Abstract
Background.
An excessive amount of adipose tissue may contribute to sarcopenia and may be one mechanism underlying accelerated loss of muscle mass and strength with aging. We therefore examined the association of baseline total body fat with changes in leg lean mass, muscle strength, and muscle quality over 7 years of follow-up and whether this link was explained by adipocytokines and insulin resistance.
Methods.
Data were from 2,307 men and women, aged 70–79 years, participating in the Health, Aging, and Body Composition study. Total fat mass was acquired from dual energy X-ray absorptiometry. Leg lean mass was assessed by dual energy X-ray absorptiometry in Years 1, 2, 3, 4, 5, 6, and 8. Knee extension strength was measured by isokinetic dynamometer in Years 1, 2, 4, 6, and 8. Muscle quality was calculated as muscle strength divided by leg lean mass.
Results.
Every SD greater fat mass was related to 1.3 kg more leg lean mass at baseline in men and 1.5 kg in women (p < .01). Greater fat mass was also associated with a greater decline in leg lean mass in both men and women (0.02 kg/year, p < .01), which was not explained by higher levels of adipocytokines and insulin resistance. Larger fat mass was related to significantly greater muscle strength but significantly lower muscle quality at baseline (p < .01). No significant differences in decline of muscle strength and quality were found.
Conclusions.
High fatness was associated with lower muscle quality, and it predicts accelerated loss of lean mass. Prevention of greater fatness in old age may decrease the loss of lean mass and maintain muscle quality and thereby reducing disability and mobility impairments.
Keywords: Adipose tissue, Muscle mass, Muscle strength, Obesity, Aging
AGING is associated with loss of muscle mass, muscle strength, and muscle quality, and studies show that the decline in muscle strength exceeds the decline in mass (1–4). Furthermore, previous studies show that muscle strength is a stronger predictor of functional limitation and poor health than muscle mass (2,5,6). To prevent and alleviate accelerated age-related declines in skeletal muscle mass, strength, and quality, we need to understand the underlying mechanisms. An excessive amount of adipose tissue may contribute to sarcopenia and may be one mechanism underlying accelerated loss of muscle mass and strength with aging.
The influences of body fat on skeletal muscle are likely complex. Numerous studies show that negative energy balance induced by either diet or exercise leads to loss of both fat and lean mass, and one study suggested that the amount of body fat at baseline was negatively associated with the proportion of weight lost as lean mass (7). This notion is in accordance with the function of fat mass as energy storage. Age-related loss of muscle mass is accompanied by fat gain in older adults (8–10). Therefore, fat mass may play a role in age-related muscle loss through many metabolic consequences of adipose tissue (11,12). Excess adiposity depresses anabolic action of insulin in stimulating protein synthesis (13), which may contribute to progressive loss of muscle mass, strength, and quality. In addition to its function in energy storage, fat tissue also secretes many adipocytokines such as interleukin-6, tumor necrosis factor-α, and leptin (14) that may have a catabolic effect on muscle, thus decreasing muscle mass and strength (15–18). Therefore, cytokines may mediate the link between higher fat mass and loss in muscle mass and strength.
The Health, Aging, and Body Composition study, with a large sample of community-based older adults and repeated dual energy X-ray absorptiometry (DXA) measures of body composition and muscle strength over time, provides a unique opportunity to assess the interplay between fat and lean mass, muscle strength, and muscle quality. We examined the association of baseline total body fat with changes in leg lean mass, muscle strength, and muscle quality over 7 years of follow-up. Additionally, we determined whether the potential link between fat mass and muscle mass, muscle strength, and muscle quality loss was explained by increased levels of adipocytokines and insulin resistance.
METHODS
Study Population
The Health, Aging, and Body Composition study is a longitudinal cohort study consisting of 3,075 initially well-functioning, community-dwelling, 70- to 79-year-old black and white men and women. Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible black residents in designated zip code areas surrounding Memphis, Tennessee, and Pittsburgh, Pennsylvania. Participants were eligible if they reported no difficulty in walking one quarter of a mile, going up 10 steps without resting, and performing basic activities of daily living. Participants were excluded if they reported a history of active treatment for cancer in the prior 3 years, planned to move out of the study area in the next 3 years, or were currently participating in a randomized trial of a lifestyle intervention. Baseline data, collected between April 1997 and June 1998, included an in-person interview and a clinic-based examination, with evaluation of body composition, clinical and subclinical diseases, and physical functioning. We selected participants with at least two leg lean mass and muscle strength measurements (n = 2,593). Participants with missing data on baseline fat mass (n = 9) and on all cytokines or insulin resistance (n = 277) were excluded, leaving 2,307 participants for the current analyses. All participants signed informed written consent forms approved by the institutional review boards of the clinical sites.
Measures
Body composition.—
Body weight was measured annually to the nearest 0.1 kg with a standard balance beam scale. Body height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Total fat mass and total bone–free lean mass were acquired from total body scans using fan-beam dual energy X-ray absorptiometry (Hologic QDR 4500A; Hologic, Bedford, MA). The validity and reproducibility of the dual energy X-ray absorptiometry scanner have been reported previously (19,20). Longitudinal performance of the two scanners was monitored with Hologic whole-body phantoms. The Pittsburgh scanner overestimated total mass by about 2% compared with scale weight. It has also been determined that the Hologic 4500 overestimates fat-free mass compared with criterion methods by about 5% (21). Total and regional participant body composition data were corrected accordingly. Leg lean mass was assessed in Years 1, 2, 3, 4, 5, 6, and 8.
Muscle strength and muscle quality.—
Knee extension strength was measured concentrically at 60° per second on an isokinetic dynamometer (Kin-Com dynamometer, 125 AP; Chattanooga, TN). The right leg was tested unless there was a contraindication such as joint replacement or knee pain. The maximum muscle torque (Newton meters) was calculated from the average of three reproducible and acceptable trials from a maximum of six. Participants with a systolic blood pressure greater than or equal to 200 mmHg, diastolic blood pressure greater than or equal to 110 mmHg, or who reported a history of cerebral aneurysm, cerebral bleeding, bilateral total knee replacement, or severe bilateral knee pain were excluded from testing (12.7% of original cohort). Muscle strength was assessed in Years 1, 2, 4, 6, and 8. Muscle quality was calculated as muscle strength divided by leg lean mass of the tested leg ( Newton meters per kilogram).
Adipocytokines.—
Measures of the cytokines interleukin-6 and tumor necrosis factor-α and for C-reactive protein were obtained from frozen-stored plasma or serum. Fasting blood samples were obtained in the morning, and after processing, the specimens were aliquoted into cryovials, frozen at −70°C, and shipped to the Health ABC Core Laboratory at the University of Vermont. Cytokines were measured in duplicate by enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN). The detectable limit was 0.10 pg/mL for interleukin-6 (by HS600 Quantikine Kit) and 0.18 pg/mL for tumor necrosis factor-α (by HSTA50 Kit). Serum levels of C-reactive protein were also measured in duplicate by enzyme-linked immunosorbent assay based on purified protein and polyclonal anti-C-reactive protein antibodies (Calbiochem, San Diego, CA). The C-reactive protein assay was standardized according to the World Health Organization First International Reference Standard with a sensitivity of 0.08 μg/mL. Serum leptin and adiponectin concentrations were measured by radioimmunoassay (Linco Research Inc., St. Charles, MO). Plasma plasminogen activator inhibitor-1 was measured by a two-site enzyme-linked immunosorbent assay (Collen Laboratory, Leuven, Belgium).
Insulin resistance.—
The degree of insulin resistance was calculated as fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5 according to the homeostasis model assessment (HOMA-IR) (22), a valid measure of insulin resistance (23).
Covariates.—
Sociodemographic variables included age, race, study site, and birth cohort. On the basis of age (70–79 years) at baseline, 10 successive annual birth cohorts were defined. Lifestyle factors included smoking (current, former, and never) and physical activity. Physical activity in the previous 7 days was assessed at baseline; time and intensity level were reported for activities including gardening, heavy household chores, light house work, grocery shopping, laundry, climbing stairs, walking for exercise, walking for other purposes, aerobics, weight or circuit training, and moderate- and high-intensity exercise activities. Approximate metabolic equivalent unit values were assigned to each activity category to calculate a weekly activity energy expenditure estimate in kilocalories per kilogram per week (24). Three categories were created: “exercise”: greater than or equal to 1,000 kcal/wk exercise; “lifestyle active” less than 1,000 kcal/wk exercise and greater than or equal to 2,719 kcal/wk total physical activity; and “inactive” less than 1,000 kcal/wk exercise and less than 2,719 kcal/wk total physical activity (25). Presence of diabetes and heart disease were determined using standardized algorithms considering self-report, use of specific medications, and clinical assessments.
Statistical Analysis
Multilevel analyses were used to examine the association between fat mass at baseline and change in leg lean mass, muscle strength, and muscle quality in men and women separately. A multilevel analysis is a suitable technique for repeated measurement analyses because it takes into account the correlation between measurements and allows utilizing all available information when examining change in the dependent variable. We defined a two-level hierarchy to form random regression models to describe individual variability in longitudinal change in lean mass, muscle strength, and muscle quality. The first level was defined by age, as the longitudinal time variable, and the second level by respondent. Models included fat mass as well as the interaction between fat mass and age to determine how the association of fat mass with lean mass, muscle strength, and muscle quality changes with time. The first model was adjusted for age, birth cohort, race, site, and height. Physical activity, smoking, diabetes, and heart disease were added to Model 2. Model 3 was adjusted for all variables of Model 1 and adipocytokines, and Model 4 was adjusted for all variables of Model 1 and HOMA-IR. Finally, Model 5 included all variables of the previous models. All models included the individual variable as well as the interaction terms of each variable with age. The intercepts and slope (interaction with age) of fat mass of each model are presented. Analyses were performed using SPSS, version 17.0 (SPSS Inc., Chicago, IL).
RESULTS
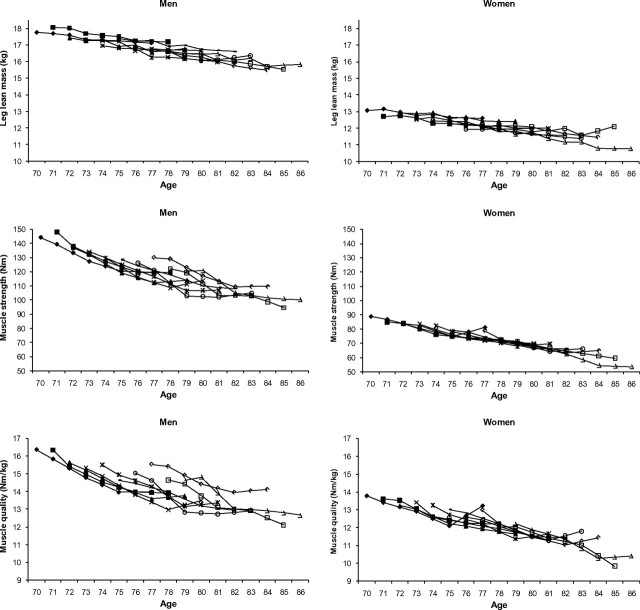
Baseline characteristics of the study population are presented in Table 1. Figure 1 shows changes in lean mass and muscle strength over time by birth cohort. On average, men lost 145 g (0.8%) of leg lean mass per year and women 88 g (0.7%) per year (Table 2). Men lost 4.1 Nm (3.1%) and women on average 2.2 Nm (2.6%) of muscle strength per year. The annual decline in leg lean mass and strength did not markedly differ by birth cohort.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Men, n = 1,129 | Women, n = 1,178 |
| Age, M (SD) | 74.2 (2.8) | 73.9 (2.8) |
| Race, white, % | 68.0 | 59.8 |
| Site, Memphis, % | 50.0 | 51.5 |
| Weight (kg), M (SD) | 81.1 (13.0) | 69.8 (14.2) |
| BMI (kg/m2), M (SD) | 27.1 (3.8) | 27.5 (5.4) |
| Total body fat (kg), M (SD) | 24.1 (7.1) | 28.9 (9.1) |
| Total lean mass (kg), M (SD) | 54.3 (7.0) | 39.1 (5.8) |
| Leg lean mass (kg), M (SD) | 17.2 (2.6) | 12.4 (2.4) |
| Muscle strength (Nm), M (SD) | 134.1 (34.5) | 82.1 (22.0) |
| Muscle quality (Nm/kg), M (SD) | 15.6 (3.3) | 13.2 (3.2) |
| Interleukin-6 (pg/mL), median (25%, 75%) | 1.8 (1.3, 2.8) | 1.7 (1.1, 2.5) |
| C-reactive protein (μg/mL), median (25%, 75%) | 1.4 (0.9, 2.5) | 1.8 (1.1, 3.4) |
| Tumor necrosis factor-α (pg/mL), median (25%, 75%) | 3.3 (2.5, 4.2) | 3.0 (2.3, 3.9) |
| Leptin (ng/mL), median (25%, 75%) | 5.9 (3.3, 9.7) | 17.9 (9.8, 28.4) |
| Adiponectin (μg/mL), median (25%, 75%) | 9.0 (6.0, 15.0) | 11.0 (7.0, 16.0) |
| PAI-1 (ng/mL), median (25%, 75%) | 20.0 (12.0, 33.5) | 22.0 (13.0, 38.0) |
| HOMA-IR, median (25%, 75%) | 1.7 (1.1, 2.5) | 1.6 (1.0, 2.5) |
| Physical activity, % | ||
| Exercise | 34.8 | 17.8 |
| Lifestyle | 46.0 | 59.3 |
| Inactive | 19.2 | 22.9 |
| Smoking, % | ||
| Never | 30.7 | 57.5 |
| Current | 10.1 | 8.8 |
| Former | 59.2 | 33.7 |
| Heart disease, % | 20.7 | 10.5 |
| Diabetes mellitus, % | 13.5 | 8.0 |
Note: HOMA-IR = homeostasis model assessment; PAI-1 = plasminogen activator inhibitor-1.
Figure 1.
Changes in leg lean mass and muscle strength with aging by birth cohort.  , birth cohort 70;
, birth cohort 70;  , birth cohort 71;
, birth cohort 71;  , birth cohort 72;
, birth cohort 72;  , birth cohort 73;
, birth cohort 73;  , birth cohort 74;
, birth cohort 74;  , birth cohort 76;
, birth cohort 76;  , birth cohort 77;
, birth cohort 77;  , birth cohort 78;
, birth cohort 78;  , birth cohort 79.
, birth cohort 79.
Table 2.
Age-Related Changes in Leg Lean Mass and Muscle Strength
| Leg Lean Mass |
Muscle Strength |
Muscle Quality |
|||||
| kg/year (SE)* | %/year | Nm/year (SE)* | %/year | (Nm/kg)/year (SE)* | |||
| %/year | Men | −0.145 (0.005) | −0.8 | −4.13 (0.14) | −3.1 | −0.37 (0.02) | |
| −2.4 | Women | −0.088 (0.004) | −0.7 | −2.16 (0.11) | −2.6 | −0.26 (0.02) | −2.0 |
Note: *Adjusted for race, site, birth cohort, and physical activity.
The association between baseline fat mass (per SD) and changes in leg lean mass, muscle strength, and muscle quality is shown in Table 3. Every SD greater fat mass was related to 1.3 kg more leg lean mass at baseline in men and 1.5 kg in women (p < .01). When leg lean mass was normalized for total body weight (leg lean mass/weight), a greater fat mass was related to significantly less normalized lean mass at baseline in both men and women (p < .01, not tabulated). In addition, greater fat mass was associated with a significantly greater decline in leg lean mass in both men and women (0.02 kg per year in men and women, p < .01). This difference in decline remained significant after including health-related factors (Model 2), adipocytokines (Model 3), and HOMA-IR (Model 4). After additional adjustment for weight at baseline and follow-up, the difference in decline remained significant (not tabulated). Larger fat mass was related to significantly greater muscle strength but significantly lower muscle quality in both men and women at baseline. No significant differences in decline of muscle strength and quality were found. In additional analysis, we excluded participants with diabetes, which did not change the results.
Table 3.
Fat Mass (per SD* increase) and Changes in Leg Lean Mass (kilograms), Muscle Strength (Newton meters), and Muscle Quality (Newton meters per kilogram)
| Model 1† | Model 2‡ | Model 3§ | Model 4‖ | Model 5¶ | |||||||||||
| B | SE | p Value | B | SE | p Value | B | SE | p Value | B | SE | p Value | B | SE | p Value | |
| Leg lean mass (kg) | |||||||||||||||
| Men | |||||||||||||||
| Total body fat | 1.28 | 0.06 | <.01 | 1.25 | 0.07 | <.01 | 1.44 | 0.09 | <.01 | 1.24 | 0.07 | <.01 | 1.37 | 0.09 | <.01 |
| Total Body Fat × Age | −0.022 | 0.005 | <.01 | −0.022 | 0.006 | <.01 | −0.015 | 0.007 | .04 | −0.019 | 0.006 | <.01 | −0.014 | 0.007 | .08 |
| Women | |||||||||||||||
| Total body fat | 1.51 | 0.05 | <.01 | 1.50 | 0.05 | <.01 | 1.66 | 0.07 | <.01 | 1.46 | 0.05 | <.05 | 1.61 | 0.07 | <.01 |
| Total Body Fat × Age | −0.015 | 0.004 | <.01 | −0.016 | 0.004 | <.01 | −0.018 | 0.006 | <.01 | −0.015 | 0.005 | <.01 | −0.018 | 0.006 | <.01 |
| Muscle strength (Nm) | |||||||||||||||
| Men | |||||||||||||||
| Total body fat | 3.80 | 1.32 | <.01 | 3.64 | 1.35 | <.01 | 4.66 | 1.81 | .09 | 2.96 | 1.47 | .04 | 3.28 | 1.86 | .08 |
| Total Body Fat × Age | −0.098 | 0.14 | .50 | −0.082 | 0.14 | .56 | −0.18 | 0.18 | .33 | −0.034 | 0.15 | .82 | −0.12 | 0.19 | .53 |
| Women | |||||||||||||||
| Total body fat | 2.73 | 0.88 | <.01 | 2.01 | 0.89 | .03 | 2.99 | 1.29 | .02 | 2.63 | 0.92 | <.01 | 1.76 | 1.32 | .18 |
| Total Body Fat × Age | −0.041 | 0.11 | .72 | −0.031 | 0.12 | .27 | −0.15 | 0.17 | .39 | −0.13 | 0.12 | .28 | −0.14 | 0.18 | .42 |
| Muscle quality (Nm/kg) | |||||||||||||||
| Men | |||||||||||||||
| Total body fat | −0.66 | 0.13 | <.01 | −0.64 | 0.14 | <.01 | −0.79 | 0.18 | <.01 | −0.70 | 0.15 | <.01 | −0.86 | 0.19 | <.01 |
| Total Body Fat × Age | 0.025 | 0.01 | .09 | 0.025 | 0.02 | .11 | 0.021 | 0.02 | .28 | 0.028 | 0.02 | .09 | 0.024 | 0.02 | .25 |
| Women | |||||||||||||||
| Total body fat | −0.98 | 0.13 | <.01 | −1.05 | 0.14 | <.01 | −1.02 | 0.20 | <.01 | −0.93 | 0.14 | <.01 | −1.11 | 0.20 | <.01 |
| Total Body Fat × Age | 0.021 | 0.02 | .27 | 0.021 | 0.02 | .28 | 0.006 | 0.03 | .85 | 0.007 | 0.02 | .73 | 0.003 | 0.03 | .92 |
Notes: CRP = C-reactive protein; HOMA-IR = homeostasis model assessment; IL-6 = interleukin-6; PAI-1 = plasminogen activator inhibitor-1; TNF-α = tumor necrosis factor-α.
SD for fat mass in men: 7.07, SD in women: 9.10.
Model 1 adjusted for age, birth cohort, race, site, height, and interaction between each covariate and age.
Model 2 adjusted for age, birth cohort, race, site, height, physical activity, smoking, diabetes, heart disease, and interaction between each covariate and age.
Model 3 adjusted for age, birth cohort, race, site, height, IL-6, CRP, TNF-α, leptin, adiponectin, PAI-1, and interaction between each covariate and age.
Model 4 adjusted for age, birth cohort, race, site, height, HOMA-IR, and interaction between each covariate and age.
Model 5 adjusted for age, birth cohort, race, site, height, physical activity, smoking, diabetes, heart disease, IL-6, CRP, TNF-α, leptin, adiponectin, PAI-1, HOMA-IR, and interaction between each covariate and age.
Figure 2 shows changes in leg lean mass, muscle strength, and muscle quality by quartile of baseline fat mass. Those within the upper three quartiles of fat mass had significantly more lean mass at baseline than those in the lowest quartile (p < .01). Additionally, the group of men and women in the highest two quartiles of fat mass lost significantly more lean mass compared with those in the lowest quartile (all p < .04). Muscle strength was also greater in people with more fat mass; however, the rate of decline in muscle strength was not significantly different. Muscle quality, however, was significantly lower in those in the highest quartile of fat mass compared with the lowest quartile of fat mass. Men in the highest quartile of fat mass lost significantly less muscle quality compared with those in the lowest quartile of fat mass (p = .02). There were no significant slope differences in muscle quality in women. Results with muscle strength remained similar after including leg lean mass in the models.
Figure 2.
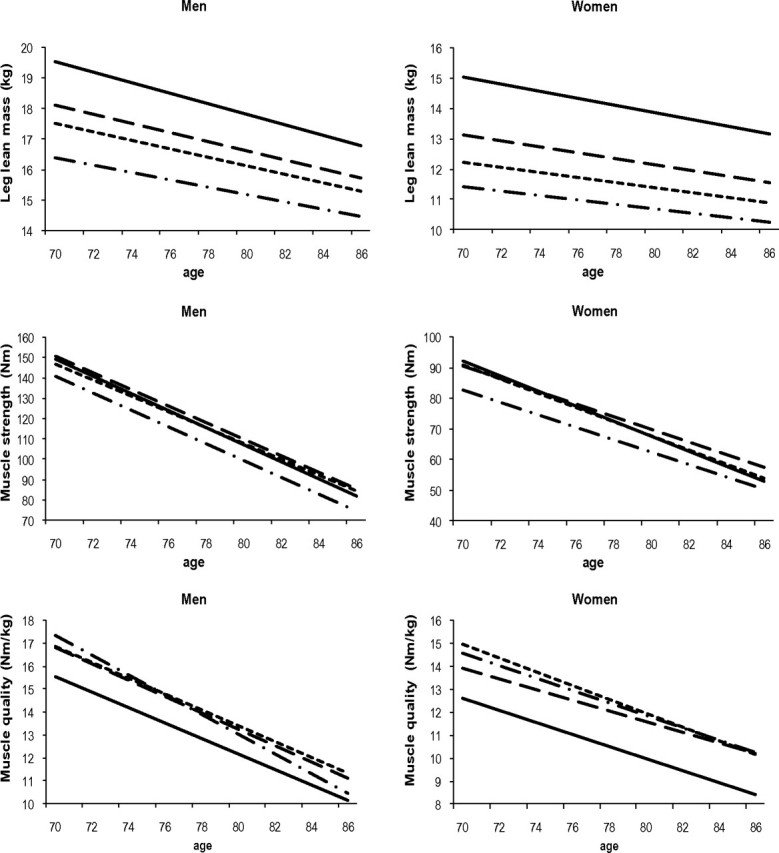
Change in leg lean mass, muscle strength, and muscle quality according to quartiles of fat mass. Adjusted for age, birth cohort, race, site, height, and interaction between each covariate and age. 1 = low = -.-.-.-.-.; 2=  ; 3=
; 3=  ; 4 = high = solid line.
; 4 = high = solid line.
DISCUSSION
This study shows the association between fat mass and 7-year change in leg lean mass and muscle strength. As shown before, the decline in muscle strength exceeds the decline in muscle mass (4,5,26). Over 7 years of follow-up, men lost on average 145 g of leg lean mass (0.8%) per year and women 88 g (0.7%) per year. Furthermore, men lost 3.1% of muscle strength in knee extensors per year and women 2.6% per year. Greater fat mass was related to more leg lean mass at baseline but a significantly greater loss of leg lean mass. The accelerated loss of leg lean mass with greater fat mass was not explained by higher levels of adipocytokines or insulin resistance. Large fat mass was also related to a significantly greater muscle strength but lower muscle quality (strength normalized for leg lean mass). Large fat mass was not associated with a greater rate of decline in muscle strength or muscle quality.
Muscle strength was greatest among the people with the greatest fat mass. Obese individuals not only have a larger fat mass but also greater lean mass and muscle strength; however, as shown in this study, the group with the highest fat mass had the lowest muscle quality at each age. Muscle quality takes into account both muscle strength and muscle mass and is therefore a better indicator for muscle impairment, especially among obese older adults (3). Low muscle strength is a strong predictor of function limitations and disability (2,5,6). The combination of obesity with muscle impairment has been termed sarcopenic obesity and seems to predispose older adults for negative health and functional consequences (27,28). A recent study showed that older obese persons with low muscle strength have a particularly high risk of a decline in walking speed and risk of developing mobility limitation (29). The equal rate of in muscle strength and muscle quality in the different fatness groups observed in this study suggests that differences in muscle strength due to varying fatness level may have occurred before the age of 70. A greater fat mass may have been a result of reduced muscle strength through, for example, reduced physical activity. A recent study shows that long-term exposure to obesity is associated with poor handgrip strength later in life and that the earlier the obesity onset had been, the lower was strength in old age (30). More studies are needed to disentangle the relationship between fat mass and muscle strength and quality during the life course.
We hypothesized that a link between fat mass, muscle mass, strength, and quality could be explained by higher levels of adipocytokines or insulin resistance. Adipose tissue is a metabolically active endocrine organ that secretes many adipocytokines (14). Cytokines may have a catabolic effect on muscle, and studies have related cytokines to decline in muscle mass (31) and strength (16,32). Furthermore, obesity is associated with reduced insulin action that may have a procatabolic effect on muscle (33). In the present study, adipocytokines and insulin resistance did not explain the accelerated decline in muscle mass that was associated with greater fat mass nor the association between fat mass and muscle strength. There are also other pathways through which fatness might be related to lean mass and muscle strength. Ectopic adipose tissue may exert a paracrine function (34) on muscle mass (and thereby muscle strength), for example, increasing local inflammation that is not necessarily translated into increased systemic inflammatory marker levels. Furthermore, hormones, such as testosterone and growth hormone, have also been related to both fatness and lean mass and strength (35,36) and could possibly mediate the association between fat mass and lean mass and muscle strength. Finally, physical inactivity is related to both fatness and low lean mass and muscle strength (37–40) and may therefore mediate the association between fat mass and lean mass and muscle strength. In our study, however, including physical activity together with other potential confounders/mediators did not significantly alter our results.
Some limitations of our study have to be considered. Our results cannot be generalized to all older adults because study participants were 70–79 years old and well functioning at baseline. Second, using multilevel analyses, we included all participants with at least two measurements of leg lean mass and muscle strength and included people with missing observations. People with full data on muscle mass and strength on all measurements were significantly younger and had more leg lean mass (men only), greater muscle strength, and greater muscle quality at baseline compared with persons with one or more missing observations (data not shown). Persons died during the follow-up, and people had missing data because they were not able to come to the clinic for the assessments because of health problems. It is likely that body composition changes were more unfavorable in people with missing follow-up assessments due to health problems; therefore, we may have underestimated the association between fatness and changes in leg lean mass and muscle strength.
Factors that predict age-related decline in lean mass and muscle strength are not well understood. In this study, we show that obesity (high fatness) is associated with lower muscle quality, and it predicts accelerated loss of lean mass. Prevention of greater fatness in old age may decrease the loss of lean mass and maintain muscle quality and thereby reducing disability and mobility impairments.
FUNDING
This study was supported by National Institute on Aging contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106. This research was supported (in part) by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
CONFLICT OF INTEREST
None.
References
- 1.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol Biol Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 5.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 6.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 7.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann N Y Acad Sci. 2000;904:359–365. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94:2368–2374. doi: 10.1152/japplphysiol.00124.2002. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–878. doi: 10.1093/ajcn/82.4.872. quiz 915–916. [DOI] [PubMed] [Google Scholar]
- 11.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 12.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 13.Chevalier S, Gougeon R, Choong N, Lamarche M, Morais JA. Influence of adiposity in the blunted whole-body protein anabolic response to insulin with aging. J Gerontol A Biol Sci Med Sci. 2006;61:156–164. doi: 10.1093/gerona/61.2.156. [DOI] [PubMed] [Google Scholar]
- 14.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 15.Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 16.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:e9–e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 18.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 19.Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study—Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol. 1999;87:1513–1520. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- 20.Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol. 2000;89:345–352. doi: 10.1152/jappl.2000.89.1.345. [DOI] [PubMed] [Google Scholar]
- 21.Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–1025. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 25.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 26.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 27.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 29.Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes (Lond) 2009;33:635–644. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenholm S, Sallinen J, Koster A, et al. Association between obesity history and hand grip strength in older adults—exploring the roles of inflammation and insulin resistance as mediating factors. J Gerontol A Biol Sci Med Sci. 2011;66:341–348. doi: 10.1093/gerona/glq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payette H, Roubenoff R, Jacques PF, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51:1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- 32.Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol Med Sci. 2000;55:M716–M724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 34.Lee DE, Kehlenbrink S, Lee H, Hawkins M, Yudkin JS. Getting the message across: mechanisms of physiological cross talk by adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E1210–E1229. doi: 10.1152/ajpendo.00015.2009. [DOI] [PubMed] [Google Scholar]
- 35.Waters DL, Qualls CR, Dorin RI, Veldhuis JD, Baumgartner RN. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontol A Biol Sci Med Sci. 2008;63:536–541. doi: 10.1093/gerona/63.5.536. [DOI] [PubMed] [Google Scholar]
- 36.Schaap LA, Pluijm SM, Smit JH, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 2005;63:152–160. doi: 10.1111/j.1365-2265.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- 37.Di Francesco V, Zamboni M, Zoico E, et al. Relationships between leisure-time physical activity, obesity and disability in elderly men. Aging Clin Exp Res. 2005;17:201–206. doi: 10.1007/BF03324597. [DOI] [PubMed] [Google Scholar]
- 38.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 39.Kyle UG, Morabia A, Schutz Y, Pichard C. Sedentarism affects body fat mass index and fat-free mass index in adults aged 18 to 98 years. Nutrition. 2004;20:255–260. doi: 10.1016/j.nut.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]