Abstract
Bone marrow stem cells from diabetes mellitus patients exhibit functional impairment, but the relative molecular mechanisms responsible for this impairment are poorly understood. We investigated the mechanisms responsible for diabetes-related functional impairment of bone marrow stem cells by extensively screening the expression levels of inflammatory factors, cell cycle regulating molecules, extracellular matrix molecules and adhesion molecules. Bone marrow cells were collected from type 2 diabetic (db/db) and healthy control (db/m+) mice, and c-kit+ stem cells were purified (purity>85%) for experiments. Compared with the healthy control mice, diabetic mice had significantly fewer c-kit+ stem cells, and these cells had a lower potency of endothelial differentiation; however, the production of the angiogenic growth factor VEGF did not differ between groups. A pathway-focused array showed that the c-kit+ stem cells from diabetic mice had up-regulated expression levels of many inflammatory factors, including Tlr4, Cxcl9, Il9, Tgfb1, Il4, and Tnfsf5, but no obvious change in the expression levels of cell cycle molecules. Interestingly, diabetes-related alterations of the extracellular matrix and adhesion molecules were varied; Pecam, Mmp10, Lamc1, Itgb7, Mmp9, and Timp4 were up-regulated, but Col11a1, Fn1, Admts2, and Itgav were down-regulated. Some of these changes were also confirmed at the protein level by flow cytometry analysis. In conclusion, c-kit+ bone marrow stem cells from diabetic mice exhibited an extensive enhancement of inflammatory factors and disorders of the extracellular matrix and adhesion molecules. Further intervention studies are required to determine the precise role of each molecule in the diabetes-related functional impairment of c-kit+ bone marrow stem cells.
Introduction
In the past decade, a number of studies have demonstrated that stem cells of bone marrow origin play very important roles in repairing/regenerating various organs [1]–[7], including the injured heart and vessels, through direct regeneration (cell differentiation/maturation) or indirect mechanisms (paracrine effects) [8]–[11]. Clinical trials have also attempted to treat ischemic heart diseases and peripheral arterial diseases by implanting autologous bone marrow-derived stem cells [12]–[17]. Some of these clinical trials have reported improvements in the clinical symptoms and regional perfusion of ischemia after treatment, but the therapeutic benefits observed were very marginal and mild, especially in patients of advanced age and those with diabetes and other diseases.
The inadequate efficiency of stem-cell-based therapy in patients with advanced age and other complications may be at least partially associated with the poor quality of cells used for implantation because aging and diabetes have previously been demonstrated to decrease the number and impair the function of bone marrow stem cells [18]–[22]. Therefore, attenuation of the impaired function of bone marrow stem cells should be a new approach to enhance the benefit of stem-cell-based therapy in these patients.
Our previous study found that oxidative stress likely contributes to the functional impairment of bone marrow stem cells in type 2 diabetic mice [23], but the precise mechanisms are not clearly understood. Using a pathway-focused microarray, we extensively compared the expression levels of inflammatory cytokines, cell cycle regulating factors, cell adhesion molecules and extracellular matrix molecules in c-kit+ bone marrow stem cells from diabetic mice and normal healthy mice, and we then attempted to further uncover the complex molecular mechanisms responsible for the diabetes-associated functional impairment of bone marrow stem cells.
Materials and Methods
Animals
We used 12-week-old male C57BLKS/J Iar-+Lepdb/+Lepdb (db/db) mice (SLC, Japan); this mouse strain is characterized by the spontaneous development of type 2 diabetes mellitus (DM). C57BLKS/J Iar-m+/+Lepdb (db/m+) mice were used as healthy controls. We measured the body weight and blood glucose levels of all mice before sacrifice for the collection and isolation of bone marrow stem cells. All experiments were approved by were approved by the Institutional Animal Care and Use Committee of Yamaguchi University (#2006012), and animal procedures were performed in accordance with institutional and national guidelines.
Collection, purification, and culture of c-kit+ bone marrow stem cells
Bone marrow cells were collected from the femur and tibia, and mononuclear cells were isolated by density gradient centrifugation. Stem cells expressing c-kit (c-kit+) were separated using a magnetic cell sorting system, as described previously [10], [19], [23]. Approximately 1.5% (0.86% to 2.12%) of the bone marrow mononuclear cells expressed c-kit, and the purity of sorted cells was approximately 90%.
Purified c-kit+ stem cells were seeded on 4-well chamber culture slides (Nalge Nunc International) coated with 10 µg/ml fibronectin (Invitrogen) at a density of 2×105 cells/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone), 100 units/ml penicillin, and 100 µg/ml streptomycin (Gibco). Cells were incubated at 37°C in 5% CO2 [10], [19].
ELISA
To measure the production of growth factors from c-kit+ stem cells, the supernatant was collected after 3 days of cell culture and stored at −80°C. The concentrations of VEGF, bFGF, and IL-1β in conditioned medium were measured with ELISA kits (R&D Systems), as described previously [10], [19].
Immunocytochemistry
For immunostaining analysis of endothelial differentiation, the cells were fixed in 1% formaldehyde after 7 days of culture. After blocking with Protein Block Serum-free (Dako), cells were reacted with phycoerythrin-conjugated anti-mouse vascular endothelial (VE)-cadherin antibody (Santa Cruz). Nuclei were stained with 4,6-diamino-2-phenylindole dihydrochloride (DAPI). The numbers of positively stained cells were counted under fluorescence microscopy with 200-fold magnification. Twenty random microscopic fields were selected in each chamber, and the mean numbers of positively stained cells per field were used for statistical analysis [10], [19], [23].
Gene expression analysis by pathway-focused GEArray
We compared the expression of inflammatory cytokines, cell cycle regulating factors, cell adhesion molecules and extracellular matrix molecules in c-kit+ bone marrow stem cells from diabetic and normal healthy mice using signaling pathway-specific gene expression GEArray systems (SuperArray Bioscience Corporation). The microarrays were used according to the manufacturer's instructions. Briefly, total RNA was extracted from freshly purified c-kit+ bone marrow stem cells. Using the reagents provided, cDNA was prepared from a mixture of purified total RNA of four independent samples by reverse transcription, biotinylated with Biotin-16-dUTP (Roche), and then hybridized under precisely specified conditions to a positively charged nylon membrane containing the arrayed DNA. The arrays were visualized using a chemiluminescent detection system (LAS-1000, FUJIFILM). Loading differences were accounted for on the basis of the intensity of the hybridization signals compared to the housekeeping genes, and then gene expression was quantified by scanning densitometry [24].
Flow cytometry
To confirm the expression levels of genes observed using the pathway-focused GEArray at the protein level, we collected mononuclear cells from mice. Cells were blocked with 0.5% BSA for 10 min and labeled with FITC-conjugated anti-mouse c-kit+ antibody for 30 min. After washing, cells were then incubated with antibodies against TNFR2, TLR4, integrin-αv, PECAM-1, fibronectin, or laminin (BD Bioscience). Respective isotype controls were used as a negative control. Quantitative flow cytometry analysis was performed using a FACSCalibur (Becton Dickinson) [7], [21].
Statistical analysis
All results are presented as the mean ± SD. Statistical significance between two groups was determined using the 2-tailed unpaired t-test (Dr. SPSS II, Chicago, IL). Differences were considered significant when p<0.05.
Results
Diabetes decreased the number and impaired the function of c-kit+ bone marrow stem cells
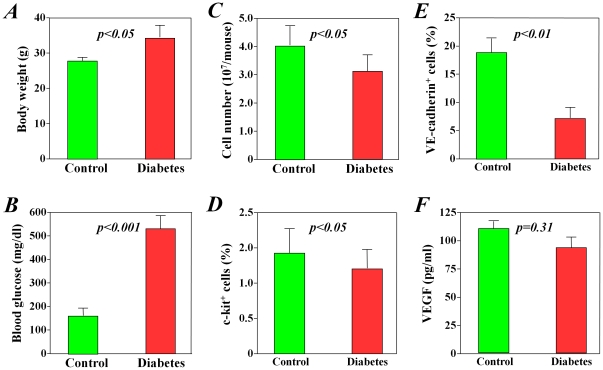
When compared with healthy mice, the db/db diabetic mice had significantly higher body weights (p<0.05, Figure 1A ) and blood glucose levels (p<0.001, Figure 1B ), confirming that these mice had early-stage type 2 diabetes mellitus (body weight loss at late-stage in these obesity type 2 diabetes mice). Although all procedures of cell collection and isolation were performed in the same way, significantly fewer bone marrow mononuclear cells were collected from diabetic mice than from healthy mice (p<0.05, Figure 1C ). Furthermore, the subpopulation of c-kit+ stem cells in freshly collected bone marrow mononuclear cells was also significantly smaller in diabetic mice than that from healthy control mice (p<0.05, Figure 1D ).
Figure 1. Differences in number and function of bone marrow stem cells between diabetic and healthy mice.
Compared with healthy control mice, diabetic mice had significantly lower body weights (A) and higher blood glucose levels (B). Significantly fewer total bone marrow mononuclear cells were harvested from diabetic mice than from healthy control mice (C), and the subpopulation of c-kit+ stem cells was significantly decreased in diabetic mice (D). After 7 days of culture, the c-kit+ stem cells from diabetic mice showed significantly less differentiation into VE-cadherin-positive endothelial cells than did cells from healthy control mice (E), but the production of VEGF did not differ between the two groups (F).
After 7 days of culturing purified c-kit+ stem cells, we found that cells from diabetic mice had poorer attachment and expressed lower levels of VE-cadherin when compared with cells from healthy control mice (p<0.05, Figure 1E ). However, the production of VEGF from c-kit+ stem cells did not differ significantly between groups at 3 days after culture (p = 0.31, Figure 1F ), in agreement with the results of our recent study using bone marrow mononuclear cells from diabetic patients [25]. The production of bFGF and IL-1β was not detectable by ELISA.
Diabetes increased the expression of inflammatory-related factors in c-kit+ bone marrow stem cells
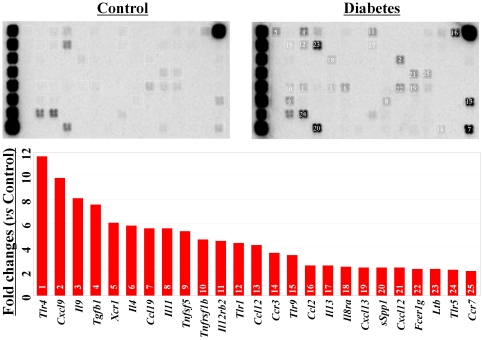
We compared the expression levels of inflammatory-related factors in freshly purified c-kit+ stem cells between diabetic and healthy control mice. Among the 84 genes included in the pathway-focused GEArray system, 25 genes were up-regulated more than 2-fold in diabetic mice when compared with healthy control mice ( Figure 2 ). These genes included Tlr4, Cxcl9, Il9, Tgfb1, Il4, ccl19, Il11, and Tnfsf5. No inflammatory-related gene was down-regulated more than 2-fold in the c-kit+ stem cells of diabetic mice.
Figure 2. Pathway-focused GEArray analysis of the expression of inflammatory-related factors.
Compared with the healthy control mice, the expression of inflammatory-related genes was extensively up-regulated in the c-kit+ bone marrow stem cells from diabetic mice.
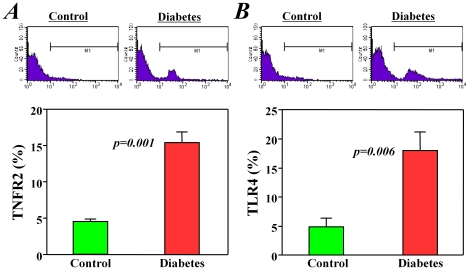
Furthermore, we confirmed some of the GEArray data at the protein level using flow cytometry analysis. Only approximately 4.5% of c-kit+ stem cells from healthy control mice positively expressed TNF receptor 2, but the expression of this protein was observed in approximately 15% of c-kit+ stem cells from diabetic mice (p<0.01, Figure 3A ). Similarly, the expression of toll-like receptor 4 was observed in approximately 20% of c-kit+ stem cells from diabetic mice but in only 5% of those from healthy control mice (p<0.01, Figure 3B ).
Figure 3. Expression of tumor necrosis factor receptor 2 (TNFR2) and toll-like receptor 4 (TLR4) in bone marrow stem cells.
Flow cytometry analysis showed that the positive expression levels of TNFR2 and TLR4 in the c-kit+ bone marrow stem cells from diabetic mice were significantly higher (>3-fold) than in cells from healthy control mice.
Diabetes did not change the cell cycle-related factors in c-kit+ bone marrow stem cells
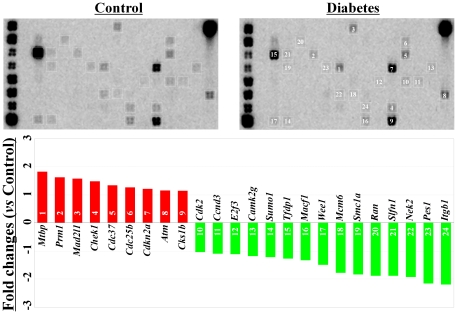
The expression of cell cycle-related factors in c-kit+ stem cells was also compared between diabetic and healthy control mice. Although many of the cell cycle genes were clearly detected by the GEArray system ( Figure 4 ), only two genes, Pes1 and Itgb1, were changed (down-regulation) more than 2-fold (2.2- and 2.3-fold, respectively) in diabetic mice when compared with healthy control mice ( Figure 4 ). Itgb1 is a well-known cell adhesion molecule, and we will discuss the data for this protein in detail below.
Figure 4. Pathway-focused GEArray analysis of cell cycle-related factors.
The expression of cell cycle-related genes was clearly detected in the c-kit+ bone marrow stem cells from both diabetic mice and healthy control mice. However, the difference observed in the expression levels between groups was very mild (<2-fold).
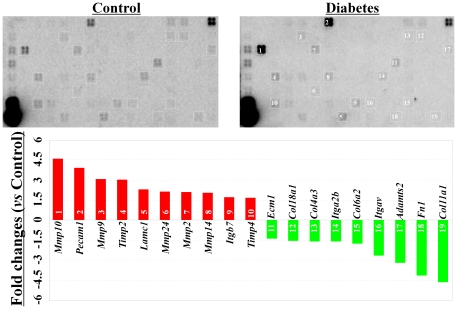
Diabetes induced varying changes in the expression levels of ECM and adhesion molecules in c-kit+ bone marrow stem cells
Interestingly, diabetes induced varying changes in the expression of ECM and cell adhesion molecules in c-kit+ stem cells. Among the 84 genes included in the GEArray system, 10 genes (MMP10, Pecam1, MMP9, Timp2, Lamc1, Mmp24, Mmp2, Mmp14, Itgb7, and Timp4) were up-regulated, and 9 genes (Col11a1, Fn1, Adamts2, Itgav, Col6a2, Itga2b, Col4a3, Col18a1, and Ecm1) were down-regulated more than 1.5-fold in diabetic mice when compared with those from healthy controls ( Figure 5 ).
Figure 5. Pathway-focused GEArray analysis of the gene expression of extracellular matrix and adhesion molecules.
Compared with the healthy control mice, 10 genes were up-regulated and 9 genes were down-regulated in the c-kit+ bone marrow stem cells from the diabetic mice.
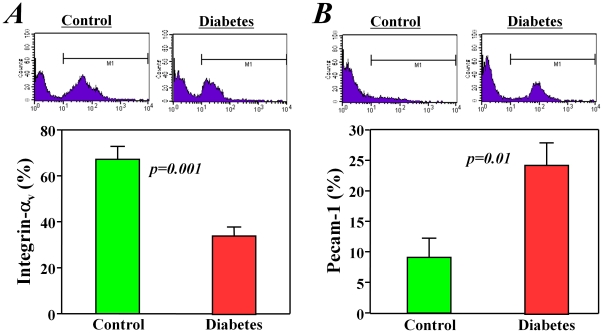
Furthermore, we examined some of these changes at the protein level using flow cytometry analysis. We confirmed that the expression of one important cell adhesion molecule, integrin-αv, was significantly decreased (p<0.01, Figure 6A ), but the expression of another important cell adhesion molecule, Pcam-1, was increased (p<0.01, Figure 6B ) in c-kit+ stem cells from diabetic mice when compared with healthy controls.
Figure 6. Expression of the adhesion molecules integrin-av and Pecam-1 in bone marrow stem cells.
Flow cytometry analysis showed that the expression of integrin-av (A) was decreased but that of Pecam-1 (B) was increased in the c-kit+ bone marrow stem cells from diabetic mice when compared with those from healthy control mice.
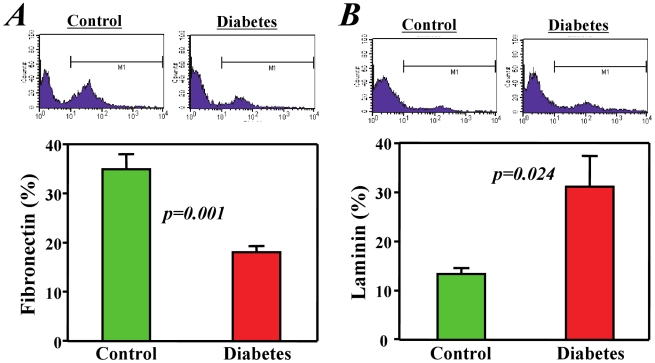
Similarly, diabetes induced varying changes in ECM protein expression in c-kit+ bone marrow stem cells. One important ECM protein, fibronectin, was significantly decreased (p<0.01, Figure 7A ), but laminin, another important ECM protein, was increased (p<0.01, Figure 7B ) in c-kit+ stem cells from diabetic mice when compared with healthy controls.
Figure 7. Expression of the extracellular matrix molecules fibronectin and laminin in bone marrow stem cells.
Flow cytometry analysis showed that the expression of fibronectin (A) was decreased but that of laminin (B) was increased in the c-kit+ bone marrow stem cells from diabetic mice when compared with cells from healthy control mice.
Discussion
Diabetes is well known to induce renal failure, impair hematopoiesis, and increase the risk of cardiovascular diseases [26], [27], but the precise mechanisms responsible for these diabetes-associated complications are not understood. In addition to hematogenesis, increased evidence has shown that bone marrow stem cells likely play important roles in repairing various tissues/organs [1]–[7]. Therefore, the reduced number and impaired function of bone marrow stem cells may contribute to the development of many complications. If we can understand the molecular mechanisms associated with the functional impairment of bone marrow stem cells, new approaches may be found to prevent and treat these diabetes-associated complications.
In this study, we purified c-kit+ bone marrow stem cells from early-stage diabetic mice (12 weeks old) for analysis and confirmed that diabetes is associated with a decrease in the number of c-kit+ bone marrow stem cells and that diabetes partially impairs their endothelial differentiation function [18]–[23].
Using a screening analysis of three of the most interesting pathways in this study, we obtained several interesting findings. First, the extensive enhancement of inflammatory factors in c-kit+ bone marrow stem cells from diabetic mice supported our speculation that systemic and local inflammatory microenvironments play central roles in the decrease in the number and functions of bone marrow cells. In fact, diabetes is considered an inflammatory disease that is characterized by increases in oxidative stress (e.g., the oxidation of glucose) and advanced glycation end-products (AGE) [23], [28], which induce the up-regulation of many inflammatory cytokines, such as MCP-1, TNF-α, IL-1, and IL-6 [29], [30]. We found that expressions of TNF receptor 2 and toll-like receptor 4 were significantly increased in these c-kit+ bone marrow stem cells from diabetic mice. Although it has reported that TNF receptor 2 is required for angiogenesis in response to ischemia [31], TNF receptors as mediators of apoptosis have been classically considered as negative regulators in the immune and hematopoietic systems to suppress hematopoietic stem and progenitor cell function [32]. Toll-like receptor 4 is primarily identified as an innate immune receptor, but it has been recently found to play a negative role in stem cells [33], [34]. Although further intervention experiments are required to identify the key mediators and the pathway signaling in detail, inflammatory stimulation likely contributes to the functional impairment of bone marrow stem cells in diabetes patients.
Because the number of c-kit+ bone marrow stem cells was decreased in diabetic mice, the proliferative activity of stem cells may be enhanced to maintain the homeostasis of the stem cell pool [35], [36]. However, the expression levels of cell cycle-related factors in c-kit+ bone marrow stem cells did not obviously change between diabetic and healthy control mice. This result may due to that fact that the cells used for analysis were harvested at the early-stage of diabetes, at which the number of stem cells in the bone marrow may not have dramatically decreased. Alternatively, the proliferative activity of bone marrow stem cells in diabetic mice may be suppressed by the inflammatory microenvironment or other unknown mechanisms.
The pattern of changes in the expression levels of ECM and adhesion molecules was quite interesting. As inflammation is known to change the expression of ECM and adhesion molecules in various types of cells, it is not difficult to conjecture that the systemic and local inflammatory microenvironment of diabetes may induce these changes in the expression levels of bone marrow stem cells. Inflammatory stimulation usually enhances the expression levels of ECM and adhesion molecules. However, approximately half of these ECM and adhesion molecules were down-regulated in bone marrow stem cells of diabetic mice. Thus, the mechanism responsible for the diabetes-induced disorder of ECM and adhesion molecules in bone marrow stem cells is not likely as simple as inflammatory stimulation. The down-regulation of Itgb1 was detected in the stem cells of diabetic mice (Figure 4). This result agreed well with results of our previous study that functional impairment of ex vivo expanded bone marrow stem cells was related to decreased expression of integrin-β1 [37]. Although the ECM and adhesion molecules are generally known to be critical for cell growth, it is poorly understood on their precise role in regulating the growth and differentiation of stem cells. In this study, we found that the expression of integrin αv was decreased in these c-kit+ bone marrow stem cells from diabetic mice. Agree well with our data, it has been demonstrated that oxidized low density lipoprotein induce functional impair of endothelial progenitor cell by down-regulation of E-selectin and integrin αvβ5 [38]. However, it has reported that the αv subunit of integrin does not appear to be involved with the attachment of embryonic stem cells to fibronectin [39]. An interventional approach of inflammatory inhibition will help us to demonstrate the causal relationship between inflammation and the disorder of ECM and adhesion molecules.
Several limitations of this study need to be mentioned. First, we detected extensive changes in the expression levels of many inflammatory factors, ECM molecules, and adhesion molecules, but we did not identify the factor(s) that would be critical to induce the functional impairment of bone marrow stem cells. Second, we only screened three pathways in this study, but diabetes may also induce changes in many other pathways that contribute to the functional impairment of bone marrow stem cells. Further in vitro and in vivo studies will be needed to elucidate how these changes affect the growth and function of bone marrow stem cells.
In summary, c-kit+ bone marrow stem cells from diabetic mice exhibited extensive enhancement of inflammatory factors and changes in the expression of ECM and adhesion molecules, which might contribute to the decrease in the number of bone marrow stem cells and the impairment of their function for endothelial differentiation. The identification of key factor(s) in future studies will enable us to find novel approaches to prevent or reverse the diabetes-related functional impairment of stem cells.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilchrist ES, Plevris JN. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transpl. 2010;16:118–129. doi: 10.1002/lt.21965. [DOI] [PubMed] [Google Scholar]
- 3.Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 4.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, et al. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22:1226–1236. doi: 10.1096/fj.07-8076com. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Westendorf JJ, Mödder UI. Concise review: Insights from normal bone remodeling and stem cell-based therapies for bone repair. Stem Cells. 2010;28:2124–2128. doi: 10.1002/stem.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng M, Zhou J, Wu M, Boriboun C, Thorne T, et al. CXCR4-mediated bone marrow progenitor cell maintenance and mobilization are modulated by c-kit activity. Circ Res. 2010;107:1083–1093. doi: 10.1161/CIRCRESAHA.110.220970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li TS, Suzuki R, Ueda K, Murata T, Hamano K. Analysis of the origin and population dynamics of cardiac progenitor cells in a donor heart model. Stem Cells. 2007;25:911–917. doi: 10.1634/stemcells.2006-0497. [DOI] [PubMed] [Google Scholar]
- 8.Dimmeler S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol. 2010;30:1088–1093. doi: 10.1161/ATVBAHA.109.191668. [DOI] [PubMed] [Google Scholar]
- 9.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li TS, Hayashi M, Ito H, Furutani A, Murata T, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–2445. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 12.Li TS, Murakami M, Kobayashi T, Shirasawa B, Mikamo A, et al. Long-term efficacy and safety of the intramyocardial implantation of autologous bone marrow cells for the treatment of ischemic heart disease. J Thorac Cardiovasc Surg. 2007;134:1347–1349. doi: 10.1016/j.jtcvs.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Esato K, Hamano K, Li TS, Furutani A, Seyama A, et al. Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant. 2002;11:747–52. [PubMed] [Google Scholar]
- 14.Fuchs S, Satler LF, Kornowski R, Okubagzi P, Weisz G, et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: a feasibility study. J Am Coll Cardiol. 2003;41:1721–1724. doi: 10.1016/s0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 15.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 16.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 17.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 18.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 19.Li TS, Furutani A, Takahashi M, Ohshima M, Qin SL, et al. Impaired potency of bone marrow mononuclear cells for inducing therapeutic angiogenesis in obese diabetic rats. Am J Physiol. 2006;290:H1362–1369. doi: 10.1152/ajpheart.00766.2005. [DOI] [PubMed] [Google Scholar]
- 20.Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, et al. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:57–66. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li TS, Kubo M, Ueda K, Murakami M, Ohshima M, et al. Identification of risk factors related to poor angiogenic potency of bone marrow cells from different patients. Circulation. 2009;120:S255–261. doi: 10.1161/CIRCULATIONAHA.108.837039. [DOI] [PubMed] [Google Scholar]
- 22.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 23.Ohshima M, Li TS, Kubo M, Qin SL, Hamano K. Antioxidant therapy attenuates diabetes-related impairment of bone marrow stem cells. Circ J. 2009;73:162–166. doi: 10.1253/circj.cj-08-0123. [DOI] [PubMed] [Google Scholar]
- 24.Li TS, Komota T, Ohshima M, Qin SL, Kubo M, et al. TGF-beta induces the differentiation of bone marrow stem cells into immature cardiomyocytes. Biochem Biophys Res Commun. 2008;366:1074–1080. doi: 10.1016/j.bbrc.2007.12.095. [DOI] [PubMed] [Google Scholar]
- 25.Li TS, Kubo M, Ueda K, Murakami M, Mikamo A, et al. Impaired angiogenic potency of bone marrow cells from patients with advanced age, anemia and renal failure. J Thorac Cardiovasc Surg. 2010;139:459–465. doi: 10.1016/j.jtcvs.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 26.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark CM, Jr, Lee DA. Prevention and treatment of the complications of diabetes mellitus. N Engl J Med. 1995;332:1210–1217. doi: 10.1056/NEJM199505043321807. [DOI] [PubMed] [Google Scholar]
- 28.Yan SF, Yan SD, Ramasamy R, Schmidt AM. Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med. 2009;41:408–422. doi: 10.1080/07853890902806576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, et al. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 30.Kempf K, Rose B, Herder C, Kleophas U, Martin S, et al. Inflammation in metabolic syndrome and type 2 diabetes: Impact of dietary glucose. Ann N Y Acad Sci. 2006;1084:30–48. doi: 10.1196/annals.1372.012. [DOI] [PubMed] [Google Scholar]
- 31.Goukassian DA, Qin G, Dolan C, Murayama T, Silver M, et al. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 33.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186:5367–5375. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shechter R, Ronen A, Rolls A, London A, Bakalash S, et al. Toll-like receptor 4 restricts retinal progenitor cell proliferation. J Cell Biol. 2008;183:393–400. doi: 10.1083/jcb.200804010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima H, Ito M, Smookler DS, Shibata F, Fukuchi Y, et al. TIMP-3 recruits quiescent hematopoietic stem cells into active cell cycle and expands multipotent progenitor pool. Blood. 2010;116:4474–4482. doi: 10.1182/blood-2010-01-266528. [DOI] [PubMed] [Google Scholar]
- 36.McClellan KA, Vanderluit JL, Julian LM, Andrusiak MG, Dugal-Tessier D, et al. The p107/E2F pathway regulates fibroblast growth factor 2 responsiveness in neural precursor cells. Mol Cell Biol. 2009;29:4701–4713. doi: 10.1128/MCB.01767-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li TS, Ito H, Hayashi M, Furutani A, Matsuzaki M, et al. Cellular expression of integrin-beta 1 is of critical importance for inducing therapeutic angiogenesis by cell implantation. Cardiovasc Res. 2005;65:64–72. doi: 10.1016/j.cardiores.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Di Santo S, Diehm N, Ortmann J, Völzmann J, Yang Z, et al. Oxidized low density lipoprotein impairs endothelial progenitor cell function by downregulation of E-selectin and integrin alpha(v)beta5. Biochem Biophys Res Commun. 2008;373:528–532. doi: 10.1016/j.bbrc.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 39.Baxter MA, Camarasa MV, Bates N, Small F, Murray P, et al. Analysis of the distinct functions of growth factors and tissue culture substrates necessary for the long-term self-renewal of human embryonic stem cell lines. Stem Cell Res. 2009;3:28–38. doi: 10.1016/j.scr.2009.03.002. [DOI] [PubMed] [Google Scholar]