Abstract
The extent of catecholaminergic receptor and respective behavioral alterations associated with prenatal cocaine exposure varies according to exogenous factors such as the amount, frequency, and route of maternal exposure, as well as endogenous factors such as specific brain regions under consideration and sex of the species. The goal of the current study was to use autoradiography to delineate possible moderators of dopaminergic and adrenergic receptor expression in adult rat offspring exposed to cocaine in utero. The current study demonstrated sex-dependent D1 receptor, α2, and noradrenergic transporter binding alterations in prelimbic, hippocampus, and anterior cingulate regions of adult rat brains exposed to cocaine during gestational days 8–21. Of further interest was the lack of alterations in the nucleus accumbens for nearly all receptors/transporters investigated, as well as the lack of alterations in D3 receptor binding in nearly all of the regions investigated (nucleus accumbens, prelimbic region, hippocampus, and cingulate gyrus).
Thus, the current investigation demonstrated persistent receptor and transporter alterations that extend well into adulthood as a result of cocaine exposure in utero. Furthermore, the demonstration that sex played a mediating role in prenatal cocaine-induced, aberrant receptor/transporter expression is of primary importance for future studies that seek to control for sex in either design or analysis.
Keywords: Prenatal Cocaine, Dopamine, Adrenergic, Receptor, Autoradiography, NET
Introduction
The effects of cocaine on the catecholamine systems in the adult brain have been studied extensively (e.g., Peris et al., 1990; Wallace et al., 1996) and multiple theories are more or less well established (e.g., Pierce and Kalivas, 1997). However, less is known about long-term alterations in dopaminergic (DA) and noradrenergic (NE) systems following exposure to cocaine in utero. Nevertheless, it is apparent that in utero cocaine exposure alters the developmental trajectory of DA and NE systems (Mayes et al., 2003; Stanwood et al., 2001a). The extent of alterations depends not only on exogenous factors like the amount, frequency, and route of maternal exposure, but also endogenous factors such as specific neuronal systems under consideration and even sex of the subjects (Gendle, 2002). A meta-analysis of up to 16 independent studies and multiple independent effect sizes indicated that striatal DA receptor affinities and striatal DA levels are moderated by both sex and age in prenatal cocaine exposed animals (Glatt et al., 2000).
There were two goals for the current investigation: First, to continue and extend recent autoradiography work to determine if systematic alterations in brain receptor expression that have been identified during adolescence (Booze et al., 2006; Silvers et al., 2006) extend into adulthood as a result of in utero cocaine exposure. This initial goal stems from multiple studies, including a meta-analysis, which demonstrated age moderation of receptor expression in animals exposed to cocaine in utero (Glatt et al., 2000; Henderson et al., 1991). Since investigations of the effects of prenatal cocaine exposure in early postnatal (Collins and Meyer, 1996; Spear et al., 1989), preweanling (Mactutus, 1999), and adolescent rats (Booze et al., 2006; Silvers et al., 2006) may not continue into adulthood (for review, see Riley and LaFiette, 1996), we chose to utilize rats that had matured to postnatal day (P)395, well into adulthood. The second purpose of this research was to investigate prenatal cocaine mediated brain alterations that are potentially responsible for systematic behavioral differences demonstrated in preweanling (Foltz et al., 2004; Mactutus, 1999) and adult rats (Bayer et al., 2000; Gendle et al., 2003, 2004a). Some behavioral and molecular studies suggest sex may be a moderating - and perhaps even mediating - factor in the expression of alterations resulting from prenatal cocaine exposure (Nordstrom Bailey et al., 2005; Snow et al., 2004; Levin and Seidler, 1993). Specifically, the current investigation seeks to characterize sex-moderated alterations in receptor binding in the context of adult rats exposed to cocaine in utero. This investigation focuses on alterations in receptors from both the DA (D1 & D3) and NE (α2) systems, as well as the transporter from the NE system (NET).
Biogenic amines and catecholaminergic neurons are known to be present prenatally, and have been shown to play an important role in prenatal brain development (Mayes, 1999; Winzer-Serhan et al., 1997, 1999). The soma of DA and NE neurons have been observed in the rat brainstem as early as gestational day (GD) 14 (Olson & Seiger, 1972) as NE neurons in the locus coeruleus (LC) are formed between GD11 and 13 (Bayer et al., 1993). Neurogenesis of DA neurons in the substantia nigra (SN)/ventral tegmental area (VTA) initiates between GD13 and 16 (Gates et al., 2006; Bayer et al., 1993).
Given that neurotransmitters have been shown to play a role in prenatal brain development, the actions of cocaine on the DA and NE systems proximal to the time of their neurogenesis may have potentially profound effects on the developing brain (for review see Levitt et al., 1997). Cocaine binding sites are evident in the fetal rat brain as early as gestational day 15, and by gestational day 20 the Kd of [3H]cocaine binding is similar to the Kd values observed in adulthood (Meyer et al., 1993). Thus, it appears that cocaine may act as a neuroteratogen in early development of the NE system (Mayes, 1999). For example, it is now well established that neurotransmitters such as NE can act as growth factors in early development (Weiss et al., 1998). NE acts as a growth promoting factor in the CNS by controlling such processes as cell proliferation, growth, survival, differentiation, motility and gene expression (Laifenfeld et al., 2002; Sieber-Blum and Ren, 2000). Cocaine, via inhibition of NE reuptake, may therefore interfere with critical developmental processes (Mayes, 1999). For example, recent studies have shown that cocaine alters process outgrowth in locus coeruleus neurons in vitro (Dey and Snow, 2007; Dey et al., 2006, 2007; Snow et al., 2004), and may initiate pro-apoptotic cascades and cytokine response (Dey and Snow, 2007, Dey et al., 2007). With respect to receptors and transporters, profound effects of prenatal cocaine exposure may be expressed as either a delay in development of these proteins, change in receptor subtype expression, or possibly altered levels of receptor/transporter expression.
Brain regions were selected based on physiological density of receptors, and relevance of the region in mediating those behaviors which have been shown to be altered in offspring exposed to cocaine in utero. Specifically, we chose the hippocampus (CA1 and CA2), nucleus accumbens (NAcc; shell and core), cingulate gyrus (CG1 and CG2) and the prelimbic region, which have been implicated in memory/learning (Clark et al., 2005; Broadbent et al., 2005; Hannesson et al., 2001), drug-seeking/motivation (Schmidt et al., 2005; Di Chiara et al., 2004), and/or attention processes (Seamans et al., 1995; Ragozzino et al., 1999; Arnsten et al., 1996). Interestingly, research has shown that tasks which heavily recruit attention resources or reactivity to stress are sensitive enough to highlight sex differences (Gendle et al., 2004a; 2004b), therefore attention may be one cognitive indicator for many of these sex dependent disruptions resulting from prenatal cocaine exposure. This finding supports the idea that receptor alterations in regions thought to subserve attention may differ as a function of sex of the subject. While D1 receptors are located abundantly throughout the CG, prelimbic region and throughout the core and shell of the NAcc, D3 receptors are dense in the NAcc (Levesque et al., 1992). For this reason, we limited D3 receptor autoradiography to the core and shell of the NAcc, whereas D1 receptor autoradiography encompassed the NAcc, prelimbic, CG1, and CG2. NET and α2 autoradiography encompassed all the aforementioned regions (NAcc, prelimbic, CG1, CG2, CA1, and CA2).
Research on sex moderated differences in behavioral expression of prenatal cocaine-induced alterations (Nordstrom Bailey et al., 2005; Delaney-Black et al., 2004; Levin and Seidler, 1993), in combination with evidence for long-term alterations in prefrontal brain areas as a result of prenatal cocaine exposure (Stanwood et al., 2001b; Jones et al., 2000; 1996), predicts that D1 receptors in the prelimbic and CG regions ought to be particularly sensitive to prenatal cocaine exposure, and sex differences. Although evidence for alterations in NE receptor binding in adolescent rats has been demonstrated (Booze et al., 2006), this alteration may not extend to adulthood as evidenced by the attenuation of NE alterations over time (Henderson et al., 1991). Nevertheless, based on α2 receptor adolescent data (Booze et al., 2006), alterations in α2 and NET binding were predicted in our adult sample.
2. Experimental Procedures
2.1. Animals
Female Sprague-Dawley rats were surgically implanted with an intravenous access port (Mactutus et al., 1994) and allowed one week to recuperate before being paired with male rats for breeding. Females were considered to be at gestational day 0 (GD0) when sperm was detected by vaginal lavage. Beginning on GD8, animals received saline or 3.0 mg/kg IV cocaine either once a day (1/day) or twice a day (2/day) until GD21, delivered as a bolus injection. Following delivery litters were culled to 10 pups, balanced by sex. Male and female pups were raised with their dams and post-weaning, were group-housed with same-sex littermates. Pups were weighed daily confirming no differences in body weight between treatment groups, similar to other studies using this model (Mactutus et al. 1994; Mactutus, 1999).
2.2. Tissue Preparation
Autoradiography was performed in accord with a previously established methodology (see Silvers et al., 2006; Harrod et al., 2004; Wallace et al., 1996; Booze and Wallace, 1995). On postnatal day 395, animals were injected with sodium pentobarbital (60 mg/kg i.p.) and transcardially perfused with 50 ml of 0.9% NaCl (37 °C) and 150 ml 0.9% NaCl. The brains were rapidly dissected and immediately frozen for storage (−80°C). Frozen brains were cryostat sectioned (−20°C, 20 µm thick) in the standard coronal plane and thaw-mounted onto Super Frost Plus glass slides (Fisher Scientific, USA). Sections were systematically collected, using stereotaxic coordinates (Paxinos and Watson, 1997), throughout the prelimbic region (2.7 to 3.2 mm), the hippocampus (−3.3 to −3.8 mm), and the cingulate gyrus and the nucleus accumbens (1.2 to 1.7 mm), relative to Bregma. All sections were stored at −80°C until 24 hours prior to processing, when they were switched to −20°C storage.
2.3. D1 receptor (SCH23390) autoradiography
Frozen tissue sections were brought to room temperature and immersed in Tris buffer (pH = 7.4) containing 50mM Tris-HCl, 120mM NaCl, 5mM KCl, 2mM CaCl2, and 1mM MgCl2 for 5 minutes. The sections were then transferred to incubation vials containing Tris buffer (with 1mM ascorbic acid) and 1.0 nM [3H] SCH 23390 (total binding) (Amersham Pharmacia Biotech Inc, NJ, USA), and incubated to equilibrium at room temperature for 60 minutes, according to prior established methods (Silvers et al., 2006; Harrod et al., 2004; Wallace et al., 1996; Booze and Wallace, 1995). Ketanserin (1µM; Research Biochemicals International (RBI), MA, USA) was added to block binding to 5-hydroxytryptamine, 5-hydroxytrytomine, and alpha-2 adrenergic sites. Nonspecific binding was determined in the presence of 5 µM (+) Butaclamol (RBI). Following incubation, binding was terminated by rinsing slides in 4°C assay buffer, 2 × 20 seconds.
2.4. D3 receptor (7-OH-PIPAT) autoradiography
Frozen tissue sections were brought to room temperature and immersed in 50mM Tris buffer (30°C; pH = 7.4) for 30 minutes. Sections were then incubated in buffer containing 50mM Tris-HCl, 40mM NaCl, 100 µM GPP (Sigma), 0.5 µM DTG (RBI), and 0.05 nM [125I]-7-OH-PIPAT (PerkinElmer Life Sciences, Inc., MA, USA (PLS)) for total binding, and incubated at room temperature for 60 minutes. Nonspecific binding was determined in the presence of 10 µM unlabeled 7-OH-DPAT (RBI), using prior established methods (Silvers et al., 2006; Harrod et al., 2004; Wallace et al., 1996; Booze and Wallace, 1995). Following incubation, binding was terminated by the transfer of slides to vials of 4°C assay buffer containing 50mM Tris-HCl (pH 7.4) and 40mM NaCl. In this buffer, sections were washed and agitated for 3 hours, refreshing the buffer every hour.
2.5. α2 adrenergic receptor (Paraiodoclonidine) autoradiography
Frozen tissue sections were brought to room temperature and immersed in 50mM Tris buffer (pH = 7.4) for 30 minutes. The sections were then transferred to incubation buffer containing 50mM Tris-HCl, 5mM MgCl2, 2mM EGTA, 1mM ascorbic acid, 0.01 mM pargyline (Sigma), and 0.45nM [125I] Paraiodoclonidine (PLS) for total binding. Nonspecific binding was determined with the addition of 0.01mM unlabeled RX821002 (RBI), according to prior methods (Silvers et al., 2006; Harrod et al., 2004; Wallace et al., 1996; Booze and Wallace, 1995). Incubation proceeded at room temperature for 90 minutes. Following incubation, binding was terminated by the transfer of slides to vials of 4°C assay buffer where sections were washed 2 × 5 minutes.
2.6. NET (Nisoxetine) autoradiography
Frozen tissue sections were brought to room temperature and immersed in Tris buffer (pH 7.4) containing 50mM Tris-HCl, 300mM NaCl, and 5mM KCl for 20 minutes. The sections were then transferred to incubation buffer containing 3.0 nM [3H] nisoxetine for total binding. Nonspecific binding was determined in alternate sections by the addition of 1 µM mazindol (Sigma), according to prior methods (Silvers et al., 2006; Harrod et al., 2004; Wallace et al., 1996; Booze and Wallace, 1995). Incubation proceeded for 4 hours at 4°C. Binding was terminated by transfer of slides into 4°C buffer and washing 3 × 5 min.
2.7. Imaging
Immediately following the final buffer rinse, all slides were quickly dipped in ice-cold distilled water to remove excess salts, and dried under a stream of cold air for 15 minutes. Dried, radio-labeled tissue sections and appropriate micro-scales (Amersham, Arlington Heights, IL) were apposed to Kodak MS film in light-tight, x-ray cassettes. Slides were apposed to film for 48 hours ([125I] Paraiodoclonidine), 72 hours ([125I]-7-OH-PIPAT), or 5 weeks ([3H] nisoxetine and [3H] SCH 23390) at −80°C. A low-energy intensifying screen (Kodak Biomax Transcreen LE) was included for 3H ligands. The autoradiographic films were developed using the Kodak D-19 Developer for 2 minutes at 20°C under safelight darkroom conditions following a 30 second rinse in running water. Films were fixed in Kodak Rapid Fixer for 5–10 minutes at 20°C followed by a 20 minute rinse in running water (Booze et al., 1996). Autoradiography images were examined with a video camera based densitometric system (MCID-7 imaging software, InterFocus Imaging, Linton, UK) (Silvers et al., 2006; Booze et al., 2006; Harrod et al., 2004). Regional optical density data were expressed as fmol/mg wet weight (Silvers et al., 2006; Booze and Wallace; 1995; Unnerstall et al., 1984; Geary and Wooten., 1983).
Relevant regions of interest for D1 receptor autoradiography included the prelimbic areas, Cingulate Gyrus 1 (CG1), Cingulate Gyrus 2 (CG2), and the core and shell of the Nucleus Accumbens (NAcc). Relevant brain regions of interest for D3 receptor autoradiography included the core and shell of NAcc. Regions of interest for α2 receptor and NET autoradiography included the prelimbic area, CG1, CG2, and Hippocampus (CA1, CA2).
2.8. Data Analyses
First, a mixed model analysis of variance (ANOVA), using specific binding (fmol/mg) in the saline control group as the dependent variable, and with brain region as the within-subjects factor and sex as the between-subjects factor, was used to investigate possible differences in saline baseline densities for each receptor/transporter of interest. Next, given our primary interest in understanding the differential effects of prenatal cocaine exposure on receptor binding for male and female rats, difference scores were calculated for each sex by subtracting mean saline density for each region from individual scores in the 1/day and 2/day cocaine groups. Thus, averaged difference scores represent change in receptor density for a particular region upon administration of cocaine (1/day, 2/day), relative to the saline baseline. Each region of interest was analyzed separately using a 2 (male vs. female) × 2 (1/day vs. 2/day cocaine) between subject ANOVA on change scores as the dependent measure. Planned contrasts were utilized to investigate specific trends, using change scores as the dependent measure. All data analyses were run using SYSTAT 11 statistical software (Systat Software, Inc., San Jose, CA).
3. Results
3.1. D1 receptor (SCH23390) autoradiography
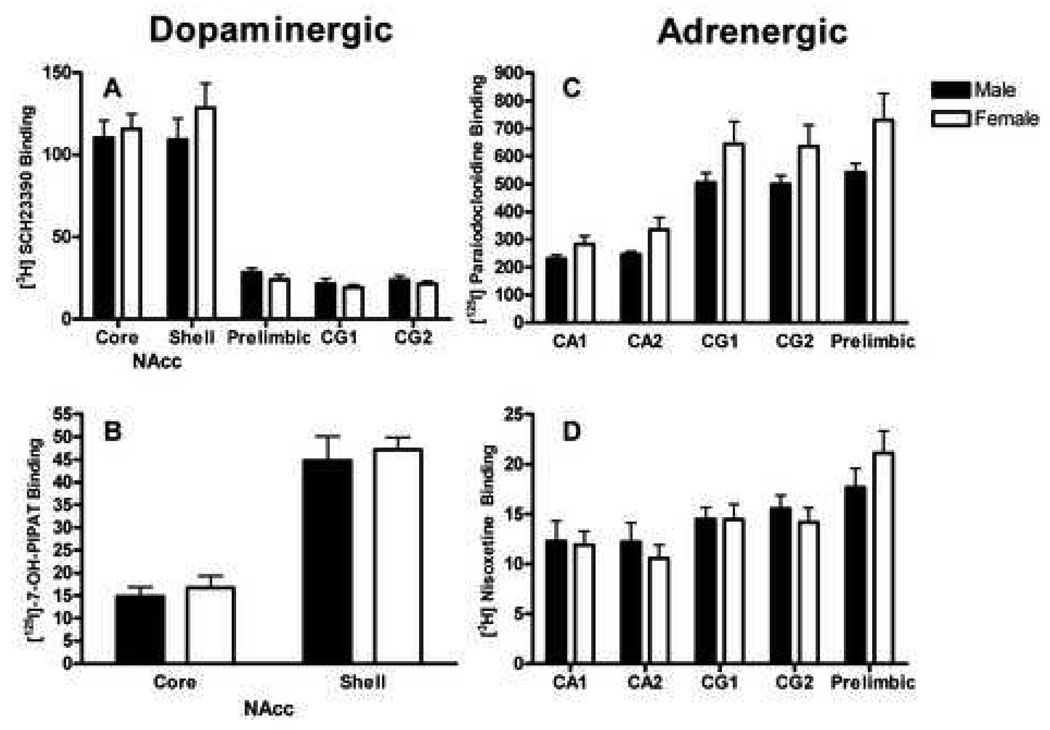
A repeated measures ANOVA on binding in the saline control group indicated a main effect of region, F(4, 88) = 114.83, p ≤ .0001. Figure 1a shows the greatest amount of D1 receptor binding was observed in the NAcc, followed by prelimbic region and CG. There was no Sex × Region interaction, and no main effects of sex in any region within the saline control group.
Figure 1. Saline Baselines.
Specific binding (fmol/mg) for D1 receptors (A), D3 receptors (B), α2 adrenergic receptors (C), and NET (D) in the saline control group. There was a main effect of region for each receptor, however, no main effects of sex or Sex × Region interactions were present for any receptors and regions investigated.
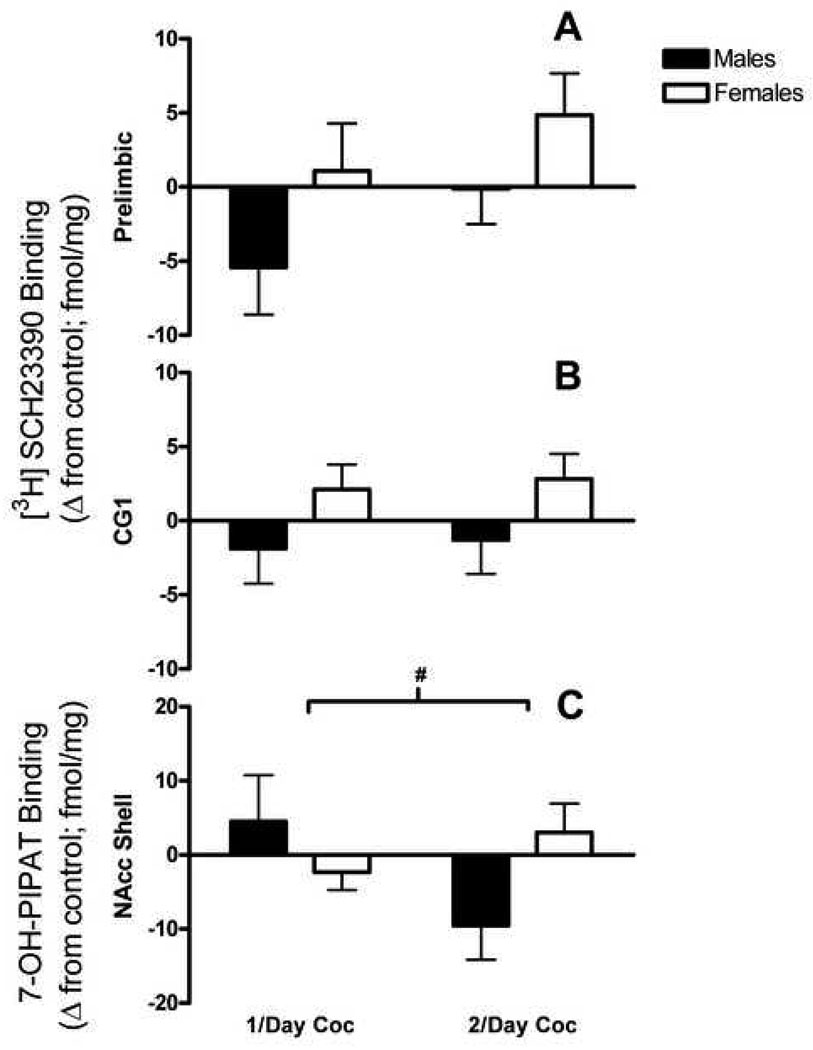
Due to the differences in receptor density for each region in saline animals, separate analyses were performed on each region. A 2 × 2 factorial ANOVA using difference scores as the dependent measure (see section 2.8.) on the prelimbic region revealed a main effect of sex, F(1, 39) = 3.83, p ≤ .05 (Fig 2a), but no main effect of dose or Sex × Treatment interaction. Since the main effect of sex collapses both treatment levels, planned comparisons were utilized to investigate whether an effect of sex is maintained in both the 1/day and 2/day treatment groups. The main effect of sex is maintained only when collapsing the two treatment groups.
Figure 2. SCH23390 and 7-OH-PIPAT (D1 and D3 receptors).
Change scores for males and females relative to saline baseline (fmol/mg) for SCH23390 and 7-OH-PIPAT (D1 and D3 receptors) in the prelimbic region (A), CG1 (B), and NAcc shell (C) for both 1/day and 2/day cocaine groups. For D1 receptors, main effect of sex only present when collapsing treatment groups. For D3 receptors, # = Sex × Treatment interaction.
Similar results were found in areas CG1 and CG2. For CG1, the 2 × 2 ANOVA revealed a main effect of sex, F(1, 39) = 4.16, p ≤ .05. Planned comparisons indicate that the main effect of sex was present only when collapsing the two treatment groups (Fig 2b). For CG2, the ANOVA indicated no main effect of sex, F(1,39) = 2.23, p = .143, dose, or Sex × Dose Interaction. Finally with respect to D1 receptor binding, no main effects or interactions were present in the core and shell of nucleus accumbens (NAcc).
3.2. D3 receptor (7-OH-PIPAT) autoradiography
D3 receptors in the NAcc are significantly lower than D1 receptor binding in the same area, F(1,18) = 102.22, p ≤ .0001 (Fig. 1b). Furthermore, core D3 receptor binding is significantly lower compared to that in the shell, F(1, 18) = 333.06, p ≤ .0001. Despite these regional differences, there are no main effects of treatment or sex, and no interactions in D3 receptor binding in the core of the NAcc. Nevertheless, a significant Sex × Drug Interaction is present in the shell of the NAcc, F(1, 32) = 4.51, p ≤ .05 (Fig 2c).
3.3. α2 receptor (Paraiodoclonidine) autoradiography
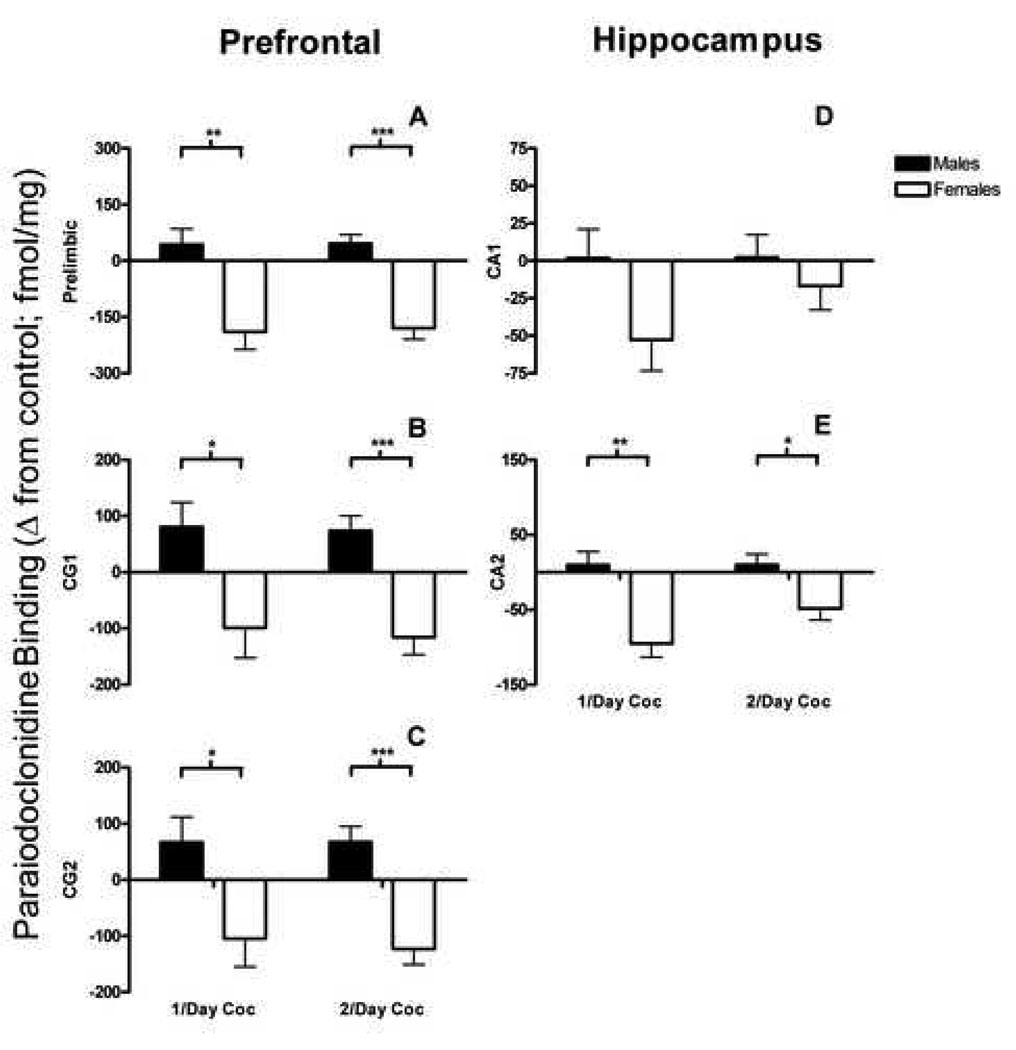
A repeated measures ANOVA on receptor density in the saline control group indicated a main effect of region, F(4, 88) = 68.69, p ≤ .0001. Figure 1c shows the greatest amount of α2 receptor binding was observed in the prelimbic region and CG, followed by the hippocampus (CA) for saline control animals. The 2 × 2 factorial ANOVA using difference scores as the dependent measure (see section 2.8.) on the prelimbic region revealed a main effect of sex, F(1, 41) = 38.73, p ≤ .0001. Since the main effect of sex collapses both treatment levels, planned comparisons were utilized to investigate whether an effect of sex is maintained in both the 1/day and 2/day treatment groups. The main effect of sex is maintained for both the 1/day treatment group, t(20) = 3.69, p ≤ .001, and the 2/day treatment group, t(21) = 5.73, p ≤ .0001. (Fig. 3a).
Figure 3. Paraiodoclonidine (α2 receptors).
Change scores for males and females relative to saline baseline (fmol/mg) for Paraiodoclonidine (α2 receptors) in the prelimbic region (A), CG1 (B), CG2 (C), CA1 (D), and CA2 (E) for both 1/day and 2/day cocaine groups. * = p < .01, ** = p < .001 *** = p < .0001.
Similar results were found for areas CG1 and CG2. For CG1, the 2 × 2 ANOVA revealed a main effect of sex, F(1, 41) = 21.34, p ≤ .0001, but no main effect of dose or Sex × Treatment interaction. Planned comparisons indicate that the main effect of sex was present in the 1/day treatment group, t(20) = 2.60, p ≤ .01, and the 2/day treatment group, t(21) = 4.57, p ≤ .0001 (Fig 3b). For CG2, the ANOVA indicated a main effect of sex, F(1,41) = 22.29, p ≤ .0001. Planned comparisons indicated a main effect for the 1/day group, t(20) = 2.56, p ≤ .01, and the 2/day group, t(21) = 4.90, p ≤ .0001 (Fig. 2=3c).
Significant effects of sex were present for CA1 and CA2, although effect sizes were somewhat attenuated. For CA1, the 2 × 2 ANOVA revealed a main effect of sex, F(1, 40) = 4.23, p ≤ .05, but no main effect of dose or Sex × Treatment interaction. Planned comparisons indicate that the main effect of sex was only present when collapsing the 1/day and 2/day treatment groups (Fig 3d). For CA2, the ANOVA indicated a main effect of sex, F(1,40) = 24.32, p ≤ .0001. Unlike CA1, however, planned comparisons indicated a main effect for the 1/day group, t(20) = 4.06, p ≤ .001, and the 2/day group, t(20) = 2.82, p ≤ .01 (Fig. 3e).
3.4. NET (Nisoxetine) autoradiography
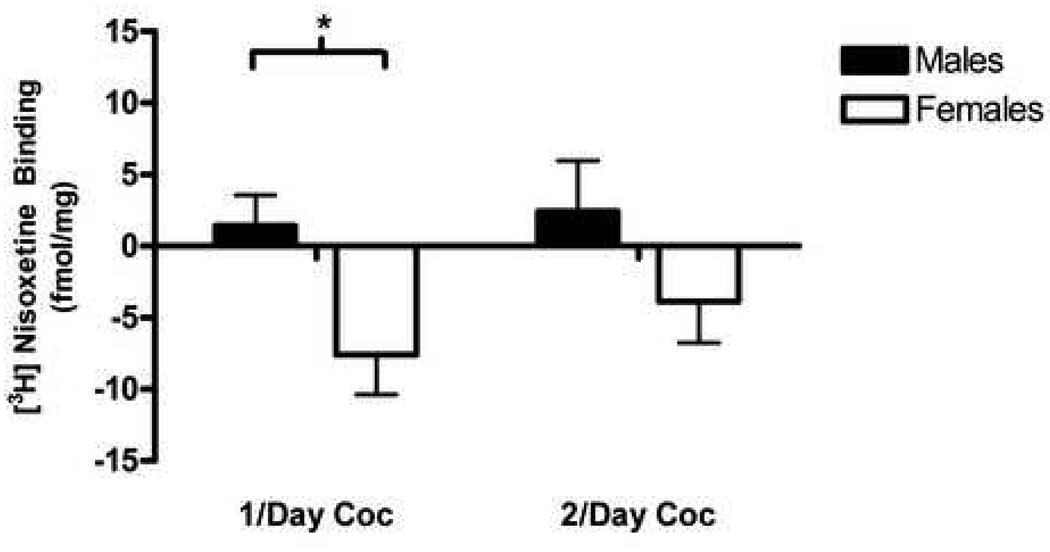
Repeated measures ANOVA on receptor density in the saline control groups indicated a main effect of region, F(4, 88) = 14.44, p ≤ .0001. Figure 1d shows the greatest amount of NET binding was observed in the prelimbic region, followed by CG and hippocampus (CA) for saline control animals. The 2 × 2 ANOVA on change scores indicated a main effect of sex, F(1, 37) = 7.14, p ≤ .01, for the prelimbic region only, as every other region investigated failed to show any main effects or interactions. Planned comparisons indicate that this main effect was maintained only for the 1/day treatment group t(18) = 2.61, p ≤ .01 (Fig. 4).
Figure 4. Nisoxetine (NET).
Change scores for males and females relative to saline baseline (fmol/mg) for Nisoxetine (NET binding) in the prelimbic region for both 1/day and 2/day cocaine groups. 2 × 2 ANOVA indicated a significant main effect of sex. * = p < .01
4. Discussion
4.1. DA receptors, prefrontal areas, and behavior
Age may moderate prenatal cocaine mediated alterations in behavior and brain function (Beeghly et al., 2006; Glatt et al., 2004; 2000). Henderson and colleagues (1991) demonstrated levels of DA were altered as a function of age. Specifically, levels of DA in the striatum were decreased in P30 prenatal cocaine animals while levels of DA in the striatum were increased in P60 prenatal cocaine animals. Furthermore, Glatt and colleagues (2004) have suggested that the onset of puberty (i.e., hormonal influence) may moderate, or even initiate, prenatal cocaine-dependent DA system alterations. These studies (Glatt et al., 2004; Henderson et al., 1991) support the importance of the current investigation, and indicate that DA system alterations in young rats may not be the same as in adult rats. Furthermore, research with rabbits has demonstrated that DA projections to prefrontal areas such as the anterior cingulate are particularly sensitive to prenatal cocaine exposure alterations that persist into adulthood (Levitt et al., 1997). In the current investigation, we demonstrated that CG and prelimbic D1 receptor density was differentially affected by prenatal cocaine exposure in male and female rats well into adulthood. D1 receptor density in CG and prelimbic areas differed by as much as 19% in the group receiving cocaine once a day, and by as much as 20% in the group receiving cocaine twice a day. Therefore, one administration of 3.0 mg/kg a day from GD 8–21, in a model with a pharmacokinetic profile found to be similar to human recreational cocaine abusers (Mactutus et al., 1994), is sufficient to produce long lasting sex differences in D1 receptor densities. It is important to note that these differences are not present in the saline groups. The D1 receptor alterations in these areas may partially account for attention based behavioral discrepancies (Nordstrom Bailey et al., 2005; Delaney-Black, 2004; Gendle et al., 2003; Levin and Seidler, 1993) between prenatally exposed male and female rats.
Indeed, numerous imaging and behavioral studies across a multitude of species have highlighted prefrontal and (pre)limbic area involvement in attention demanding tasks (Seamans et al., 1995; Ragozzino et al., 1999), and DA projections to CG and prelimbic areas are important for associative and attention processes (Granon et al., 2000; Williams et al., 1995). Within this system, D1 receptors have been implicated as playing an essential role in attention demanding tasks (Chudasama and Robbins, 2004; Granon et al., 2000; Williams et al., 1995). For example, Bayer and colleagues (2000) discovered that prenatal cocaine exposure altered rat offspring performance on a sustained attention task via alterations in DA systems thought to subserve attention. In Bayer’s (2000) study, cocaine exposed rats and saline exposed controls were allowed to grow to adulthood and then trained on a 3-choice light discrimination task. Briefly, this task required subjects to make 1-sec nose poke responses to LED indicator lights above the response ports to receive a food pellet reward, as well as learn vigilance tasks based on variations in cue (light) onset and duration. Prior to the testing phase in this task in which an olfactory distracter stimulus was included, both groups of rats were injected with SKF81297, a full D1 receptor agonist. The cocaine exposed group made significantly more omission errors on the task than the saline control group. While this agonist increased omission errors at high doses for both groups (as expected given the research on the negative effects of high level DA release in the prefrontal cortex (e.g., see Williams and Castner, 2006)), only cocaine exposed rats showed impairment at lower doses, demonstrating an increased sensitivity to the attention disrupting effects of SKF81297.
Our demonstration of sex-mediated alterations in D1 receptor binding in animals exposed to cocaine in utero supports the hypothesis that prenatal cocaine exposure differentially alters the DA system(s) thought to subserve sustained attention in male and female rats. These alterations are congruent with Harvey and colleagues hypothesis of morphological alterations and reduced coupling of D1 receptors in the frontocingulate cortex (Harvey, 2004); and related to studies showing adverse effects of prenatal cocaine on attention demanding tasks (Romano et al., 1996; for review see Harvey, 2004). Furthermore, these studies as well as results from the current investigation converge with research showing prenatal cocaine exposure alters “normal” D1 receptor function (e.g., Friedman & Wang, 1998), as well as research on the influence and alterations of D1 receptors associated with postnatal cocaine abuse (for review see Hummel & Unterwald, 2002). Finally with respect to D1 receptors in prefrontal areas, we have demonstrated that these changes extend into adulthood, which highlights the hypothesis that prefrontal DA systems may be enduringly sensitive to the effects of prenatal cocaine exposure when sex is included as a mediating factor.
4.2. α2 receptors, NET, and behavior
It is not always the case that NE alterations persist in aging offspring. An earlier study reported that following prenatal cocaine, a 68% increase in β2-adrenergic receptors was seen on P30, however this increase was not present on P60 or P180 (Henderson et al., 1991). Recent work was performed on adolescent rats exposed to cocaine in utero, and demonstrated cocaine-induced alterations in α2 receptors in the hippocampus, amygdala, and parietal cortex (Booze et al., 2006). These authors also demonstrated sex-dependent alterations in α2 receptor binding in the hippocampus in the 35 day old rats. Thus, one purpose of the current investigation was to understand whether α2 alterations found by Booze and colleagues (2006), as well as alterations in NET, extend into adulthood in many of the same limbic areas demonstrated earlier (in addition to several cortical areas). The robust effects on α2 receptors in all areas under investigation was expected given early evidence from work done in our laboratory (Booze et al., 2006) and among collaborators (Dey and Snow, 2007; Dey et al., 2006, 2007). In the current investigation, we demonstrated that α2 receptor densities were differentially affected by prenatal cocaine exposure in male and female rats well into adulthood. α2 receptor densities in CA, CG and prelimbic areas differed by as much as 34% in the both the group receiving cocaine once a day, and the group receiving cocaine twice a day. Therefore as with D1 receptors, one administration of 3.0 mg/kg a day throughout the second trimester is sufficient to produce long lasting sex differences in α2 receptor densities. These effects, taken together with the lack of α2 receptor alterations demonstrated by Henderson et al. (1991), may implicate α2 receptors as particularly vulnerable to cocaine exposure in utero. Furthermore, the demonstration of prenatal cocaine-induced prefrontal α2 and NET alterations is important in that it has implications for prefrontal DA transmission (Seidler and Slotkin, 1992); as NE has been shown to modulate DA activation, which in turn could have implications for attention processes (Bayer et al., 2002). For example, researchers have demonstrated that the α2 antagonist idazoxan (IDZ), which increases coeruleocortical NE activity under normal conditions, differentially affected prenatal cocaine treated and non-treated rats in measures of attention and regulation of arousal (Bayer et al., 2002). Prenatal cocaine animals were more sensitive to IDZ with respect to several dependent measures of attention. The authors note that this may be due to alterations in NE mediated DA release to prefrontal areas involved in attention; indicative of, and converging with, evidence for enduring prenatal cocaine-induced changes in either or both of these neurotransmitter systems (Seidler and Slotkin, 1992).
The current investigation is also congruent with literature demonstrating prenatal cocaine-induced alterations in the hippocampus. For example, the hippocampus from 60 day old male rats prenatally exposed to cocaine displayed significantly reduced glucose metabolism (Dow-Edwards et al., 1990). Further, electrophysiology studies reported changes following prenatal cocaine that suggest increased hippocampal excitability (Baraban & Schwartzkroin, 1997). Collectively these studies implicate changes the hippocampus as possible mechanisms of prenatal cocaine behavioral effects as early as day 60; and the current study utilized up to 395 day old rats, almost a seven-fold increase in age from many of the studies reported here (e.g., Dow-Edwards et al., 1990). Nevertheless, the relatively smaller effect sizes for α2 alterations in CA1 of the hippocampus (c.f., prefrontal areas) may not be surprising given that prenatal cocaine induced behavioral alterations have been demonstrated mostly in the context of tasks requiring attention processing, and may have less to do with memory processes per se (Gendle et al., 2004b). The hippocampus has been more strongly linked to memory processes than to attention, and research has demonstrated no effect of prenatal cocaine exposure on adult rat performance in a working memory task (Gendle et al., 2004b), but has demonstrated sex-mediated differences in sustained attention and vigilance behavioral tasks (Gendle et al., 2004b).
No main effects of prenatal cocaine exposure in the shell and core of the NAcc were found for any of the receptors/transporters investigated. The lack of any effect in the core and shell of the NAcc for DA and NE receptors is somewhat surprising given the wealth of research demonstrating persistent alterations in accumbal circuitry in the adult abuser (Di Chiara et al., 2004; Hummel & Unterwald, 2002; Pierce and Kalivas, 1997). Indeed, the core and shell of the NAcc have been studied and implicated extensively in adult drug abuse and motivation research (Di Chiara et al., 2004; Pierce and Kalivas, 1997). In fact, models of addiction and sensitization for both licit and illicit substances often includes the NAcc as a major substrate for reward/drug seeking (e.g., Kalivas & Volkow, 2005), and expression of behavioral sensitization (Pierce & Kalivas, 1997). Thus, this investigation highlights potential region based discrepancies in prenatal cocaine-induced receptor alterations, whereby prefrontal areas may be particularly vulnerable to long-term effects. Furthermore, it highlights the importance of including sex as a mediating factor in adult receptor expression.
4.3. Sex differences
Unfortunately, a large number of published investigations on the effects prenatal cocaine exposure do not account for sex as a moderating or mediating factor in either design or analysis. This may be partially responsible for disparate results so common in the literature (Gendle, 2002). For example, those studies which collapse males and females may attenuate treatment effects by decreasing differences between treatment and control groups, and increasing within-subject heterogeneity, thereby increasing within-subject error. These studies might readily report false negatives. Meta-analyses attest to the attenuation of the size of an effect (or even loss of a statistical effect) when collapsing across moderators such as sex and age (Glatt et al., 2000). Furthermore, the culmination of multiple but methodologically distinct lines of investigation within the last few years is beginning to highlight the differential effects prenatal cocaine exposure between male and female rats (Nordstrom Bailey et al., 2005; Delaney-Black et al., 2004; Brunzell et al., 2002; Levin and Seidler, 1993).
In humans, researchers have found that 6 and 7 year old boys who were prenatally exposed to cocaine had significantly more cognitive and behavioral problems than boys and girls from the same cohort who were never exposed to cocaine, as well as girls from the same cohort who were prenatally exposed to cocaine (Nordstrom Bailey et al., 2005). Similarly, others have reported behavioral discrepancies between male and female rat offspring exposed prenatally to cocaine (Gendle et al., 2003; Brunzell et al., 2002; Levin and Seidler, 1993). Gendle and colleagues (2003) demonstrated that while both males and females exposed to 0.5 mg/kg and 1.0 mg/kg were generally impaired on a visual sustained attention task relative to control male and female rats, exposed male and female rates also demonstrated disparate behavioral strategies on the task. Specifically, cocaine-exposed males engaged the task regardless of increased errors, while cocaine-exposed females did not engage the task, indicating a possible altered response to stress. Even more notably, only males exposed to 3.0 mg/kg showed sustained attention deficits relative to controls. Females at this high dose were unaffected relative to controls.
The current investigation highlights the importance of understanding aberrant receptor expression not simply as a function of being exposed prenatally to cocaine, but dependent on many endogenous and exogenous factors; including the amount and number of exposures, time since exposure (i.e., age), characteristics of sex, and brain region/system under consideration. We found little to no effect when collapsing across sex, similar to negligible effect sizes found that have been suggested in meta-analytical investigations (Glatt et al., 2000); and we found little effect even within sex in these adult rats. However, when using sex as a mediating factor of prenatal cocaine exposure, we discovered alterations in receptor expression differ between male and female rats. It is not the case, however, that control (i.e., non-exposed) male and female rats differ in receptor expression in adulthood. Interestingly, we have also reported sex differences in adolescent animals (Booze et al., 2006; Silvers et al., 2006).
4.4. Conclusion
The current investigation further demonstrates that the effect of prenatal cocaine exposure on aberrant receptor expression is contingent on multiple endogenous (sex, age) and exogenous (dose, route of administration) factors, which is likely the source of variability in effects demonstrated in the literature. This study also demonstrates that aberrant prenatal cocaine-induced receptor expression differs as a function of sex well into adulthood, and these differences may account for a significant portion of variance in sex-dependent behavioral discrepancies in offspring exposed to cocaine in utero. The findings in the current investigation are congruent with prenatal cocaine research on prefrontal DA/NE projections (Stanwood et al., 2001b; Jones et al., 2000; 1996), sex/gender moderated behavioral discrepancies (Delaney-Black et al., 2004; Gendle et al., 2003; Brunzell et al., 2002), and receptor coupling abnormalities (see Harvey, 2004) which have been shown to be among the few consistent effects of prenatal cocaine exposure found that extend into adulthood.
Acknowledgments
Supported by NIH Grants: DA013712, DA014401 (RMB), DA013965 (BJS), DA009160, and HD043680 (CFM)
List of Abbreviations
- DA
Dopamine
- NE
Noradrenergic
- NET
NE transporter
- D1, D3, α2
Dopamine and NE receptors
- GD
gestational day
- P
postnatal day
- IDZ
Idazoxan
- CA1, CA2
Hippocampal areas
- CG1, CG2
Cingulate Gyrus
- NAcc
Nucleus Accumbens
- SN
Substantia Nigra
- VTA
Ventral Tegmental Area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson NH. Empirical Direction in Design and Analysis. Mahwah, New Jersey: Lawrence Erlbaum Associates, Inc.; 2001. [Google Scholar]
- Arnsten AFT, Steere JC, Hunt RD. The contribution of alpha-2 noradrenergic mechanisms to prefrontal cortical cognitive function. Arch. Gen. Psych. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Schwartzkroin PA. Effects of prenatal cocaine exposure on the developing hippocampus: intrinsic and synaptic physiology. J. Neurophysiol. 1997;77:126–136. doi: 10.1152/jn.1997.77.1.126. [DOI] [PubMed] [Google Scholar]
- Bayer LE, Kakumanu S, Mactutus CF, Booze RM, Strupp BJ. Prenatal cocaine exposure alters sensitivity to the effects of idazoxan in a distraction task. Behav. Brain Res. 2002;133:185–196. doi: 10.1016/s0166-4328(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Bayer LE, Brown A, Mactutus CF, Booze RM, Strupp BJ. Prenatal cocaine exposure increases sensitivity to the attentional effects of the dopamine D1 agonist SKF81297. J. Neuroscience. 2000;20:8902–8908. doi: 10.1523/JNEUROSCI.20-23-08902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Beeghly M, Martin B, Rose-Jacobs R, Cabral H, Heeren T, Augustyn M, Bellinger D, Frank DA. Prenatal cocaine exposure and children’s language function at 6 and 9.5 years: moderating effects of age, birthweight, and gender. J. Pediatr. Psychol. 2006;31:98–115. doi: 10.1093/jpepsy/jsj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Wallace DR, Silvers JM, Strupp BJ, Mactutus CM. Prenatal cocaine exposure alters alpha2 receptor expression in adolescent rats. BMC Neuroscience. 2006;7:33. doi: 10.1186/1471-2202-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Wallace DR. Dopamine D2 and D3 receptors in the rat striatum and nucleus accumbens: use of 7-OH-DPAT and [125I]-iodosulpride. Synapse. 1995;19:1–13. doi: 10.1002/syn.890190102. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learn. Mem. 2005;13:187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Coy AE, Ayres JJ, Meyer JS. Prenatal cocaine effects on fear conditioning: exaggeration of sex-dependent context extinction. Neurotoxicol. Teratol. 2002;24:161–172. doi: 10.1016/s0892-0362(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Meyer JS. Prenatal cocaine alters dopamine transporter binding in postnatal day 10 rat striatum. Synapse. 1996;23:335–343. doi: 10.1002/(SICI)1098-2396(199608)23:4<335::AID-SYN12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, Chiodo L, Sokol RJ. Prenatal cocaine: quantity of exposure and gender moderation. J. Dev. Behav. Pediatr. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Dey S, Mactutus CF, Booze RM, Snow DM. Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons by altering the Bax/Bcl-2 ratio and through caspase-3 apoptotic signaling. Neuroscience. 2007;144:509–521. doi: 10.1016/j.neuroscience.2006.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Snow DM. Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons through TNG-alpha-mediated induction of Bax and phosphorylated c-Jun NH(2)-terminal kinase. J. Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04750.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dey S, Mactutus CF, Booze RM, Snow DM. Specificity of prenatal cocaine on inhibition of locus coeruleus neurite outgrowth. Neuroscience. 2006;139:899–907. doi: 10.1016/j.neuroscience.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47 Suppl. 1:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards DL, Freed LA, Fico TA. Structural and functional effects of prenatal cocaine exposure in adult rat brain. Brain Res. Dev. Brain Res. 1990;57:263–268. doi: 10.1016/0165-3806(90)90052-z. [DOI] [PubMed] [Google Scholar]
- Foltz TL, Snow DM, Strupp BJ, Booze RM, Mactutus CF. Prenatal intravenous cocaine and the heart rate-orienting response: a dose-response study. Int. J. Dev. Neuroscience. 2004;22:285–296. doi: 10.1016/j.ijdevneu.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Friedman E, Wang HY. Prenatal cocaine exposure alters signal transduction in the brain D1 dopamine receptor system. Ann. NY Acad. Sci. 1998;846:238–247. doi: 10.1111/j.1749-6632.1998.tb09741.x. [DOI] [PubMed] [Google Scholar]
- Gates MA, Torres EM, White A, Fricker-Gates RA, Dunnett SB. Re-examining the ontogeny of substantia nigra dopamine neurons. Eur. J. Neurosci. 2006;23:1384–1390. doi: 10.1111/j.1460-9568.2006.04637.x. [DOI] [PubMed] [Google Scholar]
- Geary WA, 2nd, Wooten GF. Quantitative film autoradiography of opiate and antagonist binding in rat brain. J. Pharmacol. Exp. Ther. 1983;225:234–240. [PubMed] [Google Scholar]
- Gendle MH, White TL, Strawderman M, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Enduring effects of prenatal cocaine exposure on selective attention and reactivity to errors: evidence from an animal model. Behav. Neuroscience. 2004a;118:290–297. doi: 10.1037/0735-7044.118.2.290. [DOI] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Prenatal cocaine exposure does not alter working memory in adult rats. Neurotoxicol. Teratol. 2004b;26:319–329. doi: 10.1016/j.ntt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Developmental Brain Research. 2003;147:85–96. doi: 10.1016/j.devbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gendle MH. Increases, reductions, or no effect? The puzzle of neural findings from animal models of prenatal cocaine exposure. J. Drug Issues. 2002;32:1–10. [Google Scholar]
- Glatt SJ, Trksak GH, Cohen OS, Simeone BP, Jackson D. Prenatal cocaine exposure decreases nigrostriatal dopamine release in vitro: effects of age and sex. Synapse. 2004;53:74–89. doi: 10.1002/syn.20036. [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Bolanos CA, Trksak GH, Jackson D. Effects of prenatal cocaine exposure on dopamine system development: a meta-analysis. Neurtoxicol. Teratol. 2000;22:617–629. doi: 10.1016/s0892-0362(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J. Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson DK, Mohapel P, Corcoran ME. Dorsal hippocampal kindling selectively impairs spatial learning/short-term memory. Hippocampus. 2001;11:275–286. doi: 10.1002/hipo.1042. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol. Biochem. Behav. 2004;78:1–5. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Cocaine effects on the developing brain: current status. Neurosci. Biobehav. Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Henderson MG, McConnaughey MM, McMillen BA. Long-term consequences of prenatal exposure to cocaine or related drugs: effects on rat brain monoaminergic receptors. Brain Res. Bull. 1991;26:941–945. doi: 10.1016/0361-9230(91)90261-h. [DOI] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J. Cell Physiol. 2002;191:17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- Jones LB, Stanwood GD, Reinoso BS, Washington RA, Wang H-Y, Friedman E, Levitt P. In utero cocaine-induced dysfunction of D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J. Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LB, Fisher I, Levitt P. Nonuniform alteration of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cereb. Cortex. 1996;6:431–435. doi: 10.1093/cercor/6.3.431. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Laifenfeld D, Klein E, Ben-Shachar D. Norepinephrine alters the expression of genes involved in neuronal sprouting and differentiation: relevance for major depression and antidepressant mechanisms. J. Neurochem. 2002;83:1054–1064. doi: 10.1046/j.1471-4159.2002.01215.x. [DOI] [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc. Natl. Acad. Sci. USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Seidler FJ. Sex-related spatial learning differences after prenatal cocaine exposure in the young adult rat. Neurotoxicology. 1993;14:23–28. [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Mactutus CF. Prenatal intravenous cocaine adversely affects attentional processing in preweanling rats. Neurotoxicol. Teratol. 1999;21:539–550. doi: 10.1016/s0892-0362(99)00024-0. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug (ab)use in the pregnant and/or group housed rat: An initial study with cocaine. Neurotoxicol. Teratol. 1994;16:183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Cicchetti D, Acharyya S, Zhang H. Developmental trajectories of cocaine-and-other-drug-exposed and non-cocaine-exposed children. J. Dev. Behav. Pediatr. 2003;24:323–335. doi: 10.1097/00004703-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: effects of neural ontogeny. Dev. Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Shearman LP, Collins LM, Maguire RL. Cocaine binding sites in fetal rat brain: Implications for prenatal cocaine action. Psychopharmacology. 1993;112:445–451. doi: 10.1007/BF02244892. [DOI] [PubMed] [Google Scholar]
- Nordstrom Bailey B, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, Covington C, Delaney-Black V. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol. Teratol. 2005;27:181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Olson L, Seiger A. Early prenatal ontogeny of central monoamine neurons in the rat: fluorescent histochemical observations. Anat. Ent-Gesh. 1972;137:301–316. doi: 10.1007/BF00519099. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Third Edition. CA: Academic Press; 1997. [Google Scholar]
- Peris J, Boyson SJ, Cass WA, Curella P, Dwoskin LP, Larson G, Lin LH, Yasuda RP, Zahniser N. Persistence of neurochemical changes in dopamine systems after repeated cocaine administration. J. Pharmacol. Exp. Therap. 1990;167:38–44. [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav. Neurosci. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lafiette MH. The effects of prenatal cocaine exposure on subsequent learning in the rat. NIDA Res. Monogr. 1996;164:53–77. [PubMed] [Google Scholar]
- Romano AG, Harvey JA. Prenatal exposure to cocaine disrupts discrimination learning in adult rabbits. Pharmacol. Biochem. Behav. 1996;53:617–621. doi: 10.1016/0091-3057(95)02061-6. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur. J. Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingluate regions of the rat prefrontal cortex. Behav. Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J. Pharmacol. Exp. Therap. 1992;263:413–421. [PubMed] [Google Scholar]
- Sieber-Blum M, Ren Z. Norepinephrine transporter expression and function in noradrenergic cell differentiation. Mol. Cell Biochem. 2000;212:61–71. [PubMed] [Google Scholar]
- Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM. Prenatal cocaine alters dopamine and sigma receptor binding in the nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol. Teratol. 2006;28:173–180. doi: 10.1016/j.ntt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Snow DM, Carman HM, Smith JD, Booze RM, Welch MA, Mactutus CF. Cocaine-induced inhibition of process outgrowth in locus coeruleus neurons: role of gestational exposure period and offspring sex. Int. J. Dev. Neurosci. 2004;22:899–907. doi: 10.1016/j.ijdevneu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Spear LP, Kirstein CL, Bell J, Yoottanasumpun V, Greenbaum R, O’Shea J, Hoffmann H, Spear NE. Effects of prenatal cocaine exposure on behavior during the early postnatal period. Neurotoxicol. Teratol. 1989;11:57–63. doi: 10.1016/0892-0362(89)90086-x. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001a;106:5–14. doi: 10.1016/s0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Levitt P. Identification of sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb. Cortex. 2001b;11:430–440. doi: 10.1093/cercor/11.5.430. [DOI] [PubMed] [Google Scholar]
- Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res. 1984;319:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Mactutus CF, Booze RM. Repeated intravenous cocaine administration: Locomotor activity and dopamine D2/D3 receptors. Synapse. 1996;23:152–163. doi: 10.1002/(SICI)1098-2396(199607)23:3<152::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Weiss ER, Maness P, Lauder JM. Why do neurotransmitters act like growth factors? Perspect. Dev. Neurobiol. 1998;5:323–335. [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in the prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha 2A adrenoceptors during rat neocortical development. J. Neurobiol. 1999;38:259–269. doi: 10.1002/(sici)1097-4695(19990205)38:2<259::aid-neu8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of alpha 2 adrenoceptors during rat brain development--I. Alpha 2A messenger RNA expression. Neuroscience. 1997;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]