Abstract
This study aimed to replicate and extend prior research showing that the targeted use of naltrexone is a useful strategy to reduce heavy drinking. We compared the effects of naltrexone with those of placebo in a sample of 163 individuals (58.3% male) whose goal was to reduce their drinking to safe limits. Patients received study medication (i.e., naltrexone 50 mg or placebo) and were instructed to use it either daily or targeted to situations identified by them as being high risk for heavy drinking. An interactive voice response (IVR) system was used to obtain daily reports of drinking and medication use during the 12-week trial. Analyses were conducted using hierarchical linear modeling, with sex as a potential moderator variable. On the primary outcome measure, mean drinks per day, at week 12, men in the targeted naltrexone group drank significantly less than patients in the other groups. On a secondary outcome measure, drinks per drinking day, during week 12, the targeted naltrexone group drank significantly less than the other groups, with no moderating effect of sex. These results support the use of a targeted approach to reduce drinking among heavy drinkers, particularly men, but argue for the use of additional strategies or more efficacious medications to increase the effects of such an intervention.
Keywords: Naltrexone, Alcohol Pharmacotherapy, Problem Drinkers, Targeted Medication
INTRODUCTION
The opioid antagonist naltrexone is approved in the United States for the treatment of alcohol dependence. The approved oral dosage of the medication, 50 mg/day, was based on two studies that showed the medication to be superior to placebo in reducing the rate of relapse to heavy drinking in alcohol-dependent individuals [1, 2]. Although not all subsequent studies of naltrexone have supported its efficacy in the treatment of alcohol dependence, meta-analyses have shown a clear, though modest, advantage for the drug compared with placebo on some drinking outcomes [3, 4]. The largest meta-analysis [4], which included data from 24 randomized, controlled trials, showed that daily treatment with oral naltrexone decreased the risk of relapse to heavy drinking by about 36%. Naltrexone treatment also increased the likelihood of adverse effects (e.g., nausea, dizziness, fatigue) compared with placebo.
An alternate approach to the daily use of naltrexone is for patients to take it in anticipation of a high-risk drinking situation, i.e., targeted use of the medication. Heinala et al. [5] showed that naltrexone targeted to alcohol craving during a 20-week period following daily naltrexone treatment sustained the reduced risk of relapse to heavy drinking seen during an initial 12-week period of daily treatment. Following an initial pilot study [6], we found that naltrexone reduced the risk of heavy drinking more than placebo treatment in an 8-week trial in problem drinkers. Targeted administration of naltrexone or placebo also reduced the risk of both drinking and heavy drinking [7]. In a secondary analysis of these findings, Hernandez-Avila et al. [8] found that, by the end of the study, treatment with targeted naltrexone was associated with a greater reduction in the number of drinks consumed per day than daily placebo. Daily naltrexone treatment reduced daily alcohol consumption only among men, at the level of a non-significant trend.
Karhuvaara et al. [9] examined the targeted use of nalmefene, another orally administered opioid antagonist, in combination with a minimal psychosocial intervention in a multi-center, randomized trial. In that study, patients were encouraged to use the medication when they believed drinking to be imminent. These investigators found that nalmefene was superior to placebo in reducing heavy drinking days, very heavy drinking days, and drinks per drinking day and in increasing abstinent days.
The present study compared the efficacy of naltrexone or placebo, administered daily or on a targeted schedule, as an adjunct to individual, brief skills training in a sample of problem drinkers. Based on our prior findings [8], we hypothesized that naltrexone and targeted administration would reduce the mean number of daily drinks consumed and that patients receiving targeted naltrexone would show greater reductions on this measure than the other three groups (i.e., daily naltrexone or targeted or daily placebo). The present study differs from the prior study, in which the effects of naltrexone were confounded with the schedule of administration, by using a standard dosage for the targeted condition throughout the study, extending the duration of treatment from eight to 12 weeks, and by using interactive voice response (IVR) technology in place of paper diaries to measure daily drinking.
MATERIALS AND METHODS
Overview
This 12-week treatment trial employed a factorial study design to examine the effects of medication and schedule of administration, and their interaction on mean daily drinking in problem drinkers who had a goal of reducing, but not completely stopping, their drinking. In addition to random assignment to naltrexone 50 mg/day or a matched placebo tablet, patients were randomly assigned to a daily or targeted schedule of medication administration. In counseling sessions held every two weeks, patients received brief coping skills therapy focusing on the identification and management of high-risk drinking situations to reduce their drinking to non-hazardous levels [10].
Patients in the daily conditions received 7 tablets per week and were encouraged to take one tablet daily. Patients in the targeted groups received 5 tablets per week and were encouraged to use at least 3 tablets per week by taking one tablet in anticipation of a high-risk drinking situation, with a maximum of one tablet every 24 hours. Counseling in the targeted group included the use of the medication as a coping strategy (i.e., medication to be taken 1-2 hours before a high-risk drinking situation). The counseling provided to the targeted and daily groups was, in all other respects, the same.
Patients
Recruitment was conducted predominantly through advertisements in local media, with some patients being referred for treatment by primary care physicians or other clinicians in the area. Following a telephone-screening interview, eligible participants were invited for an in-person interview. Prospective patients were given a complete description of the study procedures and potential risks, following which they gave written, informed consent to participate. The institutional review board of the University of Connecticut Health Center approved the informed consent form and the study protocol. Study participants were paid for their completion of daily reports and for research assessments conducted at the end of treatment. The study was registered as NCT00369408 on www.clinicaltrials.gov.
Prior to study enrollment, all patients received a physical examination and routine laboratory testing. Patients were included in the trial if they were 18-65 years old; reported an average weekly alcohol consumption of ≥24 standard drinks for men and ≥18 standard drinks for women (a difference based on sex differences in body weight and composition); were able to read English at an eighth grade or higher level; showed no gross evidence of cognitive impairment on a brief mental status examination conducted by a study psychiatrist; and were willing to provide signed, informed consent to participate in the study (including an expressed willingness to reduce drinking to non-hazardous levels). Women of childbearing potential had to be non-lactating, practicing a reliable method of birth control, and have a negative serum pregnancy test prior to initiation of treatment.
Patients were excluded from participation if they had a clinically significant physical or psychiatric illness requiring medical treatment, a current DSM-IV diagnosis of drug (other than nicotine) dependence or a lifetime DSM-IV diagnosis of opioid dependence, regular use of opioids or other psychoactive medications in the preceding month or a current DSM-IV diagnosis of alcohol dependence that was clinically severe.
Because there is no clear empirical basis for choosing a severity cutoff with respect to alcohol dependence criteria, we excluded patients who reported a recent unsuccessful attempt to reduce drinking or who had a history or present evidence of significant alcohol withdrawal symptoms, including recurrent use of alcohol to alleviate such symptoms. This determination was made by the physician at study entry, who based it on historical information obtained by a research nurse and the results of the clinical evaluation of the patient.
Study Medication
Study medication (naltrexone 50-mg tablets or indistinguishable placebo tablets) was dispensed in quantities adequate for a 2-week period. Patients were asked to bring any remaining capsules to each clinic visit. At each visit a self-report screening questionnaire was followed by a nurse’s inquiry concerning the presence of 11 adverse events commonly associated with naltrexone treatment.
Brief Skills Training
Biweekly counseling sessions were administered individually to patients by one of 5 therapists (3 with a master’s degree and 2 with a doctorate) who were trained to administer the intervention. The sessions, which were manualized, were developed for use in our prior study of targeted naltrexone [7]. Because it is structured, this coping skills training approach can be used in a variety of settings, including medical settings, where physicians, nurses, or other professionals can be trained to deliver it. The manual, which was designed to foster problem solving, interpersonal skills, and the means to cope with desires and urges, is available upon request of the corresponding author.
The first skills training session consisted of five minutes of simple advice followed by 20 minutes of counseling about heavy drinking. Simple advice consisted of a review of the features of hazardous drinking. Individuals were told that, on the basis of the information provided, their drinking placed them at increased risk for a variety of alcohol-related problems. They were given a brochure, which was based on one developed for use in the World Health Organization Brief Intervention Study [11]. The brochure illustrates the amounts of alcohol contained in a standard drink of beer, wine, and liquor and establishes a sensible drinking limit for men and women. Patients were urged to aim for a sensible drinking limit [i.e., no more than 2 (women) or 3 (men) drinks per day; no more than 8 (women) or 12 (men) drinks per week]. They were also encouraged to consider abstaining from alcohol completely under certain circumstances.
The therapist also gave the patient a leaflet that describes a habit-breaking plan and explained how to use it to achieve the drinking goals described in the sensible drinking brochure. The therapist reviewed the sections of the brochure that require the patient to identify high-risk drinking situations and set goals for reduced drinking.
Patients received five additional 20-minute counseling sessions at two-week intervals. At these sessions, the topics covered in the first session were reiterated and expanded upon. At each session, patients were given brochures on how to manage the desire to drink and other risks to excessive drinking, and the importance of adherence to the medication regimen to achieve beneficial effects of the medication. Specific skills that were discussed included increasing pleasant activities, adding structure to one’s life, and using alcohol-free problem solving strategies. During these sessions, the therapist also reviewed the patient’s progress in drink reduction, the identification of heavy drinking situations, and the development of alternate behavioral strategies to facilitate avoidance or reduced drinking in such situations.
The initial sessions conducted by each counselor were audio taped and rated for adherence to the prescribed guidelines with respect to both duration and content. Once therapists were determined to be adherent to the protocol, approximately 20% of subsequent sessions were recorded and analyzed to permit a comparison of the counseling as delivered to daily vs. targeted groups.
Assessments
Prior to randomization, patients underwent a series of assessments:
The Alcohol Use Disorders Identification Test (AUDIT), a ten-item test, was developed as a screening instrument for the identification of hazardous drinkers [12]. Based on evidence that it is a valid index of the severity of alcohol dependence [13], we used it to rate the severity of harmful drinking.
The Structured Clinical Interview for DSM-IV [(SCID-I; First et al. [14] was used to determine the presence or absence of mood and anxiety and alcohol and drug use disorders according to DSM-IV criteria.
The Time-Line Follow-Back Assessment Method (TLFB) was used to estimate alcohol consumption during the 90-day pre-treatment period. Patients were given a blank calendar and were asked to reconstruct their drinking behavior over the preceding three-month period [15]. The TLFB was re-administered at biweekly intervals during the treatment trials as a secondary indicator of drinking behavior and medication adherence.
The Short Inventory of Problems (SIP), a 15-item instrument derived from the Drinker Inventory of Consequences [16], measures a wide range of alcohol-related problems.
The Beck Depression Inventory (BDI), a 21-item self-report measure that yields a total score ranging from 0 to 63 [17], was used to measure depressive symptoms. Cut-offs for the BDI that are used clinically are: 0-9, not depressed; 10-18, mild-to-moderate depression; 19-29, moderate-to-severe depression; and ≥30, severe depression.
Interactive Voice Response Technology (IVR) uses the telephone to administer survey questions. The methods employed have been presented previously [18]; they are summarized briefly here. During their randomization visit, patients met with a research assistant for a brief (10–15 min) IVR training session and were provided with a toll-free number to use to contact the IVR system. Patients were asked to call the system daily from a touch-tone phone between 5:00 and 9:00 PM to report on the day’s experience. This provided consistent timing of reports across days and patients and promoted adherence to the IVR regimen. Patients were given a wallet-sized interview guide with key terms for each interview question in the order presented by the system. Patients who did not call the IVR system by 8:00 PM were called automatically at a preferred telephone number and reminded to complete the interview.
Each day, patients recorded their alcohol consumption for the previous night (i.e., after the last IVR survey) and for that day by pressing the keys on the telephone keypad, with responses entered automatically in a database. They reported the number and quantity (in standard drinks) of each of three categories of alcoholic beverages: beer, wine, and liquor. We summed the beverage categories and the two time periods to create a total number of standard drinks consumed per day. Using IVR, patients also reported whether they consumed a study tablet since the previous call.
Medication Questionnaire
At the last treatment visit, patients were asked to identify whether they believed they had received naltrexone or placebo.
Data analysis
Pretreatment demographic and clinical features were compared across the four study conditions (targeted naltrexone, daily naltrexone, targeted placebo, and daily placebo) using χ2 analysis for categorical measures and t-test or ANOVA for continuous measures.
We used a two-level hierarchical linear model (HLM) to examine the main and interaction effects of medication group (naltrexone versus placebo), schedule of administration (daily versus targeted), and time (week in treatment) on the number of standard drinks consumed during the 12-week treatment period. We chose this primary outcome measure based on a secondary analysis of the findings from our prior study [8]. We also conducted an exploratory analysis of drinks per drinking day, based on the presumed mechanism of naltrexone’s effects to reduce heavy drinking [19]. We included the following variables as covariates: sex, years of education and the mean number of drinks per day during the 90-day period immediately preceding the screening visit. Because the outcome variables are counts, we specified a Poisson model with log-link and over-dispersion. In the level one equation, we predicted the number of drinks per day or per drinking day consumed from week of treatment (coded 0-11). The level two intercept (drinking level at the beginning of the study) and slope (time-drinking association) equations included all three covariates and the medication main effect (coded naltrexone = 0, placebo = 1), the schedule main effect (coded targeted = 0, daily = 1) and a multiplicative term to test the interaction between medication and schedule. In a second step, we entered the multiplicative terms to test the moderating effects of sex on medication, schedule and their interaction. With the exception of sex (coded 0 = female, 1 = male), covariates were grand mean centered. We estimated the residual error terms for both the intercept and slope portions of the model. We report the unit-specific estimates with robust standard errors (SE).1
RESULTS
As shown in Supplemental Material – Figure A, of 192 respondents who were screened, 163 (84.9%) were randomly assigned to treatment, including 83 patients who received naltrexone (N = 38 in the targeted naltrexone group; N = 45 in the daily naltrexone group) and 80 patients who received placebo (N = 39 in the targeted placebo group; N = 41 in the daily placebo group). A total of 138 patients (85%) completed the 12-week treatment. Because all randomized patients completed at least one IVR call and HLM weights participants’ daily data based on the number of reports provided, it was possible to retain all patients in the analysis.
Descriptive statistics
The sample was middle-aged [M = 49.1 yr; standard deviation (SD) = 9.6], mostly male (58.3%), and predominantly European American (96.9%); there were no differences among treatment groups on these characteristics. Overall, the study sample was well educated (M = 15.4 yrs of school; SD = 2.4). Naltrexone-treated patients had significantly more years of education than those receiving placebo [Naltrexone: M = 15.8; SD = 2.4, Placebo: M = 15.0; SD = 2.3, equal variance t test with DF = 161, test statistic = 2.06, p = 0.041].
Although the sample consisted predominantly of individuals with current alcohol dependence (95.1%), the mean number of DSM-IV criteria met for current alcohol dependence (out of a maximum of 7) was only 3.6 (SD = 1.1). The AUDIT score (M = 19.9; SD = 4.4) was also consistent with a low level of alcohol dependence severity [11]. The score on the SIP reflected a low level of alcohol problem severity as well (M = 15.7, SD = 7.5). More than one-quarter of the patients (30.1%) had previously received alcohol treatment and less than one-quarter were current smokers (23.3%).
During the pretreatment period, patients drank on 88.3% of days (SD = 15.4); they drank heavily (men: ≥ 5 drinks in a day; women: ≥ 4 drinks in a day) on 61.1% of days (SD = 26.9). This represents a mean of 5.0 (SD = 1.9) standard drinks per day and 5.7 (SD = 2.1) standard drinks per drinking day.
Sixty-six patients (40.5%) met lifetime (but not current) criteria for a drug use disorder, including abuse or dependence on cannabis (n = 55, 33.7%), cocaine (n = 35, 21.5%), stimulants (n = 9, 5.5%), hallucinogens (n = 6, 3.7%), and sedative/hypnotics (n = 5, 3.1%). Fifty-eight patients (35.6%) met past criteria for a psychiatric disorder, including major depression (n = 38, 23.3%), social phobia (n = 14, 8.6%), panic disorder (n = 6, 3.7%), agoraphobia without panic disorder (n = 1, 0.6%), and obsessive-compulsive disorder (OCD; n = 1, 0.6%). One patient met criteria for a current drug use disorder: cannabis abuse (n = 1, 0.6%). Few patients met current criteria for a current psychiatric disorder, which included social phobia (n = 5, 3.1%), antisocial personality disorder, (n = 4, 2.5%), dysthymic disorder (n = 1, 0.6%), agoraphobia without panic disorder (n = 1, 0.6%), OCD (n = 1, 0.6%), and generalized anxiety disorder (n = 1, 0.6%). BDI scores (M = 7.7; SD = 5.5) reflected an absence of clinically significant depression. The treatment groups did not differ significantly on any of the clinical indicators examined, when considered in relation to medication condition, schedule of administration, or their interaction.
Treatment and Research Adherence
The rate of treatment completion was 84.7% overall. Based on daily IVR and biweekly TLFB data, adherence to the medication regimen (i.e., daily medication taken on a minimum of 6 days/week and targeted medication taken 3–5 days/week) was also high (86.7%). The mean number of therapy sessions (out of a maximum of 6) was 5.4 (SD = 1.4). Of the 13,692 possible IVR reports (163 patients × 84 days), we received 11,171 (81.6%). Thus, patients completed an average of 68.5 calls (SD = 19.8). None of the adherence measures differed significantly among the four treatment groups.
A total of 150 therapy sessions from 91 patients were rated for the therapist’s adherence to the treatment guidelines. As planned, the length of the sessions differed by week, with session 1 (on the day of randomization) averaging 30.6 (SD = 5.7) minutes in duration compared with averages ranging from 18.2 to 22.1 minutes for sessions 2–6. The length of the sessions did not vary significantly across the four treatment groups.
In the audiotape review, the therapy sessions were rated for the presence of the basic characteristics that were supposed to occur either in all six sessions irrespective of schedule of administration or that discriminated the two medication administration schedules. As intended for all sessions, the therapist assessed recent alcohol use, drinking goal, and medication compliance in more than 95% of the sessions, irrespective of medication administration schedule assignment. In 88% of the sessions for the daily groups, the therapist discussed daily administration of the medication, compared to only 21% of the sessions for the targeted groups. In 77% of the sessions for the daily patients, the therapist discussed the need to take the medication at the same time each day, compared with 17% of the sessions for targeted patients. Consistent with the protocol for targeted treatment, 95% of the sessions for patients in these groups included discussion of medication use in anticipation of high-risk situations. This was not done in any of the sessions for the daily patients.
Safety and Tolerability
Two patients (both in the targeted naltrexone group) experienced serious adverse events that appeared unrelated to study participation (one case of a severe recurrence of diverticulitis and one case of pneumonia that required hospitalization), following which the medication was resumed and the study completed uneventfully. Three patients (all who received naltrexone) required a reduction in dosage to one-half tablet (25 mg) due to adverse effects; one of these individuals did not complete the study. Five patients (2 daily naltrexone, 2 targeted naltrexone and 1 daily placebo) discontinued treatment due to adverse effects.
The likelihood of experiencing at least one of the 11 common adverse effects did not differ significantly by medication group, administration schedule, or the interaction of these factors. Of the individual adverse events, nausea and dizziness varied as a function of medication group [nausea: χ2(1) = 27.86, p < 0.0001; naltrexone = 37.4%, placebo = 3.8%; dizziness: χ2(1) = 11.37, p = 0.0007; naltrexone = 13.3%, placebo = 0%].
Integrity of the Masking Procedure
Analysis of the patients’ report of whether they received the active or placebo treatment revealed that those in the daily medication groups were unable to identify their treatment assignment at a rate greater than chance [χ2(1) = 1.96, p = 0.16]. However, those in the targeted condition were significantly more likely than chance to identify correctly their treatment assignment [χ2(1) = 5.40, p = 0.020]. Although patients receiving targeted naltrexone were equally divided in their assessment of their treatment (with 51.6% incorrectly identifying their treatment as placebo), 78.4% if patients in the targeted placebo group correctly identified their treatment as placebo.
Drinking Behavior During the Treatment Period
The full model results for the primary dependent variable, drinks per day, are shown in Table 1, which is divided into the intercept (drinking level) and slope (study week) portions of the model. The coefficients in the intercept portion correspond to the main or conditional (if higher level interactions are included) effects on drinking level; the coefficients in the slope portion correspond to the time (study week) × predictor interactive effects.
TABLE 1.
Multilevel Regression Results
| Drinks Per Day | Drinks Per Drinking Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | t | df | P | b | SE | t | df | P | |
| Interce pt (drinki ng level) |
1.051 | 0.116 | 9.052 | 156 | <0.001 | 1.361 | 0.067 | 20.405 | 153 | <0.001 |
| Sex | 0.041 | 0.113 | 0.363 | 156 | 0.717 | 0.237 | 0.072 | 3.293 | 153 | 0.002 |
| Educati on |
−0.010 | 0.023 | −0.424 | 156 | 0.672 | −0.016 | 0.014 | −1.165 | 153 | 0.246 |
| Pretrea tment drinkin g |
0.136 | 0.031 | 4.458 | 156 | <0.001 | 0.088 | 0.016 | 5.342 | 153 | <0.001 |
| Medica tion |
0.223 | 0.148 | 1.509 | 156 | 0.133 | 0.020 | 0.089 | 0.222 | 153 | 0.825 |
| Schedu le |
0.161 | 0.154 | 1.043 | 156 | 0.299 | 0.036 | 0.097 | 0.372 | 153 | 0.710 |
| Schedu le × medica tion |
−0.309 | 0.208 | −1.484 | 156 | 0.140 | −0.032 | 0.131 | −0.243 | 153 | 0.808 |
| Sex × medica tion |
0.641 | 0.291 | 2.204 | 153 | 0.029 | 0.358 | 0.182 | 1.958 | 150 | 0.052 |
| Sex × schedu le |
0.564 | 0.299 | 1.887 | 153 | 0.061 | 0.263 | 0.186 | 1.418 | 150 | 0.158 |
| Sex × schedu le × medica tion |
−0.752 | 0.380 | −1.977 | 153 | 0.049 | −0.203 | 0.236 | −0.861 | 150 | 0.391 |
| Slope (study wk) |
−0.018 | 0.011 | −1.594 | 156 | 0.113 | −0.017 | 0.008 | −2.068 | 153 | 0.040 |
| Sex | −0.010 | 0.010 | −1.093 | 156 | 0.277 | −0.012 | 0.006 | −2.026 | 153 | 0.044 |
| Educati on |
−0.002 | 0.002 | −0.878 | 156 | 0.382 | −0.002 | 0.001 | −1.391 | 153 | 0.166 |
| Pretrea tment drinkin g |
−0.005 | 0.002 | −2.177 | 156 | 0.031 | −0.002 | 0.002 | −1.375 | 153 | 0.171 |
| Medica tion |
−0.003 | 0.012 | −0.209 | 156 | 0.835 | 0.011 | 0.009 | 1.201 | 153 | 0.232 |
| Schedu le |
0.025 | 0.012 | 2.150 | 156 | 0.033 | 0.026 | 0.009 | 2.931 | 153 | 0.004 |
| Schedu le × medica tion |
−0.024 | 0.016 | −1.490 | 156 | 0.138 | −0.024 | 0.011 | −2.131 | 153 | 0.034 |
| Sex × medica tion |
0.039 | 0.017 | 2.314 | 154 | 0.022 | 0.039 | 0.012 | 3.280 | 151 | 0.002 |
| Sex × schedu le |
0.027 | 0.017 | 1.630 | 154 | 0.105 | 0.005 | 0.012 | 0.462 | 151 | 0.644 |
b and SE values are in log form; medication group coded 0 = naltrexone, 1 = placebo; schedule of administration coded 0 = targeted, 1 = daily; study week coded 0 to 11; pretreatment drinking refers to the corresponding value from the 90-day pretreatment period. Sex interactions were included in a second block.
Drinks Per Day
In the intercept portion of the model, the only predictor that was significantly associated with drinking during treatment was pretreatment mean drinks per day (which was elicited using the TLFB). The schedule × medication predictor was not significant, indicating that there were no study condition interaction effects during treatment week 1.2 Moreover, the time × schedule × medication interaction (i.e., the schedule × medication effect in the slope section) also was not significant, indicating that the schedule × medication interaction did not vary across the duration of the study.
In a simplified model that omitted the time × schedule × medication interaction term, there was a significant schedule × medication effect in the intercept portion of the model (b = −0.402, SE = 0.20, p = 0.044). Averaged across all weeks of treatment, the difference in mean drinks per day between the naltrexone and placebo groups differed between the targeted and daily conditions. However, a focused comparison revealed that the difference between the targeted naltrexone group (coded 1) and the mean of the other three groups (coded 0) was not significant (b = −0.18, SE = 0.13, p = 0.15; the exponentiated b indicates that across the 12 weeks of the study the targeted naltrexone group drank 16.5% less per day than the other groups). The only other significant effect in the trimmed model was a pretreatment drinking × time interaction (b = −0.004, SE = 0.002, p = 0.038), indicating that heavier drinkers (during pretreatment) showed greater decreases in drinks per day during the study period.
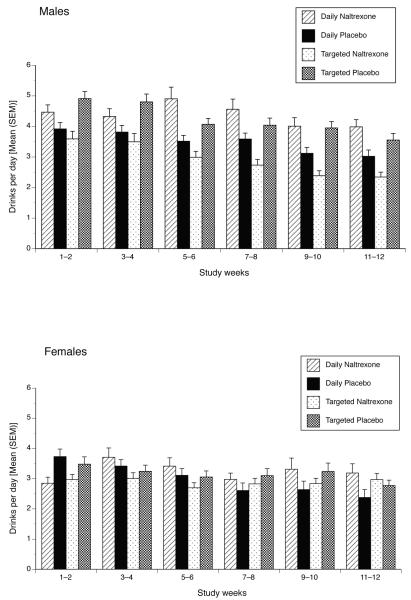
To examine whether the effects of treatment varied by sex we incorporated the sex × schedule, sex × medication and sex × schedule × medication product terms in both the intercept and slope portions of the model. The 4-way week × sex × schedule × medication interaction was not significant and was removed from the model to facilitate interpretation; the remaining coefficients are shown in Table 1. We found a significant sex × schedule × medication effect in predicting intercepts (i.e., mean drinks per day). The form of this effect is shown separately for men and women in Figure 1. Among men, the targeted naltrexone group drank less than the daily naltrexone group with the opposite pattern in evidence for the targeted and daily placebo groups. Women’s drinking was generally the same across study conditions. A focused test of targeted naltrexone versus others among men revealed a non-significant (p =0.20) difference in mean drinks per day across the entire study period. However, among men at week 12, the targeted naltrexone group drank less than the other groups (p = 0.027).
Figure 1.
Number of Standard Drinks Per Day by Study Group [Mean (SEM)], Separately by Sex. Data are presented as biweekly means (at the midpoint of 2-week period), derived from daily reports. There were no pretreatment group differences on this measure. Differences evident at period 1, i.e., during weeks 1–2, reflect differences that emerged during the first two weeks following the initiation of treatment. Significant treatment effects are described in the text.
In the slope portion of the model we also found a study week × sex × medication interaction. The form of this effect indicated that women treated with naltrexone showed no decrease in drinking over the course of the study (week effect slope: b = 0.0038, p = 0.65). In contrast, the other three groups showed study week effects: women receiving placebo (b = −0.031, p =0.009), men receiving naltrexone (b = −0.021, p = 0.023), and men receiving placebo (b = −0.024, p <0.001).
Drinks Per Drinking Day
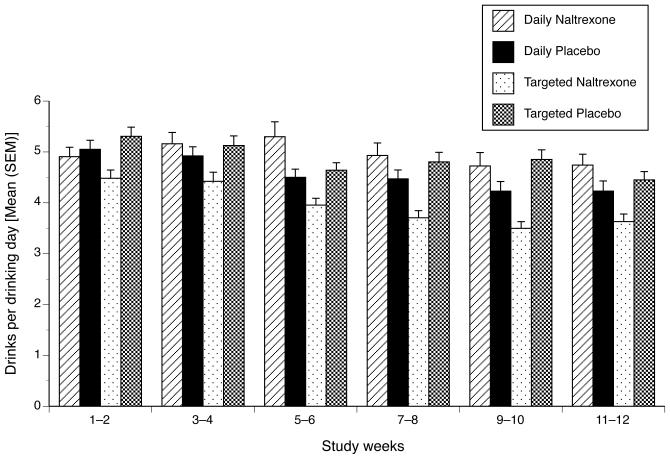
In an exploratory fashion, we also examined drinks per drinking day as an outcome. Although data from three patients who reported no drinking during the treatment period (one patient in each of the targeted naltrexone, daily naltrexone, and daily placebo groups) were excluded from this analysis, the model setup for the analysis was otherwise identical to the drinks per day model. Results for the full model are shown in Table 1. We found a significant time × schedule × medication interaction (i.e., the schedule × medication effect in the slope section), indicating that the schedule × medication interaction varied across the weeks of the study. Given the coding of study week, the schedule × medication effect shown in Table 1 (in the intercept section) shows no interaction in week 1. Thus, we recoded study week so that week 12 equaled zero (i.e., study week ranged from −11 to 0) and re-estimated the full model. This allowed us to test the schedule × medication effect at the end of the study. Results from this model indicated a significant schedule × medication effect in week 12 (b = −0.30, SE = 0.13, p = 0.027), which is shown in Figure 2. A focused comparison using the same coding as stated above revealed that the difference between the targeted naltrexone group and the mean of the other three groups was significant (b = −0.21, SE = 0.09, p = 0.014; the exponentiated b indicates that, during week 12, the targeted naltrexone group drank 19% less on drinking days than the other groups).
Figure 2.
Number of Standard Drinks Per Drinking Day by Study Group [Mean (SEM)]. Data are presented as biweekly means (at the midpoint of 2-week period), derived from daily reports. There were no pretreatment group differences on this measure. Differences evident at period 1, i.e., during weeks 1–2, reflect differences that emerged during the first two weeks following the initiation of treatment. Significant treatment effects are described in the text.
Finally, we incorporated the condition × sex interactions into the model. Similar to the drinks per day model, the 4-way week × sex × schedule × medication was not significant and thus was removed from the model; the remaining coefficients are shown in Table 1. Unlike the drinks per day model, we did not observe a significant sex × schedule × medication effect for mean levels across the study period (i.e., in the intercept portion of the model). However, similar to the drinks per day model, we found a study week × sex × medication interaction, with the form of the effect essentially the same as seen for drinks per day. Specifically, women treated with naltrexone showed no study week effect (b = 0.0059, p = 0.38). In contrast, the other three groups showed decreases in drinks per drinking day across the study period: women receiving placebo (b = −0.018, p = 0.011), men receiving naltrexone (b = −0.022, p <0.001), and men receiving placebo (b = −0.011, p = 0.015).
DISCUSSION
In this study, we compared the effects of naltrexone with placebo and daily medication with a targeted schedule of administration to reduce drinking in a sample of heavy drinkers whose goal was to reduce their consumption to sensible limits. Although nearly all of the participants met current criteria for a DSM-IV diagnosis of alcohol dependence, their severity was mild to moderate, with the mean number of DSM-IV alcohol dependence criteria across the treatment groups being less than 4 (out of a maximum of 7 [11]). Further, few participants had a current psychiatric or substance use disorder. These clinical features reflect our aim to recruit a study sample for which a goal of reduced drinking was a reasonable alternative to abstinence from alcohol, an understudied population.
The choice of a primary outcome measure (i.e., the mean number of drinks consumed per day) was based on the findings from a re-analysis of the data from a completed trial of targeted naltrexone [8]. In the present study, we obtained findings consistent with those from the prior analysis [8], with the targeted naltrexone group drinking about 16.5% less than the other groups; in the present study this effect did not reach significance. However, when we examined the moderating effect of sex on response to treatment, there was evidence that men were more responsive to the beneficial effects of targeted naltrexone, particularly at the end of the treatment trial. Although this finding differs somewhat from the moderating effect of sex seen in the previous study [8], there was a substantial difference between studies in the design of the targeted intervention. Consequently, the difference between studies in the moderating effect of sex between is difficult to interpret. The greater benefit seen among men (i.e., a relative lack of response among women) is consistent with the moderating effect of sex seen in a large study of long-acting naltrexone for the treatment of alcohol dependence [20].
An exploratory analysis showed that, at the end of the study, patients in the targeted naltrexone group drank approximately 19% less on drinking days than individuals in the other three treatment groups combined, which was statistically significant. As in the drinks per day model, examination of the moderating effect of sex also showed that women did not reduce the intensity of their drinking in response to naltrexone over the course of the treatment period while the other groups did.
Overall, there was a high rate of adherence to the study procedures in this sample, as evidenced by high rates of study completion, daily reports of drinking and related measures, and medication use. In addition, naltrexone was well tolerated, with no significant group differences on the rate of premature discontinuation of treatment or on the likelihood of experiencing at least one adverse event. As might be expected, review of individual adverse events revealed an excess of complaints of nausea and dizziness in relation to naltrexone treatment.
In addition to medication treatment, all participants in the study received brief counseling at biweekly intervals. Significant reductions in drinking have been observed using a variety of brief treatment approaches and may have contributed in this study to the overall decrease in drinking during the 12 weeks of the study. Since additional brief counseling was necessary to instruct patients in the application of the targeted approach, perhaps this contributed to the better outcomes in the targeted groups compared with daily treatment. The effects of counseling, however, appear specific to the interaction of schedule of administration with medication group, such that the targeted naltrexone group reported drinking less on drinking days than the targeted placebo group.
This study had several strengths, foremost among which was the use of a factorial design that made it possible to examine the main and interactive effects of medication group and administration schedule. More than eighty percent of patients completed the 12-week study and there was good adherence to both the medication regimen and the daily IVR reports of drinking behavior and medication intake. We have previously shown the validity of self-reported medication intake and self-reported drinking behavior measured using daily reports [21]. Several daily diary studies, including a study of problem drinkers [22], indicate that the repeated data collection method used in the current investigation does not affect the behavior being measured.
There are, however, limitations in this study that should be acknowledged. Specifically, the select patient sample was highly educated and adherent, features that are not typical of the larger population of problem drinkers, thereby limiting the capacity to generalize the findings here to the larger group. The study duration was brief (i.e., 12 weeks), particularly in view of the chronic nature of heavy drinking. In addition, the drinking data are based on self-report, rather than on objective measures, such as biological measures or collateral informant report. The 50-mg dosage of naltrexone evaluated in this study is only half that used in the COMBINE Study [23], suggesting that a higher dosage of the medication may be more efficacious in reducing drinking. Finally, the masking procedure appears not to have been fully successful, as evidenced by the ability of targeted placebo group to guess correctly the treatment that they received. The fact that patients in the targeted naltrexone group were not able to do so, nor were those in the daily treatment groups, suggests that the observed effects on drinking behavior are not likely to have been due to expectancy effects.
The findings reported here are consistent with those from our prior study showing that targeted naltrexone reduces drinking in problem drinkers. The importance of the targeted approach lies both in its utility as a general strategy for the treatment of heavy drinking (including reducing the number of drinks per day and drinks per drinking day) and its specific relevance to problem drinkers, a subgroup of patients for whom medication may augment the effects of brief psychotherapeutic interventions to reduce drinking. The low level of comorbid psychopathology in our patients and the high rate of treatment adherence and completion suggest that this is a population that is amenable to treatment efforts. Further, because problem drinkers represent a large proportion of potential recipients of medication to reduce drinking, this has important public health implications, since even small reductions in drinking in a large segment of the population could substantially reduce the overall prevalence of alcohol-related problems. Additional efforts are warranted to enhance the efficacy of this approach, particularly among women.
Supplementary Material
Figure A: Flow Sheet of Patient Recruitment, Randomization and Study Completion.
Acknowledgments
Supported by NIH grants P50 AA03510, K24 AA13736, and M01 RR06192. The staff of the Clinical Research and Evaluation Unit of the UConn Alcohol Research Center (particularly Lynn McLaughlin, R.N. and Kristen Tremblay, M.P.H.) was instrumental in the conduct of this study. Although not directly related to this study, Dr. Kranzler has received research support from Ortho-McNeil Pharmaceuticals and Bristol-Myers Squibb Co. and has been a paid consultant for Forest Pharmaceuticals; Alkermes, Inc.; Ortho-McNeil Pharmaceuticals; elbion NV; sanofi-aventis; Solvay Pharmaceuticals; and H. Lundbeck A/S.
Footnotes
In a separate analysis, we substituted drinking reports from the bi-weekly TLFB for missing IVR reports. Because the results were unchanged, we present findings based on IVR data only.
This is a conditional effect given that the higher-order interaction with time was included in the model.
REFERENCES
- 1.Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 2.O’Malley S, Jaffe AJ, Chang G, et al. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–7. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 3.Bouza C, Angeles M, Munoz A, et al. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–28. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 4.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–80. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 5.Heinala P, Alho H, Kiianmaa K, et al. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21:287–92. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kranzler HR, Tennen H, Penta C, et al. Targeted naltrexone treatment of early problem drinkers. Addict Behav. 1997;22:431–6. doi: 10.1016/s0306-4603(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 7.Kranzler HR, Armeli S, Tennen H, et al. Targeted naltrexone for early problem drinkers. J Clin Psychopharmacol. 2003;23:294–304. doi: 10.1097/01.jcp.0000084030.22282.6d. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Avila CA, Song C, Kuo L, et al. Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcohol Clin Exp Res. 2006;30:860–5. doi: 10.1111/j.1530-0277.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Karhuvaara S, Simojoki K, Virta A, et al. Targeted nalmefene with simple medical management in the treatment of heavy drinkers: a randomized double-blind placebo-controlled multicenter study. Alcohol Clin Exp Res. 2007;31:1179–87. doi: 10.1111/j.1530-0277.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health. 1995;85:823–8. doi: 10.2105/ajph.85.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babor TF, Grant M, Acuda W, et al. A randomized clinical trial of brief interventions in primary care: summary of a WHO project. Addiction. 1994;89:657–660. doi: 10.1111/j.1360-0443.1994.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 12.Babor TF, Higgins-Biddle JC, Saunders J, et al. Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. 2nd ed. World Health Organization; Geneva: 2001. Publication WHO/MSD/MSB/01.6a. [Google Scholar]
- 13.Donovan DM, Kivlahan DR, Doyle SR, et al. Concurrent validity of the Alcohol Use Disorders Identification Test (AUDIT) and AUDIT zones in defining levels of severity among out-patients with alcohol dependence in the COMBINE study. Addiction. 2006;101:1696–1704. doi: 10.1111/j.1360-0443.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- 14.First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) New York Biometrics Research: New York State Psychiatric Institute; 2001. [Google Scholar]
- 15.Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption. Humana Press; Clifton, NJ: 1992. [Google Scholar]
- 16.Miller W, Tonigan J. The Drinker Inventory of Consequences (DrInC) Vol. 4. 1995. (NIAAA Project MATCH Monograph Series). NIH Publ. No. 95-3911. [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 18.Kranzler HR, Abu-Hasaballah K, Tennen H, et al. Using daily interactive voice response technology to measure drinking and related behaviors in a pharmacotherapy study. Alcohol Clin Exp Res. 2004;28:1060–4. doi: 10.1097/01.alc.0000130806.12066.9c. [DOI] [PubMed] [Google Scholar]
- 19.Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–25. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- 20.Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 21.Feinn R, Tennen H, Cramer J, et al. Measurement and prediction of medication compliance in problem drinkers. Alcohol Clin Exp Res. 2003;27:1286–92. doi: 10.1097/01.ALC.0000080670.59386.6E. [DOI] [PubMed] [Google Scholar]
- 22.Hufford MR, Shields AL, Shiffman S, et al. Reactivity to ecological momentary assessment: an example using undergraduate problem drinkers. Psychol Addict Behav. 2002;16:205–11. [PubMed] [Google Scholar]
- 23.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A: Flow Sheet of Patient Recruitment, Randomization and Study Completion.