of fragments, and those in which more than 50% of the fragments showed growth.
Statistical Analysis
Of the 1764 biopsy samples from 131 patients available for these analyses, 94 (5.3%) were excluded because they were inadequate, and 12 (0.7%) from 3 patients were excluded because they were obtained after the crossover of a patient from CLI to tacrolimus. The remaining 1658 (93.9%) biopsy specimens were included in this study.
For the three patients whose treatment crossed over from CLI to tacrolimus, no data obtained after the crossover were included in the statistical analysis. Because this was not a randomized clinical trial and the baseline comparability of the two groups is unknown, we chose not to analyze these data on the basis of “intention to treat.” Regarding time to event analyses, none of these three experienced any of the outcomes of interest (death, grade 3A or greater rejection, or Quilty lesion) after the crossover. Thus no adverse outcomes have been omitted as a result of censoring. Similarly, for the comparisons of the histopathologic findings, all biopsy specimens obtained after crossover were excluded to preclude the confounding of the comparisons of the two treatment regimens.
Patients who received tacrolimus, in general, underwent transplantation before those who received CLI and have longer total observed follow-up. To ensure appropriate comparisons between the two groups for all time-to-event analyses, we censored follow-up at 2 years after transplantation. The time-to-event curves were generated by use of the Kaplan-Meier survival analysis method, and the Wilcoxon test was used to compare the curvys. In addition, incidence rates for rejection were estimated as the number of biopsy specimens showing grade 3A or greater rejection per 100 patient-days, and these rates were compared by use of a two-sample test for incidence density measures. 28
Contingency table analyses were used to compare the histopathologic findings belween the two treatment groups and between early and late biopsies. Statistical comparisons were performed by use of a chi-square test for trend when the data were ordinal. Exact methods were used when required because of expected values less than 5 in individual cells of any contingency tables generated during these analyses. The Wilcoxon rank-sum test was used to compare the distribution of creatinine at both 1 and 2 years after transplantation between the two treatment groups.
RESULTS
Cardiac Histopathology
Findings attributable to ischemic/preservation injury, 29 such as focal areas of myocyte dropout, focal neutrophilia, edema, and hemorrhage without any associated mononuclear inflammation, were more commonly present or severe ill biopsy specimens obtained between 10 and 30 days after transplantation than at 1 year (p < 0.001). However, no differences were observed between the treatment groups for any of those features at either time point. Previous biopsy sites, characterized early by partially organized endocardial fibrin masses and later by granulation tissue containing neutrophils, lymphocytes, and hemosiderin-laden macrophages and eventually fibrosis and myofiber disarray, were also more commonly detected early versus late (30.6% vs 3.0%; p < 0.0001) after transplantation. Again, they were seen with similar frequency in both treatment groups. Table II shows data for the histopathologic findings that were different between the two treatment groups. In the early biopsy specimens, the only statistically significant histopathologic differences between the two treatment groups were in the percentage of inflamed fragments, which was higher in the patients treated with tacrolimus (p = 0.02), and the distribution of the inflammation (p = 0.02). There also was a trend toward slightly more severe inflammation (p = 0.09) among the patients treated with tacrolimus. Late after transplantation, nearly the opposite trend was observed (Table II): the percentage of inflamed fragments was lower (p = 0.03); and overall, there was less severe inflammation (p = 0.03) in the tacrolimus group. Perivascular and interstitial fibrosis were both Significantly (p ≤ 0.01) more severe among patients treated with tacrolimus, although in general, the difference observed was between none and mild fibrosis.
TABLE II A.
Histopathologic data that showed a difference between the two treatment groups at 10 to 30 days and at 1 year after transplantation: percent of inflamed fragments
| CLI |
Tacrolimus |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | N | 0% | 1%-50% | 51%-100% | N | 0% | 1%-50% | 51%-100% | p Value |
| 10-30 days | 49 | 28.6 | 26.5 | 44.9 | 75 | 10.7 | 28.0 | 61.3 | 0.02 |
| 1 year | 40 | 30.0 | 40.0 | 30.0 | 70 | 50.0 | 34.3 | 15.7 | 0.03 |
The values listed are the percentage of patients showing the finding at that time. The p values refer to a comparison of tacrolimus to CLI-treated cases at the same time point.
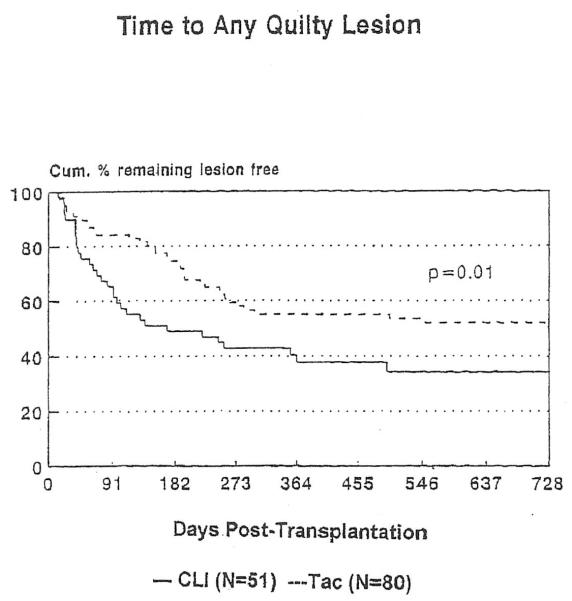
A separate analysis of the time to any Quilty lesion was performed (Figure 1). By 4 months after transplantation, the cumulative probability of the development of a Quilty lesion was 44.9% among the patients treated with CLI compared with only 17.1% among the patients treated with tacrolimus. By 2 years after transplantation, the corresponding probabilities are 65.6% and 49.5%, respectively. This difference in the incidence of a first Quilty lesion during the first 2 years after transplantation is statistically significant (p = 0.01).
FIGURE 1.
Analysis of time until onset of any Quilty lesions.
Interestingly, in spite of the limited number of differences between drug regimens, there were significant differences for almost all of the histopathologic features examined when early biopsy specimens were compared with those obtained later, regardless of the immunosuppressive treatment. The only parameters that did not show a significant difference in this comparison were the percentage of biopsy specimens containing plasma cells (p = 0.5), and the percentage of biopsy specimens showing mild perivascular fibrosis (p = 0.4). The presence or severity of endocardial fibrosis, interstitial fibrosis, and Quilty lesions were significantly (p < 0.001) greater on late biopsy specimens versus early biopsy specimens. All other histologic features were significantly (p = 0.01 for endocardial inflammation, p < 0.001 for all others) more common or severe early versus late.
Grading of Rejection
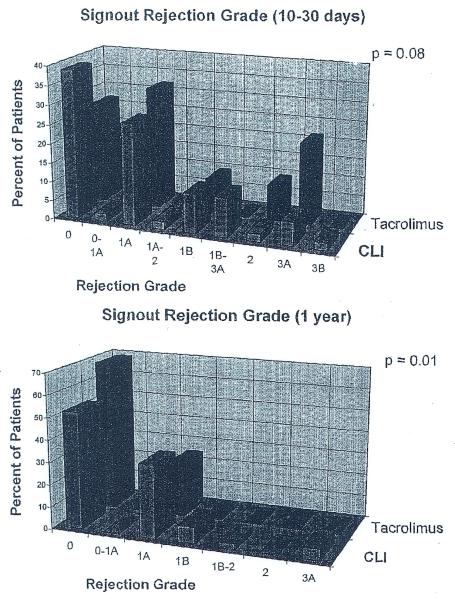
Freedom from histopathologic grade 3A or higher rejection, on the basis of the retrospective grading of rejection was higher in the CLI group during the first 2 years after transplantation (p = 0.02). At 6 weeks after transplantation, 62.1% of the patients treated with CLI and 49.1 % of the tacrolimus-treated recipients were free of grade 3A or higher rejection. The magnitude of this difference did not change at 2 years (41.1% vs 28.5). The subtle differences in histopathologic findings noted above also translated into differences in the distribution of histopathologic sign-out grades of rejection. Early after transplantation, the sign-out grade of rejection (Figure 2,A) tended to be slightly higher among patients treated with tacrolimus (p = 0.08). Late after transplantation the sign-out grade of rejection was Significantly higher in the patients treated with CLI (p = 0.01), although it was still not enough to warrant additional immunosuppression in most patients (Figure 2,B). The estimate of a “linearized” rejection rate during the first 2 years after transplantation for each group revealed that there were 0.212 episodes per 100 patient-days in the CLI group and 0.216 episodes per 100 patient days in the patients treated with tacrolimus (p = 0.98).
FIGURE 2.
Distribution of sign-out grade of acute rejection between patients treated with CLI and tacrolimus early (A) and late (B) after transplantation.
It must be emphasized that this is not a study to compare the efficacy of tacrolimus versus CLI, because the two drugs were used differently. Tacrolimus was used in combination with prednisone alone. Azathioprine was added if there was persistent rejection (grade 2 or higher on two consecutive biopsy specimens) or if there was renal insuffiCiency (creatinine >2.5 mg/dl). Cyclosporine on the other hand, was used in combination with azathioprine, steroids and lyrnpholytic induction therapy. Even thougb these differences existed, three patients treated with CLI were switched to tacrolimus 3, 4, and 12 months after transplantation for persistent and relapsing acute rejection. In all three, rejection was ultimately controlled when the next biopsy specinlen was obtained 11, 18, and 20 days after conversion to tacrolimus, and they have had an uncomplicated course since conversion to tacrolirnus.
In total there were six episodes of clinically serious rejection that were pathologically graded as moderate or severe (grade 3A or 3B). Four of these episodes failed to respond to primary or secondary therapy, including the use of OKT3 (n = 4), and all four patients died. In three of the patients who died (tacrolimus, n = 2; CLI, n = 1), endomyocardial biopsy specimens obtained before death underestimated the severe rejection first documented with certainty at autopsy. One other patient treated with CLI died during OKT3 therapy for severe rejection recognized before death.
Lymphocyte Growth
In spite of the histopathologic difference in the intensity of inflammation and the grading of rejection between the two groups, there were no statistically significant differences in the rate of biopsy growth when comparing patients treated with the different drug regimens either early or late after transplantation. However, lymphocyte growth from biopsy fragments was significantly greater (p < 0.0001) in those biopsy specimens obtained early, in comparison to late biopsy specimens after transplantation, regardless of the treatment regimen.
Patient and Allograft Survival
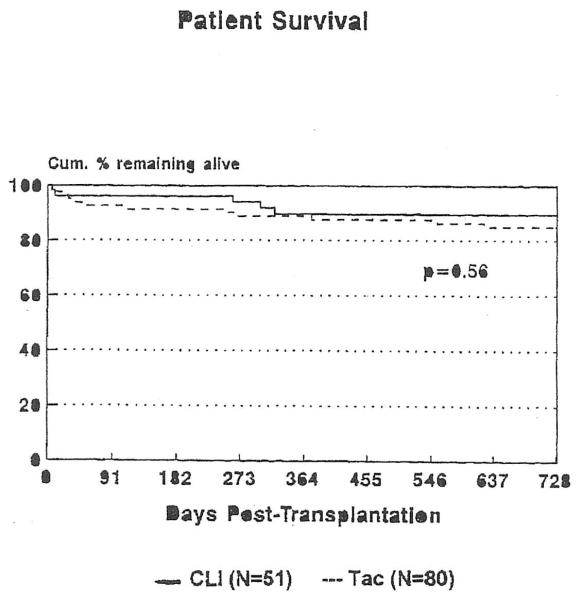
No patients underwent heart retransplantation, and those experiencing allograft failure died. The cumulative percent surviving 2 years after transplantation was 84.9% among patients treated with tacrolimus and 90.0% among patients treated with CLI (p = 0.56; Figure 3). A complete listing of the causes for death in both groups is given in Table III. It should be stressed that follow-up for the tacrolimus group was longer than for the CLI group. For statistical analysis, a common follow-up time is important. However, we wanted to provide the maximum amount of information, so all follow-up information until closure of the study is included in Table III.
FIGURE 3.
Cumulative percentage of patients remaining alive after heart transplantation, according to treatment regimen.
TABLE III.
Causes of death after transplantation, listed according to the primary immunosuppressive regimen
| Cause of death | Tacrolimus (N-80) |
CLI (N-51) |
|---|---|---|
| Acute rejection | 2* | 2 |
| Chronic rejection | 3 | 2 |
| Sepsis | 4 | 0 |
| Pancreatitis | 2 | 0 |
| Right ventricular failure | 1 | 0 |
| Recurrent and metastatic atrial sarcoma |
0 | 1 |
| Liver failure (hepatitis type B) | 1 | 0 |
| Cardiac arrhythmia (hyperkale- mia) |
1 | 0 |
| Pulmonary and gastrointestinal hemorrhage |
1 | 0 |
| Ischemic cardiac disease | 0 | 1 |
| Died during repair of right groin | 1 | — |
| Pseudoaneurysrm | — | — |
| Not determined with certainty | 1 | — |
| Total Deaths (% of total) | 17(21)† | 6(12)† |
In one patient, the rejection was superimposed on preservation injury, characterized by multiple areas of myocyte dropout. The patient also had a large pulmonary thromboembolus and acute cor puhnonale.
In five patients treated with tacrolimus and one patient treated with CLI death occurred more than 2 years after transp1antation, which is after the cut-off follow-up time for statistical analysis (see text).
Vascular, Renal, and Hepatic Structure and Function
Arterial medial necrosis has been reported as a side effect of tacrolimus in early experimental animal studies. 30,31 However, subsequent studies found similar lesions in untreated allograft recipients and in those treated with other immunosuppressive agents.7,32-34 In this study, necrotizing or inflammatory arteritis was not detected with certainty in any biopsy specimen. At autopsy, arterial inflammation and necrosis were only seen in the cardiac allografts of patients with severe rejection.
Overall, serious nephrotoxicity requiring either dialysis or kidney transplantation was seen in four patients tTeated with tacrolimus and two patients treated with CLI before closure of the study, although the patients treated with tacrolimus had a longer follow-up. No patient from either group was treated with dialysis before transplantation. Only one patient treated with CLI who experienced kidney failure underwent a needle biopsy of her native kidney 1.8 years after transplantation. The biopsy specimen showed mild interstitial fibrosis and mild arterial and hyaline arteriolonephroscierosis, consistent with chronic cycJosporine nephrotoxicity. 35 This patient died of chronic rejection 216 days after the kidney biopsy specimen was obtained.
All other liver and kidney tissue samples from these patients were obtained at the time of autopsy. No pretransplantation kidney or liver biopsy specimens were available for comparison, to determine whether any of the changes observed progressed during tacrolimus or CLI therapy. Table IV summarizes the kidney and liver histopathologic findings and the drug blood levels immediately before death. The most common renal changes observed at autopsy in both groups were patchy interstitial fibrosis with associated tubular atrophy; mild to moderate arterial and arteriolonephrosc1erosis; focal periglomerular fibrosis and acute tubular necrosis presumably associated with agonal events. One patient treated with tacrolimlls with end-stage kidneys at autopsy had undergone dialysis for 1.5 years before death. He had normal renal function before heart transplantation 3.5 years earlier.
TABLE IV.
Native kidney and liver disease at autopsy in heart allograft recipients treated with tacrolimus
| Days after transplantation |
Cause of death | Plasma tacrolimus levels (nglml) |
Kidney histopathology | Liver histopathology |
|---|---|---|---|---|
| 10 | Gastrointestinal and intra- pulmonary hemorrhage |
0.7 | Mild ANS, mild IF, ATN | Perivenular fibrosis and passive conges- tion |
| 10 | Acute rejection superim- posed on preservation injury and pulmonary thromboembolis |
2.4 | Mild ANS, tubular vacu- olization |
Unremarkable |
| 29 | Acute vascular rejection | 1.2 | Mild IF, ANS | Mild passive conges- tion, cholestasis |
| 30 | Pancreatitis and cardio- genic shock |
30.0 | Unremarkable | Widespread acute cen- trilobular coagulative necrosis |
| 267 | Septic pulmonary throm- boembolis |
2.1 | Mild IF, ANS | Passive congestion |
| 376 | Chronic rejection | NA | Mild IF, ANS | Mild portal fibrosis, chronic portal in- flammation and mild congestion |
| 624 | Right ventricular failure from pulmonary artery stenosis |
2.8 | Minimal changes | Perivenular hemor- rhage |
| 1230* | Myocardial infarct from chronic rejection |
2.0 | Endstage kidneys | Acute midzonal hepa- tocyte dropout |
ANS, Arterial and arteriolonephrosclerosis; IF, interstitial fibrosis; ATN, acute tubular necrosis.
This patient died after the cut-off for follow-up time used in statistical analysis.
There was no direct correlation between plasma tacrolimus levels and hepatic structure. However, heart failure and ischemic injury frequently complicated hepatic histologic study at autopsy (Table IV). Although this may have obscured any histopathologic findings attributable to tacrolinms hepatic toxicity, the changes were acute in nature and likely caused by agonal events.
DISCUSSION
Single-institution and randomized multicenter reports on the use of tacrolimus in liver,8,12-14 kidney,15-18,36 lung,20 and heart transplantation19 have already shown a therapeutic advantage for this agent, compared with cyclosporine-based immunosuppression. The major benefits of tacrolimus in the above clinicopathologic studies are greater overall freedom from acute rejection; less refractory acute rejection, less chronic rejection; a lesser requirement for steroids; dose maneuverability of the baseline immunosuppressant agent as treatment for rejection; and the ability to rescue patients treated with CLI with refractory rejection, who had failed to respond to steroid and lympholytic therapy.
The primary purpose of this study was to compare the histopathologic findings in heart transplant recipients treated with tacrolimus to the findings observed in patients who received a cyclosporine-based regimen. Overall, we could not detect any significant qualitative differences in the histopathologic appearance of the various graft syndromes in patients treated with CLI versus tacrolimus. Thus one need not alter criteria used to diagnose rejection or other abnormalities seen in endomyocardial biopsies when tacrolimus is used. However, there were quantitative differences in the overall severity of inflammation and rejection between the two groups, which varied with time.
Early after transplantation the overall severity of inflammation and incidence and grade of rejection was higher in the patients treated with tacrolimus. These results are similar to the findings in our clinical report:19 a greater freedom from grade 3A or higher rejection was observed in the patients treated with CLI compared with the tacrolimus recipients.19 In contrast, late after transplantation the trend was reversed: the overall severity of inflammation and incidence and grade of rejection was higher in the patients treated with CLI. These observations are likely attributable to the antithymocyte globulin induction19 for the following reasons: the differences between the two groups appeared during the early posttransplantation period; and tacrolimus-treated patients experienced less rejection than cyclosporine-treated patients who did not receive lympholytic induction.19 We must emphasize, however, that the primary purpose of this study was not to study the effectiveness of tacrolimus compared with cyclosporine in preventing acute rejection. We did not include cyclosporine-treated patients who did not receive lympholytic induction and pediatric recipients, who do not undergo the same endomyocardial biopsy protocol as in the adults. Both of these groups, however, were included in our larger clinical analysis.19
Another more speculative explanation for these time differences is that the effect is notdrug specific. It may reflect the consequences of overimmunosuppression in the early posttransplantation period. Hepatic allograft recipients who experienced early mild acute rejection but did not have development of significant dysfunction and were not treated with increased immunosuppression had development of less chronic rejection than those who did receive additional therapy for acute rejection.37 Thus early controlled alloreactivity may have long-term benetits.38 Unfortunately, all of these explanations must remain speculative in nature, because none of them can be proven with certainty by use of the data from this study.
Another interesting observation is the higher incidence of Quilty lesions in the patientstreated with CLI compared with those maintained with tacrolimus. The importance of this difference is unknown because the immunobiologic significance of Quilty lesions has yet to be determined with certainty.39 In our recently developed experimental animal model of chronic rejection,40 Quilty lesions were associated with low-grade immunologic damage to the allograft. They were present in cardiac allografts under-going chronic rejection but absent from allografts tolerated by the host.40
Previous experimental animal studies have raised concerns about the possibility of tacrolimus enhancing or promoting the development of graft vasculopathy in cardiac allografts.41 Although the number of patients in whom the heart was examined at autopsy was small, there was no significant increase in patients treated with tacrolimus for either the incidence of chronic rejection or intimal thickening of arteries in patients who died of other causes. Coronary angiography showing a statistically nonsignificant lower incidence of graft vasculopathy in the tacrolimus group19 has also assuaged some of these concerns. In addition, tacrolimus has been the only new immunosuppressant to significantly prolong the half-life of human renal allografts,18 where chronic rejection is responsible for most late graft failures.
In two separate recent publications from the same institution, Fisher et al.42 and Hytiroglou et al.43 have suggested that tacrolimus is a direct hepatotoxin, which causes perivenular hepatocellular necrosis. Clinically significant hepatotoxicity was not a problem in this patient population. In fact, none of the patients in this study required modulation of tacrolimus doses because of hepatotoxicity. Unfortunately, all of the liver specimens were obtained at autopsy and had complicating histopathologic changes, which were attributed to multiorgan failure. A clear correlation between drug levels and structure was not possible. Therefore the question of tacrolimus-associated hepatotoxicity is probably best addressed in liver needle biopsy specimens from nonhepatic allograft patients maintained on tacrolimus.
In contrast, tacrolimus is clearly nephrotoxic.44,45 The range of histopathologic renal lesions associated with tacrolimus are quite similar to cyclosporine nephrotoxicity35,46 and have been reported in detail.44-46 We were struck, however, by the heterogeneity of kidney disease in this heart transplant population: one patient who had received tacrolimus for only 10 days before death showed interstitial fibrosis and glomerulosclerosis; whereas another patient who had been treated for 624 days showed only minimal renal histopathologic alterations. Therefore one should be aware that significant underlying kidney disease may be present before heart transplantation and that the comorbid condition might accelerate or contribute to the nephrotoxicity associated with the immunosuppressive agents.
In summary, tacrolimus is an effective immunosuppressive drug for heart transplantation. The cardiac allograft histopathologic study of patients treated with tacrolimus immunosuppression does not significantly differ from those given conventional, cyclosporine-based triple therapy.
TABLE II B.
Histopathologic data that showed a difference between the two treatment groups at 10 to 30 days and at 1 year after transplantation: inflammation intensity
| CLI |
Tacrolimus |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | N | None | Minimal | Mild | >Mild | N | None | Minimal | Mild | >Mild | p Value |
| 10-30 days | 49 | 28.6 | 10.2 | 44.9 | 16.3 | 75 | 10.7 | 24 | 38.7 | 26.7 | 0.09 |
| 1 year | 40 | 30.0 | 20.0 | 42.5 | 7.5 | 70 | 50.0 | 18.6 | 28.6 | 2.9 | 0.03 |
The values listed are the percentage of patients showing the finding at that time. The p values refer to a comparison of tacrolimus to CLI-treated cases at the same time point.
TABLE II C.
Histopathologic data that showed a difference between the two treatment groups at 10 to 30 days and at 1 year after transplantation: inflammation distribution
| CLI |
Tacrolimus |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | N | None | Diffuse | Focal | Mixed | N | None | Diffuse | Focal | Mixed | p Value |
| 10-30 days | 49 | 28.6 | 12.2 | 38.8 | 20.4 | 75 | 10.7 | 6.7 | 60.0 | 22.7 | 0.02 |
| 1 year | 40 | 30.0 | 7.5 | 60.0 | 2.5 | 70 | 50.0 | 4.3 | 45.7 | 0 | 0.06 |
The values listed are the percentage of patients showing the finding at that time. The p values refer to a comparison of tacrolimus to CLI-treated cases at the same time point.
TABLE II D.
Histopathologic data that showed a difference between the two treatment groups with interstitial fibrosis and perivascular fibrosis at 1 year
| CLI |
Tacrolimus |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | None | Mild | Moderate | N | None | Mild | Moderate | p Value | |
| Interstitial fibrosis | 40 | 72.5 | 27.5 | 0 | 70 | 48.6 | 47.1 | 4.3 | 0.01 |
| Perivascular fibrosis | 40 | 100 | 0 | 0 | 70 | 84.3 | 15.7 | 0 | 0.007 |
The values listed are the percentage of patients showing the finding at that time. The p values refer to a comparison of tacrolimus to CLI-treated cases at the same time point.
REFERENCES
- 1.Murase N, Todo S, Lee PH, et al. Heterotopic heart transplantation in the rat receiving FK-506 alone or with cyclosporine. Tranplant Proc. 1987;19(Suppl 6):71–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Murase N, Kim DG, Todo S, Cramer DV, Fung JJ, Starzl TE. FK-506 suppression of heart and liver allograft rejection II: the induction of graft acceptance in the rat. Transplantation. 1990;50:739–44. doi: 10.1097/00007890-199011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of multivisceral allografts in rats: a sequential analysts with comparison to isolated orthotopic small-bowel and liver grafts. Surgery. 1990;(108):880–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Murase N, Kim DG, Todo S, Crumer DV, Fung JJ, Starzl TE. Suppression of allograft rejection with FK506. I. Prolonged cardiac and liver survival in rats following short-course therapy. Transplantation. 1990;50:186–9. doi: 10.1097/00007890-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todo S, Demetris AJ, Ueda Y, et al. Canine kidney transplantation with FK-506 alone or in combination with cyclosporine and steroids. Transplant Proc. 1987;19(Suppl 6):57–61. [PMC free article] [PubMed] [Google Scholar]
- 6.Todo S, Podesta L, ChapChap P, et al. Orthotopic liver transplantation in dogs receiving FK-506. Transplant Proc. 1987;19(Suppl 6):64–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Todo S, Ueda Y, Demetris JA, et al. Immunosuppression of canine, monkey and baboon allografts by FK 506: with special reference to synergism with other drugs and to tolerance induction. Surgery. 1988;104:239–49. [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Fllng JJ, Demetris AJ, Venkataramanan R, Jain A. FK-506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–4. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung JJ, Todo S, Tzakis A, et al. Conversion of liver allograft recipients from cyclosporine to FK 50G-based immunosuppression: bencfits and pitfalls. Transplant Proc. 1991;23:14–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney and thoracic organ transplantation under FK506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demetris AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporine to FK506 immunosupp ressive therapy–a clinicopathologic study of 96 patients. Transplantation. 1992;53:1056–62. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group EFMLS Randomized trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver rejeclion. Lancet. 1994;344:423–8. [PubMed] [Google Scholar]
- 13.Group TUSMFLS A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppr ess ion in liver transplantation. N Engl J Med. 1994;331:1110–5. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 14.Fung JJ, Eliasziw M, Todo S, et al. The Pittsburgh randomizedtrial of tacrolimus compared to cyclosporine for hepatic transplantation. J Am Coll Surg. 1996;183:117–25. [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan ML, Shapiro R, Vivas CA, et al. FK506 “rescue” for resistant rejection of ren al allografts under primary cyclosporine immunosuppression. Transplantation. 1994;57:860–5. doi: 10.1097/00007890-199403270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro R, Jordan M, Fung J, et al. Kidney transplantation under FK 506 immunosuppression. Transplant Proc. 1991;23:920–3. [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro R, Jordan M, Scantlebury V, et al. FK 506 in clinical kidney transplantation. Transplant Proc. 1991;23:3065–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Gjertson DW, Cecka JM, Terasaki PI. The relative effects of FK506 and cyclosporine on short- and long-term kidney graft survival. Transplantation. 1995;60:1384–8. doi: 10.1097/00007890-199560120-00002. [DOI] [PubMed] [Google Scholar]
- 19.Pham SM, Kormos RL, Hattler BG, et al. A prospective trial of tacrolimus (FK506) in clinical heart transplantation: intermediate results. J Thorac Cardiovasc Surg. 1996;111:764–72. doi: 10.1016/s0022-5223(96)70336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith BP, Bando K, Hardesty RL, et al. A prospective randomized trial of FK506 versus cyciosporinc after human pulmonary transplantation. Transplantation. 1994;57:848–51. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demetris AJ, Fung JJ, Todo S, et al. Pathologic observations in human allograft recipients treated with FK 506. Transplant Proc. 1990;22:25–34. [PMC free article] [PubMed] [Google Scholar]
- 22.Armitage JM, Kormos RL, Morita S, et al. Clinical trial of FK 506 immunosuppression in adult cardiac transplantation. Ann Thorac Surg. 1992;54:205–11. doi: 10.1016/0003-4975(92)91371-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kormos RL, Armitage JM, Dummer JS, Miyamoto Y, Griffith BP, Hardesty RL. Optimal perioperative immunosuppression in cardiac transplantation using rabbit antithymocyte globulin. Transplantation. 1990;49:306–11. doi: 10.1097/00007890-199002000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Kormos RL, Armitage JM, Hardesty RL, et al. Cardiac transplantation at the University of Pittsburgh: 1980 to 1991. In: Terasaki P, Cecka J, editors. Clinical transplants. UCLA Tissue Typing Laboratory; Los Angeles: 1991. pp. 87–95. [PubMed] [Google Scholar]
- 25.Billingham ME, Cary NRB, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosiS of heart and lung rejcction. Heart rejection study group. J Heart Transplant. 1990;9:587–93. [PubMed] [Google Scholar]
- 26.Costanzo-Nordin MR, Winters GL, Fisher SG, et al. Endocardial infiltrates in the transplanted heart. J Heart Lung Transplant. 1993;12:741–7. [PubMed] [Google Scholar]
- 27.Zeevi A, Fung J, Zerbe TR, et al. Allospecificity of activated T cells from endomyocardial biopsies from heart transplant patients. Transplantation. 1986;41:620–6. doi: 10.1097/00007890-198605000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Rosner B. Fundamentals of biostatistics. 3rd ed PWS-Dent Publishing Co.; Boston: 1990. pp. 366–72. [Google Scholar]
- 29.Kottke-Marchant K, Ratliff NB. Endomyocardial biopsy: pathologic findings in cardiac transplant recipients. Pathol Annu. 1990;25:211–44. [PubMed] [Google Scholar]
- 30.Thiru S, Collier DS, Calne R. Pathological studies in canine and baboon renal allograft recipients immunosuppressed with FK-506. Transplant Proc. 1987;19(Suppl 6):98–9. [PubMed] [Google Scholar]
- 31.Collier DS, Thiru S, Calne R. Kidney transplantation in the dog receiving FK-506. Transplant Proc. 1987;19(Suppl 6):62. [PubMed] [Google Scholar]
- 32.Ochiai T, Nagata M, Nakajima K, et al. Studies of the effects of FK506 on renal allografting in the beagle dog. Transplantation. 1987;44:729–33. doi: 10.1097/00007890-198712000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Ochiai T, Nakajima K, Sakamoto K, et al. Comparative studies on the immunosuppressive activity of FK506, 15-deoxyspergualin, and cyclosporine. Transplant Proc. 1989;21(Pt 1):829–32. [PubMed] [Google Scholar]
- 34.Ochiai T, Sakamoto K, Gunji Y, et al. Effects of combination treatment with FK506 and cyclosporine on survival time and vascular changes in renal-allograft–recipient dogs. Transplantation. 1989;48:193–7. doi: 10.1097/00007890-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Young EW, Ellis CN, Messana JM, et al. A prospective study of renal structure and function in psoriasis patients treated with cyclosporin. Kidney International. 1994;46:1216–22. doi: 10.1038/ki.1994.387. [DOI] [PubMed] [Google Scholar]
- 36.Jordan ML, Shapiro R, Vivas CA, et al. FK 506 salvage of renal allografts with ongoing rejection failing cyclosporine immunosuppression. Transplant Proc. 1993;25(Pt 1):638–40. [PMC free article] [PubMed] [Google Scholar]
- 37.Farges O, Nocci KA, Sebagh M, Reynes M, Bismuth H. Low incidence of chronic rejection in patients experiencing histological acute rejection without simultaneous impairment in liver function tests. Transplant Proc. 1995;27:1142–3. [PubMed] [Google Scholar]
- 38.Demetris AJ, Murase N, Rao AS, Starzl TE. The role of passenger leukocytes in rejection and “tolerance” after solid organ transplantation: a potential explanation of a paradox. In: Touraine JL, Traeger J, Betuel H, et al., editors. Rejection and tolerance. Kluwer Academic Publishers; Netherlands: 1994. pp. 325–92. [Google Scholar]
- 39.Joshi A, Masek MA, Brown BW, Jr, Weiss LM, Billingham ME. “Quilty” revisited: a 10-year perspective. Hum Pathol. 1995;26:547–57. doi: 10.1016/0046-8177(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 40.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158–71. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meiser BM, Billingham ME, Morris RE. Effects of cyclosporin, FK506, and rapamycin on graft-vessel disease. Lancet. 1991;338:1297–8. doi: 10.1016/0140-6736(91)92594-r. [see comments] [DOI] [PubMed] [Google Scholar]
- 42.Fisher A, Mor E, Hytiroglou P, et al. FK506 hepatotoxicity in liver allograft recipients. Transplantation. 1995;59:1631–2. [PubMed] [Google Scholar]
- 43.Hytiroglou P, Lee R, Sharma K, et al. FK506 versus cyclosporine as primary immunosuppressive agent for orthotopic liver allograft recipients. Transplantation. 1993;56:1389–94. doi: 10.1097/00007890-199312000-00022. [DOI] [PubMed] [Google Scholar]
- 44.McCauley J, Fung JJ, Jain A, Todo S, Starzl T. The effects of FK506 on renal function after liver transplantation. Transplant Proc. 1991;23:3148–9. [PMC free article] [PubMed] [Google Scholar]
- 45.McCauley J, Fung JJ, Brown H, et al. Renal function after conversion from cyclosporine to FK 506 in liver transplant patients. Transplant Proc. 1991;23:3148–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506: clinical significance and comparison with cyclosporine. Amer J Surg Pathol. 1993;17:60–8. doi: 10.1097/00000478-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]