Abstract
Various lines of evidence suggest that disruptions in brain dopamine (DA) transmission produce behavioral impairments that can be overcome by salient response-eliciting environmental stimuli. We examined here whether D1 receptor blockade within striatal or frontal cortical DA target regions would differentially affect head entry responses elicited by an auditory cue compared to those occurring during non-cued inter-trial intervals. Rats received two drug-free 28-trial daily sessions in which an auditory cue was immediately followed by food delivery. On the following day, separate groups of rats received bilateral infusions of D1 antagonist SCH23390 to the dorsomedial striatum (DMS), nucleus accumbens (NAcc) core, or the medial prefrontal cortex (mPFC). SCH23390 infused into the DMS and NAcc core suppressed non-cued head entries, but had no effect on head entries in response to the auditory cue. SCH23390 infused to the mPFC did not reduce either cued or non-cued approach responses. Systemic administration of the drug, in contrast, reduced the frequency of both cued and non-cued approaches. The results are consistent with the notion that has emerged from the Parkinson’s literature that reduced DA transmission produces behavioral suppression that can be overcome by salient environmental response elicitors, and extends this notion by showing that D1 receptor transmission within the striatum strongly suppresses non-cued responses while leaving the identical behavior intact when cued by an environmental stimulus.
Keywords: dopamine, D1, SCH23390, internally-generated, cue-elicited
Introduction
Reductions in brain DA transmission produce impairments in the acquisition and performance of goal-directed behavior (Correa, Carlson, Wisniecki, & Salamone, 2002; Eyny & Horvitz, 2003; S. C. Fowler & Liou, 1998; Nicola, Taha, Kim, & Fields, 2005; R. A. Wise, 2009). However certain aspects of behavior remain relatively immune to the effects of reduced DA transmission (Choi, Balsam, & Horvitz, 2005; Ettenberg, Koob, & Bloom, 1981; Salamone, Arizzi, Sandoval, Cervone, & Aberman, 2002; Wickens, Horvitz, Costa, & Killcross, 2007). In animal subjects, behavioral responses strongly tied to discrete environmental cues often remain intact even after D1 or D2 receptor blockade, or DA depletion (Alcaro, Huber, & Panksepp, 2007; Bespalov, Harich, Jongen-Relo, van Gaalen, & Gross, 2007; Choi et al., 2005; Dowd & Dunnett, 2007; Horvitz & Eyny, 2000). Similarly, patients with Parkinson’s Disease (PD), a population suffering a degeneration of nigrostriatal DA neurons (Blaszczyk, 1998; Hornykiewicz, 1979), have been reported to walk quickly or even run out of a hospital room in response to a fire alarm, or to locomote normally when permitted to step over salient lines drawn on the ground despite pronounced locomotor impairments when response-eliciting environmental stimuli are absent (Martin, 1967).
In addition to these observations, a number of studies have reported that Parkinsonian motor impairments are reduced in the presence of strong response-eliciting stimuli (Briand, Strallow, Hening, Poizner, & Sereno, 1999; Jahanshahi & Frith, 1998; Kritikos et al., 1995; Rahman, Griffin, Quinn, & Jahanshahi, 2008; Siegert, Harper, Cameron, & Abernethy, 2002). The fact that PD symptoms are associated with particularly severe loss of substantia nigra (SN) DA neurons suggests that preferential loss of non-cued behaviors results from reduced DA transmission within brain regions innervated by SN DA, i.e., dorsal striatum (Blaszczyk, 1998; Braak et al., 1995). However PD patients also show extra-nigral pathology (Braak et al., 1995) including degeneration in select regions of A10 with reduced DA concentrations in the PFC and other frontal cortical regions (Gaspar, Duyckaerts, Alvarez, Javoy-Agid, & Berger, 1991; McRitchie, Cartwright, & Halliday, 1997), complicating identification of the anatomical locus of this impairment in non-cued behavior.
In the present study, we examined the effects of D1 antagonist R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) on rats’ likelihood of entering the head into a food compartment under cued and non-cued conditions, and asked whether selective disruption of non-cued behavior following D1 receptor blockade could be localized to striatal and/or frontal cortical target sites. We examined the effects of D1 and not D2 receptor blockade because we previously found that the key behavioral measure here, the likelihood and speed of executing a cued head entry response to a food compartment, is dependent upon D1 and not D2 receptor transmission (Choi et al., 2005; Choi, Morvan, Balsam, & Horvitz, 2009). We report here that motoric impairments produced by D1 receptor blockade within the dorsal and ventral striatum can be overcome by external response-eliciting cues, an effect resembling the paradoxical kinesia observed in Parkinson’s patients.
Materials and Methods
Subjects
Sprague Dawley rats (250-300g) were obtained from Charles River laboratories (Kinsington, NY; Wilmington, MA). The rats were housed in pairs in Plexiglas cages (22 cm high × 22 cm wide × 46 cm deep) mounted on racks within a rat colony, with food and water freely available. The colony was maintained at approximately 22°C under a 12 hr light dark cycle, with lights on at 8:00AM. The rats were gently handled for one week before being placed on a 23 hr food-restricted diet designed to maintain body weight at approximately 85% of free-feeding levels.
Apparatus
Behavioral test sessions were conducted in conditioning chambers (29 cm high × 29 cm wide × 25 cm deep: Coulbourn Instruments, Allentown, PA), individually housed within sound-and light-attenuated enclosures. Two walls of the chamber were Plexiglas, and the other two were metal. A house light was located at the top center of one of the metal walls, 2cm below the ceiling of the chamber. Recessed within the bottom center of this wall, 2 cm above the chamber floor, was a food compartment (4.0 cm high × 3.0 cm wide × 2.5 cm deep), into which food pellets (Bioserve F0021 45 mg, Frenchtown, NJ) were delivered. Activation of the food magazine produced a 400-ms, 78-dB sound that served as the conditioned stimulus (CS). The pellet settled at the bottom of the feeder trough approximately 600 ms after onset of feeder activation. An infrared photo-emitter detector, located on the sides of the food compartment, was interrupted by the rat’s head entry and signaled the presence of the rat’s head within the food compartment. Locomotor counts were assessed via an infrared activity monitor (Coulbourn H24-61) employing a differential detector to sense the animals’ emitted infrared body heat image (13 nM infrared radiation) through an array of lens facets on two detector elements, with relative changes in the energy falling on the elements defined as a movement unit. A PC running Coulbourn L2T2 or Graphic State software recorded the time of pellet deliveries, head entries and withdrawals, and locomotor counts with 50 ms resolution.
Stereotaxic Surgery
Approximately one week prior to behavioral sessions, animals in central infusion experiments were anesthetized with Nembutal (50 mg/kg, Sigm-Aldrich, St. Louis, MO), secured in a stereotaxic frame (Stoelting co., Wood Dale, IL) and implanted with bilateral chronic stainless steel guide cannulae (Plastics One, Roanoke, VA). Guide cannulae (22G) were surgically implanted to the following DA target regions (coordinates in mm from bregma) according to standard flat-skull stereotaxic procedures: mPFC/prelimbic (anterior-posterior [AP] +2.8, medial-lateral [ML] ±0.5, dorsal-ventral [DV] -3.3), DMS (AP +1.6, ML ±1.9, DV -4.2), or NAcc (AP +1.4, ML ±1.7, DV -6.0). Cannulae were cemented to the skull using dental acrylic (Stoelting co., Wood Dale, IL), and anchored with three stainless steel screws (Plastics One, VA). Stainless steel wire stylets with dust cap (Plastics One, Roanoke, VA) prevented occlusion of the guide cannulae. Rats were maintained on ad libitum food and water during 1 week of post-surgical recovery, and then placed on a 23-hr food deprivation for 6 days prior to the first conditioning session.
Drug and Microinfusions
For central infusion groups, selective D1 antagonist SCH23390 (Iorio, Barnett, Leitz, Houser, & Korduba, 1983) was dissolved in isotonic saline, and infused bilaterally in a volume of 0.5μl/side. For each anatomical site, each dose of SCH23390 was administered to a separate group of rats. This between-subjects design to assess behavioral effects of drug dose has the advantage of avoiding drug sensitization effects that can confound repeated measures designs. Drug doses were selected on the basis of preliminary work examining the effects of central SCH infusion on locomotor and approach behavior (Choi, Eyny, Matin, & Horvitz, 2000). SCH23390 was infused into the mPFC, 0 (n=9), 0.5 (n=7), 1 (n=8) or 2 (n=11) μg; DMS 0 (n=7), 0.5 (n=8) or 2 (n=8) μg; or NAcc Core 0 (n=8), 1 (n=11), or 2 (n=8) μg. Internal cannulae (28G) were attached to a Hamilton micro syringe (Hamilton Co., Reno, NV) via PE-20 tubing (Becton Dickinson, Sparks, MD), and lowered to the following DV coordinates: mPFC - 4.8; DMS - 5.6; NAcc core -8.5. Infusions were driven by a microdrive pump (Razel Pump, Stamford, CT) set to deliver 0.5 μl of fluid over 90 s. After completing infusions, internal cannulae were maintained in position for an additional 1 min before removal. Rats were tested immediately following this procedure. For intraperitoneal injections, SCH23390 (0, 0.08 or 0.16 mg/kg) was dissolved in isotonic saline and administered in a volume of 1ml/kg of body weight, thirty minutes prior to the test session. Rats were assigned randomly to one of the three systemic drug dose conditions (n=8 per dose).
Behavioral Procedure
Several seconds after placement in the conditioning chamber, a house light was illuminated, and remained lit until the session ended. During each session, rats received 28 pellets (trials) delivered individually into the food compartment on a variable-time 70-s schedule (with a minimum inter-pellet interval of 30 s). The auditory cues associated with magazine activation served as the CS. The time of each head entry into and removal from the food compartment and locomotor counts were recorded throughout each session. At the end of each session, animals were returned to their home cages and provided with food for one hour. Rats received drug-free training sessions for 2 consecutive days and on the third day were treated with either a bilateral infusion of one dose of SCH23390 or saline vehicle to the PFC, DMS or NAcc core, or an intraperitoneal injection of one dose of SCH23390 or its vehicle prior to the test session. The test session was otherwise identical to the prior training sessions.
The key behavioral measures were mean frequency of head entries into the food compartment during the 10 sec period preceding each CS presentation (non-cued head entry frequency), latency to enter the food compartment in response to the CS (cued head entry latency), the proportion of trials for which the rat failed to enter the food compartment within 10 sec after CS presentation (misses), and locomotor activity. These data were recorded throughout each training and test session for all experimental and control groups. In addition, raster plots were constructed for each rat in order to depict head entries occurring from 16 sec before to 10 sec after each CS presentation.
Histology
At the conclusion of the experiment, rats were deeply anesthetized with Nembutal and received microinfusions (0.5 μl/side) of water-proof India ink in the location matched to the SCH 23390 infusion site. The rats were then perfused transcardially with 0.9% saline followed by 10% formalin. The brains were post-fixed in a formalin mixture for a day, then transferred to 10 % sucrose for 2 days and 20% sucrose mixture for another 2 days before sectioning. Brains were cut into 50 μm sections and stained for Nissl substance with cresyl violet. The sections were examined with light microscopy, and estimated locations of infusion sites were recorded on Rat Brain Atlas Sections using Adobe Illustrator 10.0. Only the infusion sites that fell within the boundaries of the target areas (figure 1) were included in the analyses.
Figure 1.
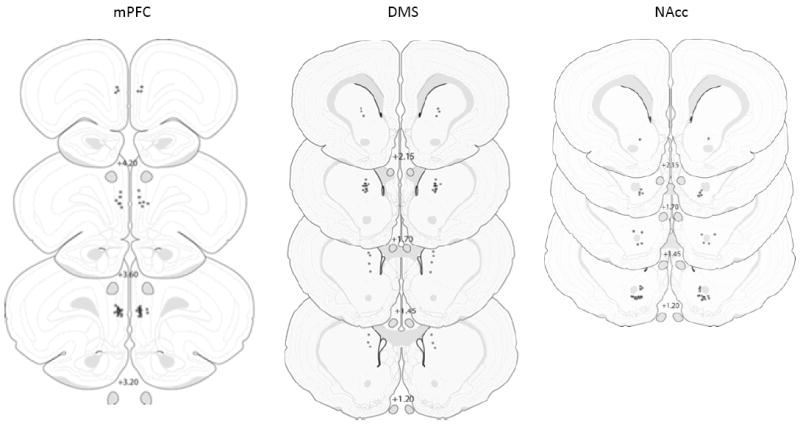
Drawing of coronal sections from animals in the mPFC (top left), DMS (top right), and NAcc core (bottom) groups. Each asterisk indicates the approximate cannula placement, and numerical values indicate distance (A-P) relative to bregma. From The Rat Brain in Stereotaxic coordinates (2nd ed.), AP 1.20 to 4.20, by G. Paxinos & C. Watson, 1986, New York, NY: Academic Press. Copyright by Elsevier Academic Press. Adapted with permission.
Data Analyses
On the basis of histological examination of brain sections, animals were excluded if cannulae did not target the DMS, NAcc core, or mPFC (prelimbic region). 1 of 23 rats in the DMS group, 4 of 27 rats in the NAcc group, and 6 of 35 rats in the mPFC group were excluded from analyses on the basis of these histological confirmations. The resulting number of animals for data analyses in each anatomical/drug infusion condition were DMS 0 (n=7), 0.5 (n=7), 2 μg (n=8); NAcc: 0 (n=7), 1.0 (n=9), 2.0 μg (n=7); mPFC 0 (n=9), 0.5 (n=5), 1.0 (n=7), 2.0 μg (n=8). 1-way ANOVAs were employed for all data analyses.
Results
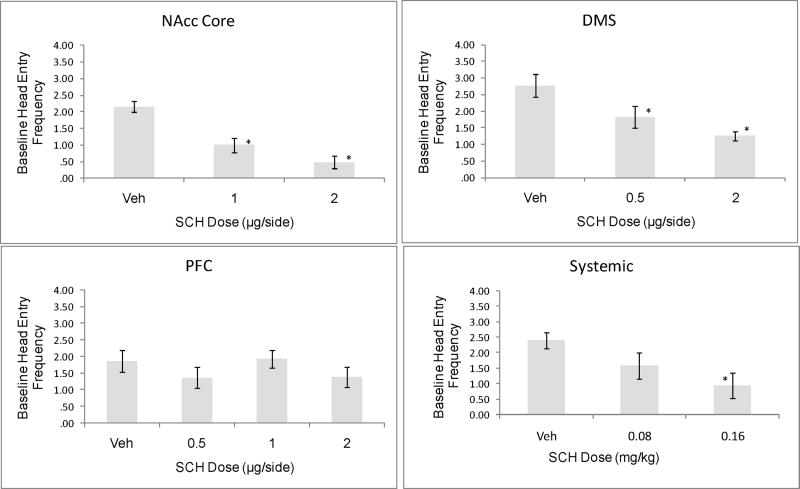
The frequency of head entries into the food compartment during the 10 s pre-CS baseline period was suppressed by SCH23390 infusion into the DMS (F [2,19]=7.58, p<.005) and NAcc core (F[2,20]=17.82, p<.0005), but not by SCH infusion to the mPFC (F[3, 25]=.009, p=ns). Systemic administration of SCH2339 also produced a dose-dependent suppression of baseline head entry frequency (F[2,21]=3.85, p<.05). These data suggest that D1 receptor blockade within the DMS and NAcc core strongly suppresses non-cued approach responses (figure 2).
Figure 2.
Frequency of head entries into the food compartment during the pre-CS baseline period. SCH23390 infusion to the NAcc core or DMS produced a dose-dependent reduction in these non-cued head entries. * = significant difference compared to Veh, Dunnett test, p<0.05.
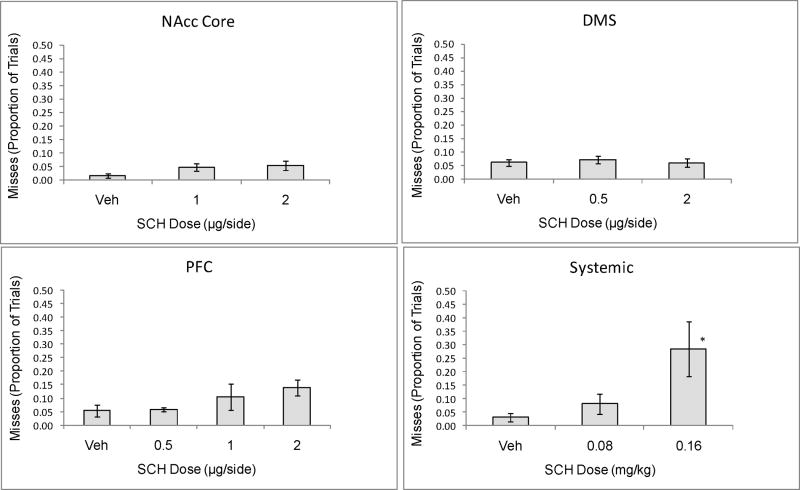
In contrast, SCH infusion to the NAcc, DMS, or mPFC failed to affect either the likelihood or latency of the head entry response to the auditory cue. As can be seen in figure 3, the proportion of trials in which the rat failed to enter the food compartment within 10 s of cue presentation (“misses”) was not significantly affected by D1 antagonist SCH infusion into the DMS (F[2,19]=0.19, p=n.s.), NAcc core (F[2,20]=1.65, p=n.s.) or mPFC (F[3, 25]=1.69, p=n.s.), although drug infusions to the mPFC produced a trend toward elevated misses. Systemic administration of SCH, however, did significantly increase the proportion of trials missed (F[2,21] = 4.52, p<.05). For trials in which animals responded within 10 s of the cue, SCH did not increase the cued head entry latency following either central or systemic administration (p=n.s. for all sites).
Figure 3.
Proportion of 28 trials in which rats failed to enter the food compartment within 10 s of the CS. Neither intra-NAcc, -DMS, or –PFC infusion of D1 antagonist SCH23390 produced the elevation in misses observed following systemic D1 antagonist administration. *=<.05 compared to VEH control, Dunnett test.
Figure 4 shows raster plots of head entries (horizontal bars) from 16 s before (-16 to 0 s) to 10 s after (0-10 s) pellet delivery for representative rats receiving infusion of either vehicle (top panel) or the high dose of SCH23390 (bottom panel) into the NAcc core. As can be seen, head entry responses occurring prior to the CS were suppressed by SCH, while head entries triggered by the cue were unaffected by the drug. A similar effect of SCH on non-cued versus cued responding was observed in the DMS, i.e., strong suppression of non-cued responding (figure 2) with no discernable effect on cued responding (figure 3).
Figure 4.
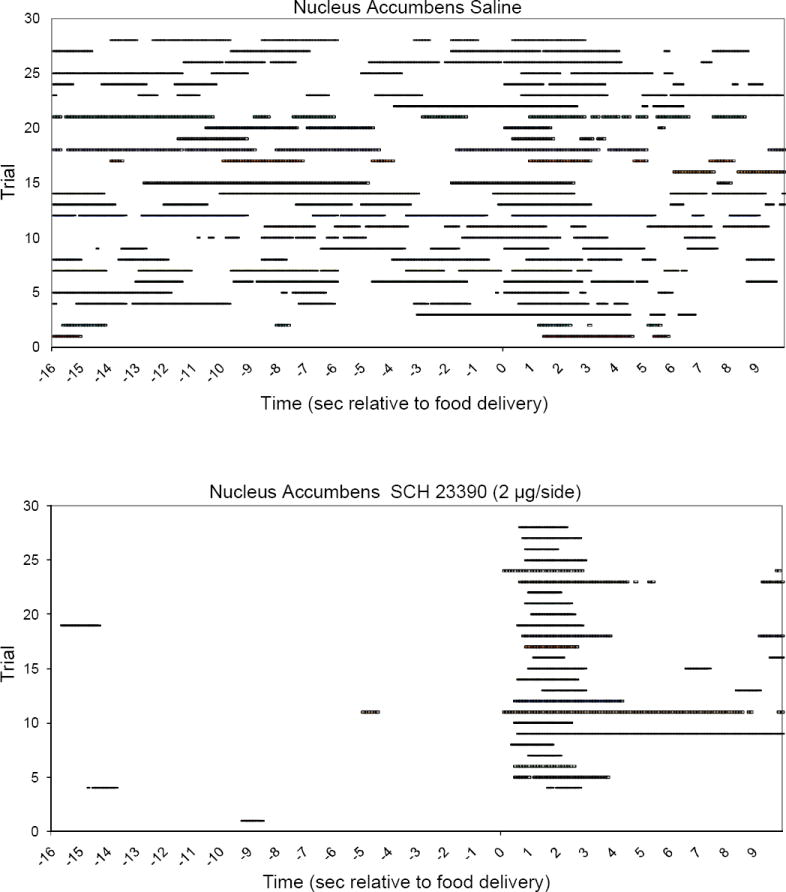
Head entries into the food compartment (horizontal bars) are shown for the period from 16 s before to 10 s after food delivery. Consecutive trials (1-28) are represented as successive rows on the y-axis (bottom to top). Time 0 indicates the presentation of the auditory cue and food delivery 600 ms later. Additional time between trials (ITI time beyond this 26 s period) is not shown. On test day, 2 μg/side SCH23390 infused to the NAcc core (bottom panel) disrupted head entries during the ITI (before s 0) but not in response to the food cue.
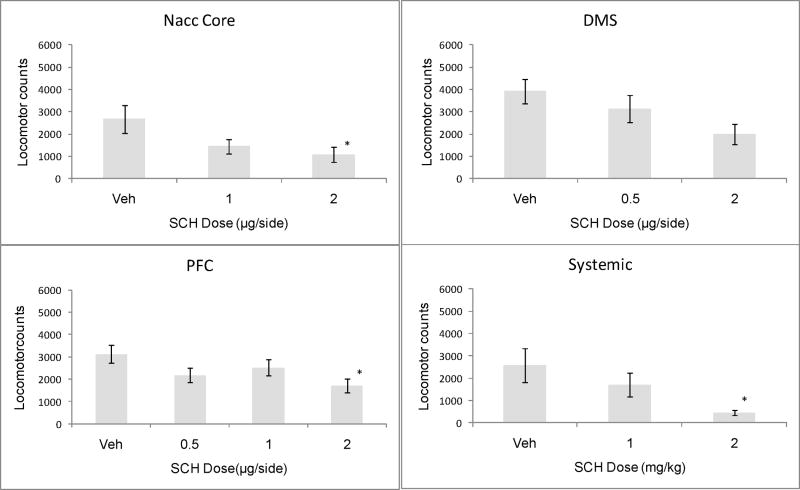
As can be seen in figure 5, the D1 antagonist suppressed locomotor behavior when infused into the mPFC (F[3,25] = 3.22, p<.05) and NAcc core (F[2,20] = 3.56, p<.05), and produced a marginally significant suppression of locomotion when infused into the DMS (F[2, 19]=3.36, p=.06). Locomotor suppression produced by systemic administration the D1 antagonist (F[2,21]= 3.97, p<.05) was particularly pronounced.
Figure 5.
Locomotor counts in rats under the influence of intra-NAcc, -DMS, -PFC or systemic SCH23390. *=<.05 compared to VEH control, Dunnett test. Locomotor suppression produced by the high dose of SCH in the DMS group was marginally significant (p=.06).
Discussion
D1 receptor blockade within the NAcc core or the DMS strongly reduced the frequency of head entries into the food compartment during non-cued baseline periods (ITIs) without reducing either the speed or the likelihood of emitting the same head entry behavior when preceded by the auditory cue. These findings are consistent with previous observations that disruptions in DA transmission produce greater impairment of non-cued compared to cue-elicited behaviors (Briand et al., 1999; Choi et al., 2005; Gramling & Fowler, 1985; Horvitz & Eyny, 2000; Jahanshahi & Frith, 1998; Jahanshahi et al., 1995). Systemic D1 receptor blockade disrupted both cued and non-cued head entries, consistent with our previous observations (Choi et al., 2005; Choi et al., 2009). The selectivity of the D1 antagonist effects to non-cued responding was only seen when the drug was infused to striatal (NAcc and DMS) target regions.
One might ask whether the suppressive effects of D1 receptor blockade on non-cued head entries were secondary to locomotor deficits. Arguing against this possibility, the high (2 μg) dose of SCH produced approximately 50% locomotor suppression when infused to either the NAcc, DMS or mPFC, while suppression of non-cued head entries was observed following SCH infusion to the NAcc and DMS but not mPFC. Thus, drug-induced suppression of the non-cued head entry response could not be predicted on the basis of locomotor-suppressing effects of the drug. Neither can the suppressive effects of SCH on the non-cued behavior be easily explained by a global motoric disruption of the head entry behavior itself, for the same head entry behavior that was suppressed by striatal SCH infusions when non-cued was unaffected by the drug when the behavior was elicited by the auditory cue. The effects of intra-striatal D1 receptor blockade appear to most strongly, (in the present results, exclusively), disrupt behavioral responding under non-cued conditions.
In light of the fact that systemic SCH23390 disrupted both cued and non-cued behavioral responding, it is reasonable to assume either that a) some DA target region(s) apart from the DMS, NAcc core and mPFC critically mediate cued responses and are responsible for the cued response disruption produced by systemic SCH23390 administration, or b) simultaneous disruption of D1 transmission within more than one DA target site (possibly including the mPFC, NAcc, and/or DMS) is required in order for cued response deficits to appear. We have not examined the effect of central D2 receptor antagonism on head entry behavior. However, under test conditions similar to those employed here, we have observed that systemic D2 receptor blockade has little effect on the likelihood of emitting either a cued or a non-cued head entry (Choi et al., 2009; Horvitz & Eyny, 2000). Initiation of the head entry response appears to be under D1 rather than D2 receptor control.
The similar behavioral effects of D1 receptor blockade within dorsomedial stratum and nucleus accumbens is in accordance with the high degree of functional overlap that has been ascribed to these dorsal and ventral striatal regions (Voorn, Vanderschuren, Groenewegen, Robbins, & Pennartz, 2004); but see (Yin, Ostlund, & Balleine, 2008). While one cannot rule out the influence of drug diffusion between dorsal striatum and accumbens, the distance between infusion sites (approximately 3 mm DV), makes this unlikely, for previous work has shown that infusion of SCH23390 at the same 0.5 ul infusion volume as that employed here does not produce detectable diffusion across such a distance (Faure, Reynolds, Richard, & Berridge, 2008).
In accordance with the effects of Parkinsonian nigrostriatal DA loss in humans (Jahanshahi & Frith, 1998), disruptions in dorsal and ventral striatal DA transmission here produced a particularly strong impairment in non-cued compared to behavioral responses elicited by salient external cues. Indeed, the food-related environmental cues to which D1 antagonist-treated rats showed normal behavioral responses were likely to have been highly salient given the animals’ food-restricted diet and food-seeking motivational state. It is of interest to note that neuronal activations in caudate and anterior putamen observed during cued versus non-cued behaviors are observed in relatively distinct populations of striatal neurons (Romo, Scarnati, & Schultz, 1992), raising the intriguing question of whether the latter population of striatal neurons may be particularly subject to DA modulation.
On the other hand, it may be difficult to draw a meaningful boundary separating the categories of behavior that are cued versus non-cued. For example, in the present paradigm, non-cued approach responses occurring during ITIs were likely to have been associated with the contextual stimuli of the chamber, and those environmental stimuli can be said to have at least indirectly contributed to response expression. An alternative framework takes into account the strength of the learned connection between environmental stimuli associated with the behavior and the behavioral response itself. Cue-elicited behaviors are typically those that have, in the past, been performed successfully, or ‘reinforced’, in the presence of the cue; behaviors that are infrequently reinforced in the presence of a cue are unlikely to be strongly ‘cue-elicited’ In the present paradigm, the response-eliciting auditory cue was always associated with the reward. To the extent that behaviors occurring during the ITI were linked to environmental context cues, these cues would have been only weakly associated with reward; they were present throughout the session both when the food was delivered and when it was not. The present results therefore can be explained by assuming simply that D1 receptor transmission within the DMS and NAcc sets a threshold for reward expectation to generate the approach response.
From this view, tonic levels of synaptic DA normally permit the expression of approach responses both during ITIs and following cue presentation. D1 receptor blockade raises the threshold for reward expectation to generate behavioral responding so that only the highest levels of reward expectation generate a goal-directed behavioral response. According to this view, behavioral responses under low reward expectation conditions were filtered out by D1 receptor blockade within striatal target sites, while those behaviors elicited under conditions of high reward expectation were intact even under conditions when striatal D1 receptor transmission was compromised.
Contrary to the present results, NAcc D1 transmission has been found, under some conditions, to disrupt cue-elicited appetitive responses. For instance, intra-accumbens infusion of SCH23390 reduced the proportion of trials for which rats performed a cue-elicited lever-press (Yun, Nicola, & Fields, 2004) or nose-poke (Nicola et al., 2005) and intra-accumbens D1/D2 antagonist alpha-flupenthixol reduced the proportion of trials for which a CS elicits an autoshaped (Pavlovian) lever-press (Di Ciano, Cardinal, Cowell, Little, & Everitt, 2001). As suggested above, the response-impairing effects of striatal (including accumbens) D1 receptor blockade may depend upon the strength of the eliciting stimulus. It is known that the associative strength of a reward-paired cue is greatest when the time between trials, i.e., between one unconditioned stimulus (US) and the next, is long and the CS- US interval brief. Acquisition speed and the strength of conditioning depends largely on the ratio of these two intervals (Balsam, Drew, & Gallistel, 2010; Balsam & Gallistel, 2009). In the present study the time between US presentations was 70 s on average, and the CS-US interval (time between feeder activation sound and pellet availability) was approximately 600 ms, a very high ratio; leading to the rapid emergence of a strong elicitor. Further, the external cue in a magazine approach task (employed here) may involve a compound of cues – including the sound of the food magazine and the presumably salient visual and olfactory cues associated with pellet delivery itself. It therefore seems likely that stimulus salience contributed to the animals’ ability to show normal approach latencies under conditions of striatal D1 receptor blockade. The finding that food and food-paired stimuli maintain their incentive properties under conditions of reduced striatal DA transmission is in accordance with previous work by Salamone and colleagues (Correa et al., 2002; Salamone et al., 2002). Finally, the limited number (28) of discrete CS-US trials employed here is likely to be relevant in light of the within-session decrements (extinction-like performance) often observed when DA transmission is disrupted (Fowler, 1990; Pitts & Horvitz, 2000; R. Wise, 1982).
D1 receptor blockade within the striatum suppressed execution of an approach response under non-cued but not under cued conditions. While D1 transmission has been shown to promote acquisition of appetitive cue-elicited behavior (Dalley et al., 2005; Eyny & Horvitz, 2003), the present results suggest that, within regions of both dorsal and ventral striatum, DA transmission may be of particular importance in the expression of appetitive behavior only when the behavior is generated in the absence of a strong response-eliciting environmental cue (Horvitz, Choi, Morvan, Eyny, & Balsam, 2007; Morvan Campbell, 2010). Under conditions of dorsal and ventral D1 receptor blockade, motoric impairments in the rat can be overcome by strong response-eliciting stimuli, an effect similar to the paradoxical kinesia observed following DA loss in Parkinson’s disease.
Acknowledgments
We gratefully acknowledge Todd Liu, Miyako Oe, Defne Amado, Sydney Rose, Kimberly Song, Mi Hae Song, Steven Chao, Lauren Friedman, Kristen Donohue, Kyle Fischer and Ruchit Kumbhani for their technical assistance. This work was supported by NIH grants R01DA023641 to JH and R01MH068073 to PB.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne.
References
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56(2):283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam PD, Drew MR, Gallistel CR. Time and associative learning. Comparative Cognition & Behavior Reviews. 2010;5:1–22. doi: 10.3819/ccbr.2010.50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends Neurosci. 2009;32(2):73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Harich S, Jongen-Relo AL, van Gaalen MM, Gross G. AMPA receptor antagonists reverse effects of extended habit training on signaled food approach responding in rats. Psychopharmacology. 2007;195(1):11–18. doi: 10.1007/s00213-007-0875-z. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW. Motor deficiency in Parkinson’s disease. Acta Neurobiol Exp. 1998;58(1):79–93. doi: 10.55782/ane-1998-1262. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, Schultz C, de Vos RA, Jansen EN. Nigral and extranigral pathology in Parkinson’s disease. J Neural Transm Suppl. 1995;46:15–31. [PubMed] [Google Scholar]
- Briand KA, Strallow D, Hening W, Poizner H, Sereno AB. Control of voluntary and reflexive saccades in Parkinson’s disease. Experimental Brain Research. 1999;129(1):38–48. doi: 10.1007/s002210050934. [DOI] [PubMed] [Google Scholar]
- Choi W, Eyny YS, Matin L, Horvitz JC. Effects of D1 antagonist SCH 23390 on spontaneous versus stimulus-elicited behavioral responses in the rat. Society for Neuroscience Abstracts. 2000;26:2252. [Google Scholar]
- Choi WY, Balsam PD, Horvitz JC. Extended habit training reduces dopamine mediation of appetitive response expression. J Neurosci. 2005;25(29):6729–6733. doi: 10.1523/JNEUROSCI.1498-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WY, Morvan C, Balsam PD, Horvitz JC. Dopamine D1 and D2 antagonist effects on response likelihood and duration. Behav Neurosci. 2009;123(6):1279–1287. doi: 10.1037/a0017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137(1-2):179–187. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102(17):6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21(23):9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E, Dunnett SB. Movement without dopamine: striatal dopamine is required to maintain but not to perform learned actions. Biochem Soc Trans. 2007;35(Pt 2):428–432. doi: 10.1042/BST0350428. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Koob GF, Bloom FE. Response artifact in the measurement of neuroleptic-induced anhedonia. Science. 1981;213(4505):357–359. doi: 10.1126/science.7244622. [DOI] [PubMed] [Google Scholar]
- Eyny YS, Horvitz JC. Opposing roles of D1 and D2 receptors in appetitive conditioning. J Neurosci. 2003;23(5):1584–1587. doi: 10.1523/JNEUROSCI.23-05-01584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. Journal of Neuroscience. 2008;28(28):7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC. Neuroleptics produce within-session response decrements: Facts and theories. Drug Dev Res. 1990;20:101–116. [Google Scholar]
- Fowler SC, Liou JR. Haloperidol, raclopride, and eticlopride induce microcatalepsy during operant performance in rats, but clozapine and SCH 23390 do not. Psychopharmacology. 1998;140(1):81–90. doi: 10.1007/s002130050742. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson’s disease. Ann Neurol. 1991;30(3):365–374. doi: 10.1002/ana.410300308. [DOI] [PubMed] [Google Scholar]
- Gramling SE, Fowler SC. Effects of neuroleptics on rate and duration of operant versus reflexive licking in rats. Pharmacol Biochem Behav. 1985;22(4):541–545. doi: 10.1016/0091-3057(85)90272-2. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Brain dopamine in Parkinson’s disease and other neurological disturbances. In: Horn AS, Korf J, Westerning BHC, editors. The Neurobiology of Dopamine. London: Academic Press; 1979. pp. 633–654. [Google Scholar]
- Horvitz JC, Choi WY, Morvan C, Eyny Y, Balsam PD. A “good parent” function of dopamine: transient modulation of learning and performance during early stages of training. Ann N Y Acad Sci. 2007;1104:270–288. doi: 10.1196/annals.1390.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC, Eyny YS. Dopamine D2 receptor blockade reduces response likelihood but does not affect latency to emit a learned sensory-motor response: implications for Parkinson’s disease. Behav Neurosci. 2000;114(5):934–939. [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226(2):462–468. [PubMed] [Google Scholar]
- Jahanshahi M, Frith CD. Willed action and its impairments. Cognitive Neuropsychology. 1998;15(6-8):483–533. doi: 10.1080/026432998381005. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118(Pt 4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Kritikos A, Leahy C, Bradshaw JL, Iansek R, Phillips JG, Bradshaw JA. Contingent and non-contingent auditory cueing in Parkinson’s disease. Neuropsychologia. 1995;33(10):1193–1203. doi: 10.1016/0028-3932(95)00036-3. [DOI] [PubMed] [Google Scholar]
- Martin JP. The basal ganglia and posture. London: Pitman Medical; 1967. [Google Scholar]
- McRitchie DA, Cartwright HR, Halliday GM. Specific A10 dopaminergic nuclei in the midbrain degenerate in Parkinson’s disease. Experimental Neurology. 1997;144(1):202–213. doi: 10.1006/exnr.1997.6418. [DOI] [PubMed] [Google Scholar]
- Morvan Campbell C. Mesolimbic dopamine involvement in Pavlovian and operant approach behaviors. Dissertation, Boston College; Boston: 2010. [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135(4):1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Pitts SM, Horvitz JC. Similar effects of D(1)/D(2) receptor blockade on feeding and locomotor behavior. Pharmacol Biochem Behav. 2000;65(3):433–438. doi: 10.1016/s0091-3057(99)00249-x. [DOI] [PubMed] [Google Scholar]
- Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. The factors that induce or overcome freezing of gait in Parkinson’s disease. Behavioural Neurology. 2008;19(3):127–136. doi: 10.1155/2008/456298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Scarnati E, Schultz W. Role of primate basal ganglia and frontal cortex in the internal generation of movements. II. Movement-related activity in the anterior striatum. Experimental Brain Research. 1992;91(3):385–395. doi: 10.1007/BF00227835. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology. 2002;160(4):371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Harper DN, Cameron FB, Abernethy D. Self-initiated versus externally cued reaction times in Parkinson’s disease. J Clin Exp Neuropsychol. 2002;24(2):146–153. doi: 10.1076/jcen.24.2.146.991. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren L, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends in Neurosciences. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27(31):8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Neuroleptics and operant behavior: The anhedonia hypothesis. Behavioral and Brain Sciences. 1982;5:39–87. [Google Scholar]
- Wise RA. Roles for nigrostriatal-not just mesocorticolimbic-dopamine in reward and addiction. Trends in Neurosciences. 2009;32(10):517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28(8):1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004;20(1):249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]