Abstract
Context
The dramatic increase in use of cellular telephones has generated concern about possible negative effects of radiofrequency signals delivered to the brain. However, whether acute cell phone exposure affects the human brain is unclear.
Objective
To evaluate if acute cell phone exposure affects brain glucose metabolism, a marker of brain activity.
Design, Setting, and Participants
Randomized crossover study conducted between January 1 and December 31, 2009, at a single US laboratory among 47 healthy participants recruited from the community. Cell phones were placed on the left and right ears and positron emission tomography with (18F)fluorodeoxyglucose injection was used to measure brain glucose metabolism twice, once with the right cell phone activated (sound muted) for 50 minutes (“on” condition) and once with both cell phones deactivated (“off” condition). Statistical parametric mapping was used to compare metabolism between on and off conditions using paired t tests, and Pearson linear correlations were used to verify the association of metabolism and estimated amplitude of radiofrequency-modulated electromagnetic waves emitted by the cell phone. Clusters with at least 1000 voxels (volume >8 cm3) and P < .05 (corrected for multiple comparisons) were considered significant.
Main Outcome Measure
Brain glucose metabolism computed as absolute metabolism (µmol/100 g per minute) and as normalized metabolism (region/whole brain).
Results
Whole-brain metabolism did not differ between on and off conditions. In contrast, metabolism in the region closest to the antenna (orbitofrontal cortex and temporal pole) was significantly higher for on than off conditions (35.7 vs 33.3 µmol/100 g per minute; mean difference, 2.4 [95% confidence interval, 0.67–4.2]; P = .004). The increases were significantly correlated with the estimated electromagnetic field amplitudes both for absolute metabolism (R = 0.95, P < .001) and normalized metabolism (R = 0.89; P < .001).
Conclusions
In healthy participants and compared with no exposure, 50-minute cell phone exposure was associated with increased brain glucose metabolism in the region closest to the antenna. This finding is of unknown clinical significance.
The dramatic worldwide increase in use of cellular telephones has prompted concerns regarding potential harmful effects of exposure to radiofrequency-modulated electromagnetic fields (RF-EMFs). Of particular concern has been the potential carcinogenic effects from the RF-EMF emissions of cell phones. However, epidemiologic studies of the association between cell phone use and prevalence of brain tumors have been inconsistent (some, but not all, studies showed increased risk), and the issue remains unresolved.1
RF-EMFs emitted by cell phones are absorbed in the brain2 within a range that could influence neuronal activity.3 Although the intensity of RF-EMFs is very low, the oscillatory frequencies correspond to some of the oscillation frequencies recorded in neuronal tissue and could interfere with neuronal activity.4 Thermal effects from RF-EMFs have also been invoked as a mechanism that could affect neuronal activity, although temperature changes produced by current cell phone technology are likely minimal.5 Studies performed in humans to investigate the effects of RF-EMF exposures from cell phones have yielded variable results.6 For example, imaging studies that used positron emission tomography (PET) to measure changes in cerebral blood flow (CBF) with RF-EMF exposures from cell phones have reported increases,7,8 decreases and increases,9,10 or no changes11 in CBF. The discrepancies among these imaging studies likely reflect their relatively small sample sizes (9–14 participants), and the potential confounding of CBF measures reflecting vascular rather than neuronal signals.12–14 This highlights the need for studies to document whether RF-EMFs from cell phone use affects brain function in humans.
The objective of this study was to assess if acute cell phone exposure affected regional activity in the human brain. For this purpose we evaluated the effects in healthy participants (N = 47) of acute cell phone exposures on brain glucose metabolism, measured using PET with injection of (18F)fluorodeoxyglucose (18FDG). Brain glucose metabolic activity is a more proximal marker of neuronal activity than measures of CBF, which reflects vascular as well as neuronal components.15 Also, because brain glucose metabolic measures obtained with 18FDG reflect the averaged brain activity occurring over a 30-minute period,16 this method allowed assessment of the cumulative effects of cell phone exposure on resting brain metabolism. Because exposure to RF-EMFs from cell phones is well localized and is highest in brain regions closest to the antenna,2 we hypothesized that the effects on brain metabolism would be greatest in inferior and anterior brain regions, the regions that would be exposed to the highest RF-EMF amplitude for the cell phone model used in this study.
METHODS
Participants
The study was conducted at Brookhaven National Laboratory from January 1, 2009, through December 31, 2009, and was approved by the local institutional review board (Committee on Research Involving Human Subjects, Stony Brook University). We enrolled 48 healthy participants recruited from advertisements in local newspapers and screened for absence of medical, psychiatric, or neurologic diseases. Special attention was given to ensure that participants did not abuse addictive substances (including alcohol and nicotine), and urine toxicology studies were performed prior to the imaging sessions to ensure lack of psychoactive drug use. For technical reasons, data from one of the participants could not be used (see below). Table 1 provides demographic characteristics and cell phone usage histories of the 47 participants whose data were used in the analysis. Participants each received $250 for their participation in the study ($200 for PET scans [$100 per scan] plus $50 for the physical examination and laboratory work). All participants provided written informed consent after receiving a complete description of the study.
Table 1.
Characteristics and Cellular Telephone Histories of Participants (N = 47)
| Characteristic | No. (%) | |
|---|---|---|
| Age, mean (SD), y | 31 (9) | |
| Sex | ||
| Men | 23 (48.9) | |
| Women | 24 (51.1) | |
| Body mass index, mean (SD)a | 26 (3) | |
| Handedness | ||
| Right-handed | 43 (91.5) | |
| Left-handed | 4 (8.5) | |
| Education mean (SD), y | 14 (2) | |
| Cell phone use, mean (SD) [range], min/mo |
1500 (1850) [15–9000] |
|
| Ear favored for use | ||
| Right | 38 (80.9) | |
| Left | 9 (19.1) | |
Calculated as weight in kilograms divided by height in meters squared.
Experimental Conditions
All participants had 2 scans performed on separate days using PET with 18FDG injection under resting conditions. For both scans 2 cell phones, one placed on the left ear and one on the right, were used to avoid confounding effects from the expectation of a signal from the side of the brain at which the cell phone was located. For one of the days both cell phones were deactivated (“off” condition). For the other day the right cell phone was on (activated but muted to avoid confounding from auditory stimulation) and the left cell phone was off (“on” condition). For the on condition the cell phone was receiving a call (from a recorded text), although the sound was muted. The order of conditions was randomly assigned, and participants were blinded to the condition. The mean time between the first and the second study was 5 (SD, 3) days.
Two Samsung model SCH-U310 cell phones, capable of transmitting at either cellular or personal communications service frequency bands with code division multiple access modulation, were used for each study. The maximum specific absorption rate in the head for this cell phone model corresponds to 0.901 W/kg. Cell phones were placed over each ear with microphones directed toward the participant’s mouth and were secured to the head using a muffler that did not interfere with the lower part of the cell phone, where the antenna is located. Activation of the right cell phone was started 20 minutes prior to 18FDG injection and maintained for 30 minutes afterward to correspond with the 18FDG uptake period. During the 50-minute period participants sat on a comfortable chair in a quiet, dimly lit room and with their eyes open, with a nurse present to ensure that they kept their eyes open and did not fall asleep.
The RF-EMF emissions were recorded once before the call (background) and every 5 minutes during the stimulation period to ensure that the call was not terminated. This was accomplished with a handheld spectrum analyzer (model FSH6; Rohde & Schwarz, Munich, Germany) connected to a cellular wide-band log periodic directional antenna (model 304411; Wilson Electronics, St. George, Utah) aimed at the head from a distance of 3 feet. The cellular band was active, with a frequency of 837.8 MHz. This frequency was monitored with a resolution bandwidth of 1 MHz. Activation of the cell phone for the experimental period was also corroborated with the records obtained from the cell phone company. For 1 participant the cell phone signal was interrupted at the time of 18FDG injection; this participant’s data were not included in the analysis.
PET Scanning
In preparation for the study, participants had 2 venous catheters placed, one in the antecubital vein for radiotracer injection and the other in a superficial vein on the dorsal surface of the hand for sampling of arterialized blood. Arterialization was achieved by warming the hand to 44°C. The participants were injected with 18FDG (148–222 MBq [to convert to millicuries, divide by 37]) and asked to refrain from moving or speaking during the 30-minute 18FDG uptake period. At the end of the sessions, the cell phones were removed and the participants were positioned in the PET scanner as previously described.17 Participants were scanned with a whole-body tomograph (ECAT HR+; Siemens/CTI, Munich, Germany), with a resolution of 4.6 × 4.6 × 4.2 mm3 as measured by National Electrical Manufacturers Association protocols. Emission scans were started 35 minutes after 18FDG injection and lasted 20 minutes. Transmission scans were performed simultaneously.
Radiofrequency Field
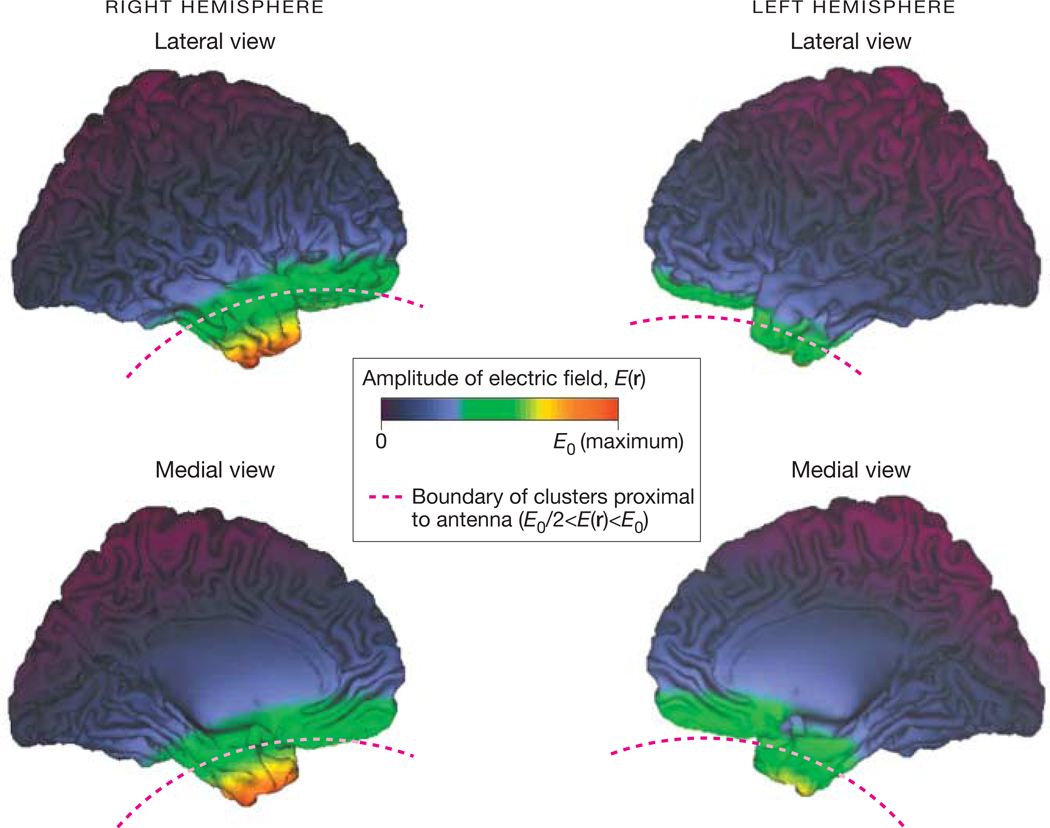
The average position of the antenna in the stereotactic space of the Montreal Neurological Institute (r0) (r0 = 21 [SD, 10] mm for x [left to right], 30 [SD, 11] mm for y [anterior to posterior], −160 [SD, 7] mm for z [superior to inferior]) was determined for 21 participants using calibrated orthogonal photography that registered orthogonal views (front and sides) of the cell phone positions on the participant’s head. The positions of the eyes were used as landmarks to determine r0 with the aid of the standard brain template (ch2.nii) provided in MRI-cron (available at http://www.sph.sc.edu/comd/rorden/mricron/). The relative amplitude of the cell phone’s electric field, E (r), at every position in the brain, r, was computed in Interactive Data Language version 6.0 (ITT Visual Information Solutions, Boulder, Colorado) using the far-field approximation, E (r) ~ ‖r−r0‖−3, of a dipole field (Figure 1).
Figure 1. Amplitude of the Electric Field Emitted by the Right Cellular Telephone Antenna Rendered on the Surface of the Human Brain.
E0 indicates maximal field value. Clusters proximal to the antenna are inferior to the red dashed line. Images created using the freeware Computerized Anatomical Reconstruction and Editing Toolkit (CARET) version 5.0 (http://brainvis.wustl.edu/wiki/index.php/Caret:About).
Image Analysis
The data were analyzed using statistical parametric mapping (SPM) in the SPM2 mapping package (Welcome Department of Cognitive Neurology, London, United Kingdom).18 The SPM analyses were performed on the absolute as well as the normalized (to whole-brain metabolism) metabolic images. For this purpose, the images were spatially normalized using the SPM2 PET template and a 2-mm3 × 2-mm3 × 2-mm3 voxel size and were subsequently smoothed with an 8-mm isotropic Gaussian kernel. Voxel-wise paired t tests were used to assess regional changes in glucose metabolism.
Because the electric field, E (r), produced by the cell phone decreases rapidly with distance to the antenna, we hypothesized that the effects of cell phones on glucose metabolism would occur in regions close to the antenna and that the regions far from the antenna would show no effects. Therefore, the corrections for multiple comparisons were restricted to brain regions in which E (r) was higher than 50% of the maximum field value, E0, in the brain (E0/2 < E (r) < E0) (Figure 1). Thus, the Bonferroni method with a searching volume (Sv) of 201.3 cm3 (Sv = 25 161 voxels) was used to correct cluster-level P values for multiple comparisons as a function of the cluster volume (Cv) (Pcorr = P × Sv/Cv). Clusters with at least 1000 voxels (Cv >8 cm3) and P < .05 (corrected for multiple comparisons) were considered significant.
A simple model assuming a linear relationship between cell phone–related increases in metabolism (Δ18FDG; average across participants) and E was used. The paired values (Δ18FDGi, Ei) from all voxels that were statistically significant in the SPM2 t test analyses contrasting on vs off conditions within Sv were sorted by E, clustered in groups of 50 voxels, and averaged. These clusters were treated as independent. The Pearson linear correlation factor, R, was used to assess the linear relationship between Δ18FDG and E in Interactive Data Language version 6.0.
The sample-size calculation was based on our preliminary study of the effect of low-frequency magnetic field gradients in glucose metabolism,19 which demonstrated metabolic differences between stimulation and sham conditions with effect size (ratio between the mean difference and the pooled standard deviation) between 0.65 and 0.80. The minimal important difference in glucose metabolism used to determine the sample size was 1 µmol/100 g per minute. For such effect sizes, to achieve a power of at least 80% using the independent-samples t test with a significance level of .05, at least 40 participants were needed.
RESULTS
Whole-brain glucose metabolism did not differ between conditions, which for the off condition corresponded to 41.2 µmol/100 g per minute (95% confidence interval [CI], 39.5–42.8) and for the on condition to 41.7 µmol/100 g per minute (95%CI, 40.1–43.3). However, there were significant regional effects. Specifically, the SPM comparisons14 on the absolute metabolic measures showed significant increases (35.7 vs 33.3 µmol/100 g per minute for the on vs off conditions, respectively; mean difference, 2.4 [95% CI, 0.67–4.2]; P = .004) in a region that included the right orbitofrontal cortex (BA11/47) and the lower part of the right superior temporal gyrus (BA 38) (Figure 2 and Table 2). No areas showed decreases. Similar results were obtained for the SPM analysis of the normalized metabolic images (normalized to whole-brain glucose metabolism), which also showed significant increases (1.048 vs 0.997 for the on vs off conditions, respectively; mean difference, 0.051 [95% CI, 0.017–0.091]; P < .001) in a region that included right orbitofrontal cortex and right superior temporal gyrus (BA 38) (Figure 2).
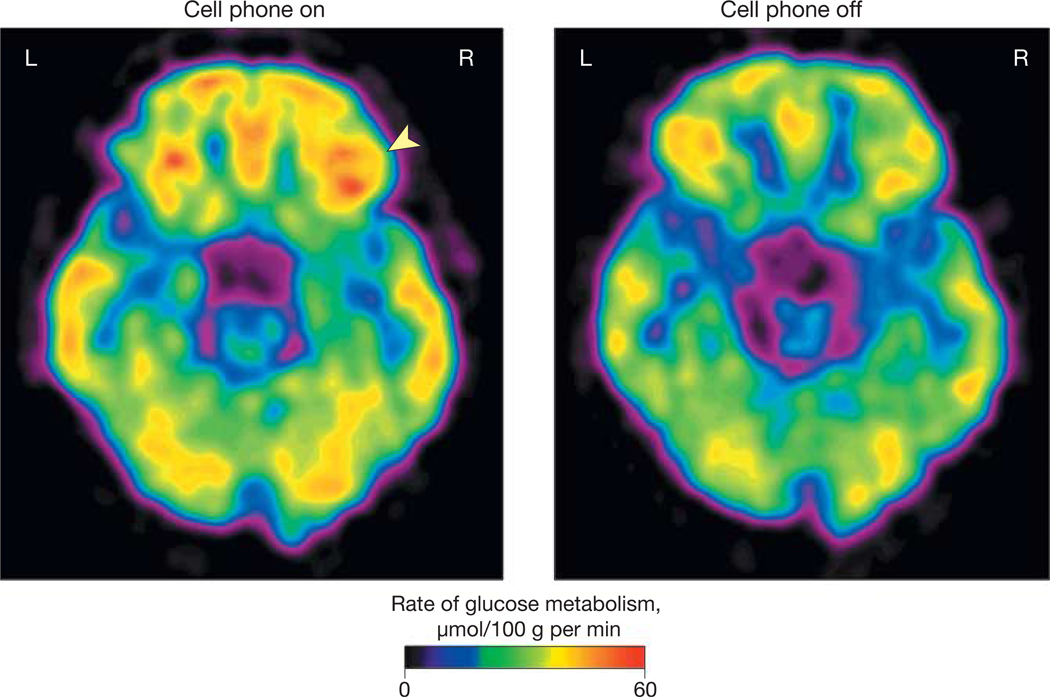
Figure 2. Brain Glucose Metabolic Images Showing Axial Planes at the Level of the Orbitofrontal Cortex.
Images are from a single participant representative of the study population. Glucose metabolism in right orbitofrontal cortex (arrowhead) was higher for the “on” than for the “off” condition (see “Methods” for description of conditions).
Table 2.
Statistical Parametric Mapping For Brain Regions Showing Higher Glucose Metabolism With Cellular Telephone On Than Off
| Brain Region | Volumea | Brodmann Area | Region Coordinates, mmb |
Z Score, On vs Off |
Pcorrc | On vs Off, Mean Difference (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| Absolute glucose metabolism | |||||||||||
| Right inferior frontal | 2649 | 47 | 18 | 23 | −18 | 2.7 | .05 | 2.4 (0.67–4.2)d | |||
| Right superior temporal | 38 | 24 | 12 | −37 | 2.6 | ||||||
| Right middle frontal | 11 | 23 | 38 | −15 | 2.6 | ||||||
| Normalized glucose metabolism | |||||||||||
| Right superior temporal | 2910 | 38 | 27 | 2 | −35 | 3.1 | .05 | 7.8 (2.7–12.9)d | |||
| Right inferior frontal | 47 | 16 | 27 | −16 | 3.1 | ||||||
| Right middle frontal | 11 | 23 | 38 | −15 | 3.1 | ||||||
Abbreviation: CI, confidence interval.
No. of voxels. One voxel = 0.008 mm3.
Coordinates on the Montreal Neurological Institute stereotactic space corresponding to distance (in mm) for x (left to right), y (anterior to posterior), and z (superior to inferior).
See “Methods” for details of calculation of Bonferroni-corrected P value.
Values for absolute metabolism reported in µmol/100 g per minute; those for normalized metabolism reported as percentages.
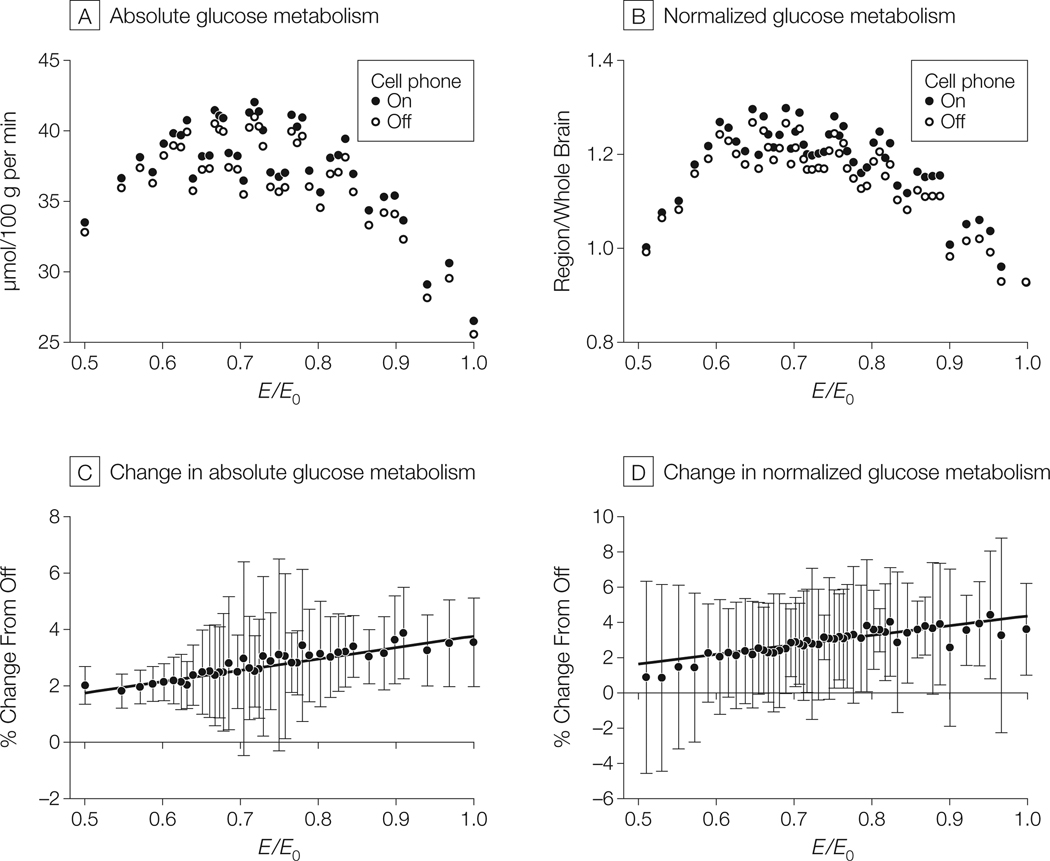
The regression analysis between cell phone–related increases in metabolism (Δ18FDG) and E revealed a significant positive correlation both for the absolute metabolic measures (R = 0.95, P < .001) and the normalized metabolic measures (R = 0.89, P < .001) (Figure 3). This indicates that the regions expected to have the greater absorption of RF-EMFs from the cell phone exposure were the ones that showed the larger increases in glucose metabolism.
Figure 3. Measures of Absolute and Normalized Glucose Metabolism and Correlation Between Estimated Electromagnetic Field Amplitudes and Increases in Measures (N = 47 Participants).
A and B, Mean measures of absolute glucose metabolism (µmol/100 g per minute) and normalized glucose metabolism (region/whole brain; units cancel) in regions with increased metabolism during “on” vs “off” conditions (see “Methods” for description of conditions) in the brain area within the spherical constraint, E0/2 < E (r) < E0 (where E0 indicates maximal field value and E (r) indicates amplitude of the theoretical electromagnetic field) and the E(r) emitted by the antenna of the right cellular telephone. Absolute = 40 clusters; 2000 voxels were activated within searching volume and grouped into clusters of 50 voxels each; normalized = 48 clusters; 2400 voxels were activated within searching volume and grouped into clusters of 50 voxels each. Range of variability (95% confidence interval [CI]): 9–21 µmol/100 g per minute (panel A) and 0.29–0.57 (panel B). C and D, Regression lines between cell phone–related increases in absolute and normalized glucose metabolism (both expressed as % change from the off condition) in brain regions within the spherical constraint, E0/2 < E (r) < E0, and the theoretical electric field, E (r), emitted by the antenna of the right cell phone. Increases significantly correlated with estimated electromagnetic field amplitudes (absolute: R = 0.95, P < .001; normalized: R = 0.89, P < .001). Data markers indicate mean metabolic measures; error bars, 95% CIs. Linear regression lines were fitted to the data using Interactive Data Language version 6.0.
CONCLUSIONS
These results provide evidence that the human brain is sensitive to the effects of RF-EMFs from acute cell phone exposures. The findings of increased metabolism in regions closest to the antenna during acute cell phone exposure suggest that brain absorption of RF-EMFs may enhance the excitability of brain tissue. This interpretation is supported by a report of enhanced cortical excitability to short transcranial magnetic stimulation pulses (1 msec) following 40-minute RF-EMF exposures.20
Although increases in frontal CBF during acute cell phone exposure had been previously reported by 2 independent PET laboratories, such increases did not occur in brain regions with the highest RF-EMF exposures.7–10 Moreover, one of these studies reported CBF decreases in the region with maximal RF-EMF exposure.10 These discrepancies are likely to reflect, among others, the methods used, particularly because the 18FDG method is optimal for detecting long-lasting effects (30 minutes) in brain activity, whereas CBF measures reflect activity over 60 seconds. In this respect, this study is an example of the value of the 18FDG method for detecting cumulative effects in brain activity that may not be observed when using more transient measures of activity. Discrepancies also could reflect uncoupling between CBF and metabolism.12–14 Moreover, the relatively large sample size (n = 47) improved our ability to detect small effects that may have been missed in prior studies with smaller sample sizes.11
The experimental setup also differed from prior studies that used cell phones for which the antenna was closest to superior and middle temporal cortices.21 However, this is unlikely to have accounted for the differences in results, because the findings in this study show increases in the region with maximal RF-EMF exposure, whereas findings from other studies have shown decreases in regions with the highest RF-EMF exposures, increases in regions far from the antenna, or both. However, the increases in frontal CBF previously reported with acute cell phone exposure possibly could reflect a downstream effect of connections with the regions that had the highest RF-EMF exposures.
The linear association between cell phone–related increases in metabolism (Δ18FDG) and E suggests that the metabolic increases are secondary to the absorption of RF-EMFs from cell phone exposures. The mechanisms by which RF-EMFs from cell phones could affect brain glucose metabolism are unclear. However, based on findings from in vivo animal and in vitro experiments, it has been hypothesized that this could reflect effects of RF-EMF exposure on neuronal activity mediated by changes in cell membrane permeability, calcium efflux, cell excitability, and/or neurotransmitter release.4 Athermal effect of cell phones on the brain has also been proposed,22 but this is unlikely to contribute to functional brain changes.5 Disruption of the blood-brain barrier has also been invoked as a potential mechanism by which RF-EMFs from cell phone exposure could affect brain activity.23 A recent clinical study reported alterations in a peripheral biomarker of blood-brain barrier integrity (transthyretin) after cell phone exposure, but the significance of this finding is unclear.24
The increases in regional metabolism induced by RF-EMFs (approximately 7%) are similar in magnitude to those reported after suprathreshold transcranial magnetic stimulation of the sensorimotor cortex (7%–8%).25 However, these increases are much smaller than the increases after visual stimulation reported by most studies (range, 6%–51%).26 The large difference in the magnitude of regional glucose metabolic increases is likely to reflect multiple factors, including differences in glycolytic rate between brain regions,27 the duration of the stimulation (transient stimulation increases glucose metabolism more than continuous stimulation26), and the characteristics of the stimulation used.28 Indeed, whereas resting glucose metabolism is predominantly supported by glucose oxidation (>90%), with acute visual stimulation the large increases in glucose metabolism appear to reflect predominantly aerobic glycolysis,29 which is used for purposes other than energy expenditures, and actual energy utilization is estimated to be 8% at most.13
Concern has been raised by the possibility that RF-EMFs emitted by cell phones may induce brain cancer.30 Epidemiologic studies assessing the relationship between cell phone use and rates of brain cancers are inconclusive; some report an association,31–33 whereas others do not.34–36 Results of this study provide evidence that acute cell phone exposure affects brain metabolic activity. However, these results provide no information as to their relevance regarding potential carcinogenic effects (or lack of such effects) from chronic cell phone use.
Limitations of this study include that it is not possible to ascertain whether the findings pertain to potential harmful effects of RF-EMF exposures or only document that the brain is affected by these exposures. Also, this study does not provide an understanding of the mechanism(s) by which RF-EMF exposures increase brain metabolism, and although we interpret these exposures as indicators of neuronal excitation, further studies are necessary to corroborate this. Lastly, this model assumes a linear relationship between the amplitude of the radiofrequency field and its effects in neuronal tissue, but we cannot rule out the possibility that this relationship could be nonlinear.
In summary, this study provides evidence that in humans RF-EMF exposure from cell phone use affects brain function, as shown by the regional increases in metabolic activity. It also documents that the observed effects were greatest in brain regions that had the highest amplitude of RF-EMF emissions (for the specific cell phones used in this study and their position relative to the head when in use), which suggests that the metabolic increases are secondary to the absorption of RF-EMF energy emitted by the cell phone. Further studies are needed to assess if these effects could have potential long-term harmful consequences.
Acknowledgments
Funding/Support: This study was carried out at Brookhaven National Laboratory (BNL) and was supported by the Intramural Research Program of the National Institutes of Health (NIH) and by infrastructure support from the Department of Energy.
Role of Sponsor: The funding agencies had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Volkow and Tomasi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Volkow.
Acquisition of data: Wang, Vaska, Telang, Alexoff, Wong.
Analysis and interpretation of data: Volkow, Tomasi, Vaska, Fowler, Telang, Logan.
Drafting of the manuscript: Volkow, Wong.
Critical revision of the manuscript for important intellectual content: Volkow, Tomasi, Wang, Vaska, Fowler, Telang, Alexoff, Logan.
Statistical analysis: Tomasi.
Obtained funding: Volkow, Fowler.
Administrative, technical, or material support: Wang, Fowler, Telang, Alexoff, Wong.
Study supervision: Wang, Fowler.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Additional Contributions: We are grateful to BNL employees Donald Warner, AA, for positron emission tomography operations; David Schlyer, PhD, and Michael Schueller, PhD, for cyclotron operations; Pauline Carter, RN, and Barbara Hubbard, RN, for nursing care; Payton King, BS, for plasma analysis; and Lisa Muench, MS, Youwen Xu, MS, and Colleen Shea, MS, for radiotracer preparation; and to NIH employees Karen Appelskog-Torres, AA, for protocol coordination; Millard Jayne, RN, for subject recruitment and nursing care; and Linda Thomas, MS, for editorial assistance. We also thank the individuals who volunteered for these studies. None of the individuals acknowledged were compensated in addition to their salaries for their contributions.
REFERENCES
- 1.Dubey RB, Hanmandlu M, Gupta SK. Risk of brain tumors from wireless phone use. J Comput Assist Tomogr. 2010;34(6):799–807. doi: 10.1097/RCT.0b013e3181ed9b54. [DOI] [PubMed] [Google Scholar]
- 2.Schönborn F, Burkhardt M, Kuster N. Differences in energy absorption between heads of adults and children in the near field of sources. Health Phys. 1998;74(2):160–168. doi: 10.1097/00004032-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kleinlogel H, Dierks T, Koenig T, Lehmann H, Minder A, Berz R. Effects of weak mobile phone–electromagnetic fields (GSM, UMTS) on event related potentials and cognitive functions. Bioelectromagnetics. 2008;29(6):488–497. doi: 10.1002/bem.20418. [DOI] [PubMed] [Google Scholar]
- 4.Hyland GJ. Physics and biology of mobile telephony. Lancet. 2000;356(9244):1833–1836. doi: 10.1016/s0140-6736(00)03243-8. [DOI] [PubMed] [Google Scholar]
- 5.Wainwright P. Thermal effects of radiation from cellular telephones. Phys Med Biol. 2000;45(8):2363–2372. doi: 10.1088/0031-9155/45/8/321. [DOI] [PubMed] [Google Scholar]
- 6.van Rongen E, Croft R, Juutilainen J, et al. Effects of radiofrequency electromagnetic fields on the human nervous system. J Toxicol Environ Health B Crit Rev. 2009;12(8):572–597. doi: 10.1080/10937400903458940. [DOI] [PubMed] [Google Scholar]
- 7.Huber R, Treyer V, Borbély AA, et al. Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. J Sleep Res. 2002;11(4):289–295. doi: 10.1046/j.1365-2869.2002.00314.x. [DOI] [PubMed] [Google Scholar]
- 8.Huber R, Treyer V, Schuderer J, et al. Exposure to pulse-modulated radio frequency electromagnetic fields affects regional cerebral blood flow. Eur J Neurosci. 2005;21(4):1000–1006. doi: 10.1111/j.1460-9568.2005.03929.x. [DOI] [PubMed] [Google Scholar]
- 9.Haarala C, Aalto S, Hautzel H, et al. Effects of a 902 MHz mobile phone on cerebral blood flow in humans. Neuroreport. 2003;14(16):2019–2023. doi: 10.1097/00001756-200311140-00003. [DOI] [PubMed] [Google Scholar]
- 10.Aalto S, Haarala C, Bruck A, et al. Mobile phone affects cerebral blood flow in humans. J Cereb Blood Flow Metab. 2006;26(7):885–890. doi: 10.1038/sj.jcbfm.9600279. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno Y, Moriguchi Y, Hikage T, et al. Effects of W-CDMA 1950 MHz EMF emitted by mobile phones on regional cerebral blood flow in humans. Bioelectromagnetics. 2009;30(7):536–544. doi: 10.1002/bem.20508. [DOI] [PubMed] [Google Scholar]
- 12.Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457(7228):475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox PT, Raichle ME, Mintun MA, Dence C. Non-oxidative glucose consumption during focal physiologic neural activity. Science. 1988;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 14.Devor A, Hillman EM, Tian P, et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J Neurosci. 2008;28(53):14347–14357. doi: 10.1523/JNEUROSCI.4307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 16.Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization. J Neurochem. 1977;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang G-J, Volkow ND, Roque CT, et al. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186(1):59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- 18.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 19.Volkow ND, Tomasi D, Wang GJ, et al. Effects of low-field magnetic stimulation on brain glucose metabolism. Neuroimage. 2010;51(2):623–628. doi: 10.1016/j.neuroimage.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreri F, Curcio G, Pasqualetti P, et al. Mobile phone emissions and human brain excitability. Ann Neurol. 2006;60(2):188–196. doi: 10.1002/ana.20906. [DOI] [PubMed] [Google Scholar]
- 21.Cardis E, Deltour I, Mann S, et al. Distribution of RF energy emitted by mobile phones in anatomical structures of the brain. Phys Med Biol. 2008;53(11):2771–2783. doi: 10.1088/0031-9155/53/11/001. [DOI] [PubMed] [Google Scholar]
- 22.Cotgreave IA. Biological stress responses to radio frequency electromagnetic radiation. Arch Biochem Biophys. 2005;435(1):227–240. doi: 10.1016/j.abb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Nittby H, Grafström G, Eberhardt JL, et al. Radiofrequency and extremely low-frequency electromagnetic field effects on the blood-brain barrier. Electromagn Biol Med. 2008;27(2):103–126. doi: 10.1080/15368370802061995. [DOI] [PubMed] [Google Scholar]
- 24.Söderqvist F, Carlberg M, Hansson Mild K, Hardell L. Exposure to an 890-MHz mobile phone-like signal and serum levels of S100B and transthyretin in volunteers. Toxicol Lett. 2009;189(1):63–66. doi: 10.1016/j.toxlet.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Siebner HR, Peller M, Bartenstein P, et al. Activation of frontal premotor areas during suprathreshold transcranial magnetic stimulation of the left primary sensorimotor cortex: a glucose metabolic PET study. Hum Brain Mapp. 2001;12(3):157–167. doi: 10.1002/1097-0193(200103)12:3<157::AID-HBM1012>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlassenko AG, Rundle MM, Mintun MA. Human brain glucose metabolism may evolve during activation. Neuroimage. 2006;33(4):1036–1041. doi: 10.1016/j.neuroimage.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 27.Vaishnavi SN, Vlassenko AG, Rundle MM, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanganahalli BG, Herman P, Hyder F. Frequency-dependent tactile responses in rat brain measured by functional MRI. NMR Biomed. 2008;21(4):410–416. doi: 10.1002/nbm.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blomqvist G, Seitz RJ, Sjögren I, et al. Regional cerebral oxidative and total glucose consumption during rest and activation studied with positron emission tomography. Acta Physiol Scand. 1994;151(1):29–43. doi: 10.1111/j.1748-1716.1994.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 30.Yakymenko I, Sidorik E. Risks of carcinogenesis from electromagnetic radiation of mobile telephony devices. Exp Oncol. 2010;32(2):54–60. [PubMed] [Google Scholar]
- 31.Lehrer S, Green S, Stock RG. Association between number of cell phone contracts and brain tumor incidence in nineteen U.S. States. J Neurooncol. 2011;101(3):505–507. doi: 10.1007/s11060-010-0280-z. [DOI] [PubMed] [Google Scholar]
- 32.Hardell L, Carlberg M. Mobile phones, cordless phones and the risk for brain tumours. Int J Oncol. 2009;35(1):5–17. doi: 10.3892/ijo_00000307. [DOI] [PubMed] [Google Scholar]
- 33.Myung SK, Ju W, McDonnell DD, et al. Mobile phone use and risk of tumors: a meta-analysis. J Clin Oncol. 2009;27(33):5565–5572. doi: 10.1200/JCO.2008.21.6366. [DOI] [PubMed] [Google Scholar]
- 34.Inskip PD, Tarone RE, Hatch EE, et al. Cellular-telephone use and brain tumors. N Engl J Med. 2001;344(2):79–86. doi: 10.1056/NEJM200101113440201. [DOI] [PubMed] [Google Scholar]
- 35.INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use. Int J Epidemiol. 2010;39(3):675–694. doi: 10.1093/ije/dyq079. [DOI] [PubMed] [Google Scholar]
- 36.Inskip PD, Hoover RN, Devesa SS. Brain cancer incidence trends in relation to cellular telephone use in the United States. Neuro Oncol. 2010;12(11):1147–1151. doi: 10.1093/neuonc/noq077. [DOI] [PMC free article] [PubMed] [Google Scholar]