Abstract
To evaluate bladder function recovery after spinal cord injury (SCI) in response to a combination treatment of an acutely administered AMPA/kainate receptor antagonist and delayed transplantation of neuronal precurors. Female rats received a contusion injury at T8/9. The AMPA/kainate receptor antagonist NBQX was directly administered into the lesion site immediately after injury. Nine days post-injury, NRP/GRP were delivered into the lesion site. Controls received NRP/GRP grafts only or no treatment (OP-Controls). Animals underwent bladder function testing during the course of the experiment and at the endpoint. Motor function was evaluated as well. After sacrifice, histological analysis of lesion site and lumbosacral spinal cord regions was performed. Rats receiving the combined treatment (NBQX&NRP/GRP) had voided volumes/micturition resembling that of normal animals and showed greater improvement of urodynamic parameters, compared to NRP/GRP alone or OP-Controls. Similarly, NBQX&NRP/GRP induced more spouting, regeneration or sparing of descending projections to the lumbosacral cord. The density of primary afferent projections at the lumbosacral spinal cord in rats with combined treatments was similar to that of NRP/GRP alone with decreased sprouting of primary afferents in lumbosacral cord, compared to OP-Control. Immunohistochemical evaluation revealed that the combined treatment reduced the size of the lesion to a greater extent than NRP/GRP alone or OP-Controls. NRP/GRP with and without NBQX produced significant recovery of hindlimb compared to OP-Controls. In conclusions, transplants of NRP/GRP combined with NBQX promote recovery of micturition function following spinal cord injury, likely through increased neuroprotection.
Keywords: spinal cord injury, bladder function, NBQX, neural stem cells, combination therapy
1. INTRODUCTION
Spinal cord injury (SCI) is a challenging and complex injury characterized by initial mechanical trauma and the ensuing cascade of secondary injury. Secondary injury processes begin immediately after the injury and continue for weeks, months and years resulting in paralysis, sensory deficits, and autonomic dysfunction that includes the loss of lower urinary tract control and sexual function [Oyinbo, 2011, Tator and Fehlings, 1991]. The lack of spontaneous repair following SCI is the result of the intrinsic inability of CNS neurons to regenerate, as well as impediments triggered by the injury, including inflammatory events and scar formation [Schwab, 2002], which cause permanent deficits of motor, sensory and autonomic function. An effective therapeutic intervention has to protect the spinal cord from the consequences of secondary injury as well as promote a repair process that may include axon regeneration across the inhibitory lesion site and reestablishment of functional connections. To date no single treatment approach is capable of such all-encompassing effects. Thus, combination therapies seeking to target more than one secondary injury process constitute increasingly attractive approaches for treating SCI. For example, combining interventions aimed at minimizing the damaging effects of an early-occurring secondary injury mechanism such as excitotoxicity together with delayed treatments that target cyst formation, loss of cells and axonal degeneration, would be of interest particularly if they promote connectivity and repair.
In previous studies, we have shown that neural precursors composed of neuronal and glial-restricted precursors (NRP/GRP) have a potential to form a neuronal relay by extending active axons across the injured spinal cord to the intended target [Bonner, et.al., 2011] and delayed transplantation of NRP/GRP into a mid-thoracic spinal cord contusion modified the intraspinal lumbosacral circuitry and significantly improved locomotion and bladder function [Mitsui, et.al., 2005b]. While we found significant recovery of bladder function associated with delayed NRP/GRP transplantation, recovery remained only partial, suggesting that additional therapeutic interventions are necessary. One target for acute treatment are AMPA/kainite receptors, which have been implicated in the excitotoxicity-mediated tissue damage that ensues within minutes following traumatic SCI. Focal microinjection of the AMPA receptor antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX) was shown to significantly decrease the amount of tissue loss following SCI [Rosenberg, et.al., 1999, Wrathall, et.al., 1994, Wrathall, et.al., 1997]. This greater tissue sparing with NBQX treatment was accompanied by a significant increase in serotonin immunoreactivity caudal to the lesion, compared to controls. Both effects were dose-dependent, and increased tissue sparing correlated with a significant increase in functional recovery [Wrathall, et.al., 1994]. Acute or delayed treatment with NBQX was effective in reducing functional deficits associated with SCI [Wrathall, et.al., 1994, Wrathall, et.al., 1997].
In the present study, we expand on our previous work on delayed transplantation of NRP/GRP into mid-thoracic contusion and examine whether the inclusion of NBQX at the acute stage of SCI will further improve the efficacy of delayed cell transplantation with respect to lower urinary tract function. We hypothesized that such a combination will provide additional protection and regeneration in the damaged spinal cord and further modify the lumbosacral circuitry for better functional outcome.
2. MATERIALS AND METHODS
Animal Groups
Thirty female Sprague-Dawley rats (225–250 g; Taconic, Germantown, USA) were studied. Ten rats received contusion alone; one was lost during surgery (OP-Controls, n=9). Ten rats received contusion and a NRP/GRP transplant 9 days post-injury; 2 animals were lost in surgery or due to incorrect injury (NRP/GRP alone, n=8). An additional ten rats received NBQX injection immediately after contusion and a delayed NRP/GRP transplant 9 days post-injury (NBQX&NRP/GRP, n=10). Rats had free access to food and water and were kept under a 12 hr light/dark cycle. All procedures were approved by the Drexel University College of Medicine's Institutional Animal Care and Use Committee and conformed to the National Institute of Health guidelines for the care and use of laboratory animals.
Preparation of NRP/GRP
Neural precursors composed of NRP/GRP were isolated from embryonic day 13.5 transgenic Fischer 344 rats that express the marker gene human placental alkaline phosphatase (AP). The transgenic animal has previously been characterized [Wrathall, et.al., 1994] and the preparation of NRP/GRP has been previously described [Han, et.al., 2002, Han, et.al., 2004, Lepore, et.al., 2004]. Following dissection, NRP/GRP were co-cultured for 5–10 days and then re-suspended at a concentration of 100,000 cells/μl for transplantation and placed on ice throughout surgery. After surgery, cell viability was assessed and found to be greater than 90% using trypan blue.
Spinal cord injury
Following anesthesia with intraperitoneal injection of XAK cocktail containing xylazine (10mg/Kg), acepromazine maleate (0.7mg/Kg) and ketamine (95mg/Kg), a laminectomy was performed at T8/9 and a contusion injury produced using the impact rod of the MASCIS device dropped from a height of 25mm [Gruner, 1992] and allowed to rest on the spinal cord for 5 seconds. Muscle and skin were closed in layers. Rats were placed on heating pads, and closely observed until awake before returning to their home cage. Ampicillin (Bristol-Myers Squibb Company, Princeton, NJ, USA) was injected twice daily for 7 days postoperatively as a prophylaxis for urinary tract infection. Bladders were manually expressed twice daily until sacrifice, except during testing in metabolic cages.
Cell transplants
All animals received subcutanteous administration of CsA injection solution (Sandimmune, Novartis Pharmaceuticals Co., East Hanover, New Jersey, USA) at a dose of 1mg/100g/24hr beginning 3 days before the transplantation and continued for 2 weeks post-transplantation. After this, oral CsA solution (50μg/ml, Neoral, Novartis Pharmaceuticals Co.) was administered through the drinking water until sacrifice. Nine days post-contusion, animals were re-anesthetized with intraperitoneal injection of XAK cocktail. Animals were placed in a spinal stereotaxic frame and spinal cords were re-exposed. Using a 10μl Hamilton syringe with 30 gauge needle, NRP/GRP were injected into the spinal cord. 5μl deposits suspended in liquid collagen (Vitrogen [Cohesion; Palo Alto, CA]) were injected at the injury center and 2.5μl deposits injected 3mm rostral and caudal to injury center. Cells or medium were injected over 5 min under the control of a microsyringe pump controller (World Precision Instruments, Sarasota, FL) and the tip was slowly withdrawn.
NBQX administration
NBQX sodium salt (Sigma) was dissolved in deionized water at a concentration of 3 mg/ml and filtered. NBQX (15 nmol) was microinjected into the center of the injury immediately after contusion over a period of 10min [Wrathall, et.al., 1994]. The volume of solution and slow injection paradigm were used to maximize focal delivery to the injury, based on preliminary dye injection experiments.
Urodynamic tests
1. Micturition pattern
Rats were placed in a metabolic cage for 24 hrs (Nalgene Metabolic Cage, Nalge Co., New York, USA) to examine voiding behavior pre-operatively and at weekly intervals after transplantation. The bladders were expressed manually before the animals were placed in the metabolic cage. The urine voided during the next 24 hours was collected on an electronic scale (FORT250, World Precision Instruments, Sarasota, USA), connected to a microcomputer, for recording micturition frequency and volume [Mitsui, et.al., 2003, Mitsui, et.al., 2005a, Mitsui, et.al., 2005b]. Data were recorded and stored using data acquisition software (WINDAQ, DATAQ Instruments, Akron, USA). The voided volume per micturition was compared between experimental groups.
2. Cystometry in awake rats
At 8 weeks post transplantation, rats were anesthetized under isoflurane inhalation and the bladder was exposed by a midline lower abdominal incision. A polyethylene catheter (PE-60, Clay-Adams, New Jersey, USA) was implanted into the bladder through the dome, as described previously [Mitsui, et.al., 2003, Mitsui, et.al., 2005a, Mitsui, et.al., 2005b]. The bladder catheter was tunneled subcutaneously and exited through the skin on the back.
Following catheter implantation, rats were placed in a restraining cage (KN-326, Natsume, Tokyo, Japan) and allowed to recover for 1–2 hours. The bladder catheter was connected to a pressure transducer (BLPR, World Precision Instruments, Sarasota, FL, USA) and a microinjection pump (STC-523, Terumo, Tokyo, Japan). Room-temperature saline was infused at a rate of 0.1 ml/min. Micturition cycles stabilized and became regular after about 30 min of saline infusion. Three micturition cycles were collected after stabilization. The averages of maximal voiding pressure, post-void residual urine, bladder capacity, and the frequency of non-voiding contraction (NVC) in these micturition cycles were compared among groups. Fluid voided from the urethral meatus was collected to determine the voided volume. Residual fluid was first withdrawn through the catheter and then the bladder was expressed manually by applying pressure on the abdominal wall to collect the remaining intravesical contents. Bladder capacity was calculated as the voided volume plus residual volume. NVC was defined as rhythmic intravesical pressure increases greater than 5 mmHg from baseline without a release of fluid from urethra [Mitsui, et.al., 2003, Mitsui, et.al., 2005a, Mitsui, et.al., 2005b]. transducer (BLPR, World Precision Instruments, Sarasota, FL, USA) and a microinjection pump (STC-523, Terumo, Tokyo, Japan). Room-temperature saline was infused at a rate of 0.1 ml/min. Micturition cycles stabilized and became regular after about 30 min of saline infusion. Three micturition cycles were collected after stabilization. The averages of maximal voiding pressure, post-void residual urine, bladder capacity, and the frequency of non-voiding contraction (NVC) in these micturition cycles were compared among groups. Fluid voided from the urethral meatus was collected to determine the voided volume. Residual fluid was first withdrawn through the catheter and then the bladder was expressed manually by applying pressure on the abdominal wall to collect the remaining intravesical contents. Bladder capacity was calculated as the voided volume plus residual volume. NVC was defined as rhythmic intravesical pressure increases greater than 5 mmHg from baseline without a release of fluid from urethra [Mitsui, et.al., 2003, Mitsui, et.al., 2005a, Mitsui, et.al., 2005b].
Tests of motor function
Rats were habituated to the open-field locomotion BBB test preoperatively and tested postoperatively at 2–3 days post-injury, and every week after transplantation. Tests were scored by trained blinded observers who had inter-rater reliability greater than 95%. Rats were placed in an open field and observed for 2 minutes. Hindlimb function was scored using the BBB locomotor rating scale [Basso, et.al., 1995].
Tissue Preparation
Animals were anesthetized with intraperitoneal injections of sodium pentobarbital (100mg/kg, Abbot Laboratories, North Chicago, IL) and sacrificed via intracardiac perfusion with 200ml of 0.1 M, pH 7.4 phosphate buffer (PB) followed by 500ml of ice cold 4% paraformaldehyde fixative in PB. The spinal cord was removed and postfixed for 24 hrs in the same fixative at 4°C followed by cryoprotection in phosphate-buffered 30% sucrose solution for 3–5 days. Tissues were serially blocked, embedded in OCT compound (Fisher Scientific) and kept at −80°C before being cut into 30μm coronal sections at the L6-S1 level or sagital sections at the level of the injury.
Lesion size
Lesion size of spared host spinal cord was estimated from Nissl-Myelin stained sections. The volumes of the lesion/transplant and spared tissue were calculated for every tenth section [Mitsui, et.al., 2005b, Neuhuber, et.al., 2008]. The total volume of the cord segment with the lesion epicenter in the middle was first determined. The volume of normal appearing spinal tissue within the 11 mm spinal segment was determined by subtracting the cyst, damaged tissue and graft volume from the volume of the entire segment.
Projection patterns at L6-S1
Coronal sections at the L6-S1 level were immersed in 0.1 M, pH 7.6, phosphate-buffered saline (PBS) for free floating staining using the avidin-biotin complex method. Sections were permeabilized with 10% goat serum in PBS for 2 hrs, then incubated with the appropriate primary antibody (Serotonin (5-HT) (1:50,000, ImmunoStar Inc., Hudson, WI), Calcitonin gene-related peptide (CGRP) (1:6000, Peninsula Laboratories Inc, San Carlos, CA)) and 2% goat serum in PBS containing 0.3% Triton X-100 at 4°C for 24–72 hrs, and finally reacted with a species-specific biotinylated secondary antibody and the ABC reagent (Vector, Burlingame, CA), each for 2 hrs at room temperature. Staining was visualized with Sigma fast DAB (Sigma Chemical). Tissue sections were mounted on gelatin-coated slides, dehydrated in graded ethanol, cleaned in xylene, and coverslipped. All sections were examined with bright-field microscopy.
Image Analysis
Images were captured using a Photometric Sensys KAF-1400 CCD camera (Roper Scientific; Trenton, NJ) attached to a Leica DMRBE fluorescence microscope (Leica Microsystems; Bannockburn, IL). Images were analyzed using NIH Image, IP Lab (Scanalytics Inc., Fairfax, VA). Densitometric analysis of images was carried out as previously described [Mitsui, et.al., 2005b].
Statistical Analysis
Cystometric data were analyzed using one-way ANOVA between groups. BBB was analyzed using two-way ANOVA between group and time, with time as a repeated measure beginning at one week post-transplantation. Post-hoc analysis was performed using Fisher's post hoc test for parametric data or Mann-Whitney U test for nonparametric data. Immunocytochemical density data were analyzed using one-way ANOVA between groups, followed by Fisher's post hoc test where appropriate. Data are presented as group mean ± standard error. Significance levels were set to 0.05 for all comparisons.
3. RESULTS
Recovery of Lower Urinary Tract Function
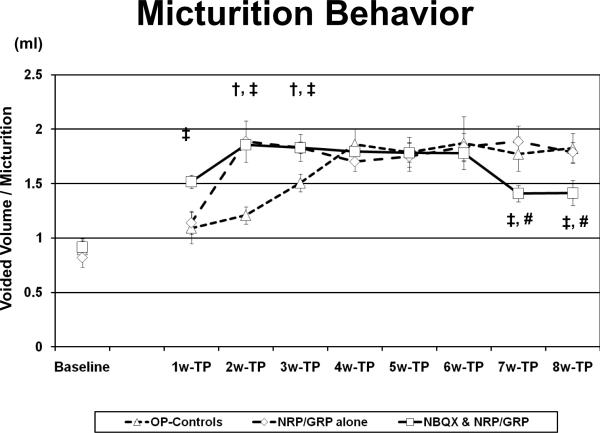
Voided volume per micturition increased in the NBQX&NRP/GRP group by week 1. In the NRP/GRP alone group, voided volume per micturition increased by week 2 after transplantation although OP-Controls reached the same level by week 4 (Fig. 1). Thus, NRP/GRP transplantation combined with NBQX administration as well as NRP/GRP transplantation alone accelerated recovery of bladder contraction from the spinal shock phase and increased voiding efficiency, compared to OP-Controls. In rats with NRP/GRP transplantation combined with NBQX administration, voided volume per micturition decreased at week 7 after transplantation, resembling preoperative or normal control values. This decrease of voided volume per micturition is related to the recovery of bladder capacity to normal value observed in cystometry (Table 1).
Fig. 1.
Micturition behavior in metabolic cages.
In the NBQX&NRP/GRP group, voided volume per micturition increased by week 1 compared to OP-controls (p<0.05). In the NRP/GRP alone group, voided volume per micturition increased by week 2 after transplantation (p<0.05), OP-Controls reached the same level only by week 4. At week 7 after transplantation, voided volume per micturition decreased in NBQX&NRP/GRP administration (p<0.05), which is close to preoperative or normal control values.
†: NRP/GRP alone vs. OP-Controls
‡: NBQX & NRP/GRP vs. OP-Controls
#: NRP/GRP alone vs. NBQX & NRP/GRP
Table 1.
Summary of urodynamic parameters
| OP-Controls | NRP / GRP alone | NBQX&NRP/GRP | |
|---|---|---|---|
| [n=9] | [n=8] | [n=10] | |
| [Parameters] | |||
| Micturition Pressure (mmHg) | 36.1±2.7 | 27.9±2.0† | 26.7±1.3‡‡ |
| Residual Urine (ml) | 0.16±0.04 | 0.14±0.08 | 0.09±0.03 |
| Bladder Capacity (ml) | 1.54±0.06 | 1.53±0.17 | 0.99±0.14‡‡,# |
| Non-voiding contraction (No. of episodes / Micturition) | 7.0±1.0 | 3.4±0.9† | 1.6±0.5‡‡ |
| Bladder Weight (mg) | 368.3±35.0 | 289.6±19.6† | 229.8±11.5‡‡ |
p<0.05,NRP/GRP alone vs. OP-Controls
p<0.01
p<0.05,NRP/GRP alone vs. NBQX&NRP/GRP
##:p<0.01
‡NBQX&NRP/GRP vs. OP-Controls
As in previous studies [Mitsui, et.al., 2005a, Mitsui, et.al., 2005b, Neuhuber, et.al., 2008], urodynamic parameters in OP-Controls showed that a number of NVC were observed during filling phase and bladder capacity, postvoid residual urine volume and micturition pressure were increased compared to un-operated normal rats. Urodynamic parameters in OP-Controls, NRP/GRP alone and NBQX&NRP/GRP rats are summarized in Table 1. Compared to OP-controls, both NRP/GRP recipients had significantly improved micturition pressure (P<0.05 in NRP/GRP alone) and fewer episodes of NVC (p<0.05 in NRP/GRP alone); rats with combined therapy of NRP/GRP and NBQX had slightly better values of micturition pressure (p<0.01) and NVC episodes (p<0.01). Although bladder capacity returned close to normal in rats with combined treatment of NBQX&NRP/GRP, there was no improvement in NRP/GRP alone. Postvoid residual urine volume was the only measure, which was not different among the three operated groups.
Bladder weight (Table 1) was significantly less in the NRP/GRP recipients compared to OP-Controls (p<0.05 in NRP/GRP alone, p<0.01 in NBQX&NRP/GRP). Combined treatment of NRP/GRP and NBQX produced further reduction of bladder weight, although it was only a trend compared to NRP/GRP alone (p=0.07). Thus, NRP/GRP transplants promote partial recovery of bladder function, and combined treatment of NRP/GRP and NBQX promotes further recovery of voiding, which contributes to the reduction in bladder hypertrophy.
Recovery of spontaneous motor function
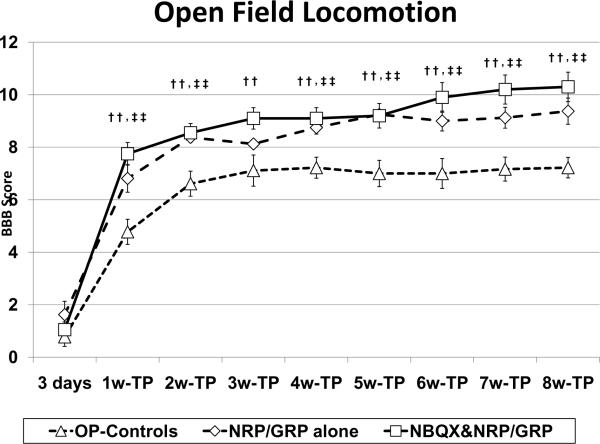
We found that open field locomotion was significantly better in the NRP/GRP alone than in OP-Controls. While OP-Control rats achieved a BBB score of 7.2±0.4, without weight support, NRP/GRP alone treated rats showed improvement to the same level in BBB scores by week 4, achieving a score of 9.4±0.5 by week 8, which indicates weight support (Fig. 2). In rats with combined treatment of NBQX&NRP/GRP, locomotor function recovered to the same degree as NRP/GRP alone until week 5 after transplantation; however, recovery continued slightly even after week 6, achieving an end score of 10.3±0.6 (Fig. 2), although this did not result in a statistically significant difference between NBQX&NRP/GRP and NRP/GRP alone. Nevertheless, the BBB score was improved in NRP/GRP alone, and optimizing the combined treatment of NBQX&NRP/GRP may have a potential to further improve recovery of locomotor function.
Fig. 2.
Function of hindlimb
Hindlimb motor function was assessed using the open-field BBB test. NRP/GRP recipients showed improvement in BBB scores, beginning at week 4 and maintained through week 8, compared to OP-Controls. (††: p<0.01, ‡‡: p<0.01)
†: NRP/GRP alone vs. OP-Controls, ‡: NBQX & NRP/GRP vs. OP-Controls
Tissue protection
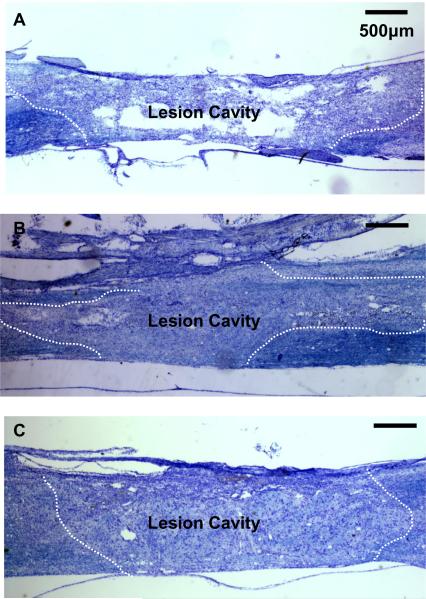
The modified-moderate contusion injury destroyed the dorsal spinal cord in the thoracic region, including the dorsal columns, the corticospinal tract and the dorsolateral funiculus, damaged the dorsal horn and impinged on the intermediolateral column. The ventral funiculi and the ventral portions of the lateral funiculi were spared. Nissl/Myelin stained sections showed cyst formation and cavitation in OP-Control animals (Fig. 3-A). In NRP/GRP alone (Fig. 3-B) and NBQX&NRP/GRP animals (Fig. 3-C), these cyst formation and cavitation were reduced with cells filling the lesion. From Nissl/Myelin stained sections we calculated the spared/total spinal cord volume in OP-Controls, NRP/GRP alone and NBQX&NRP/GRP (Table 2). The volume of spared host spinal tissue was significantly greater in NRP/GRP alone compared to OP-controls. Combined treatment of NBQX&NRP/GRP protected more host tissue than single treatment of NRP/GRP. These results indicate that NRP/GRP transplants and NBQX injection have a potential of protection of host tissue and reduction of secondary injury and this effect is greater in combined treatment of NBQX and NRP/GRP.
Fig. 3.
Nissl-Myelin staining at the lesion site. Dotted lines indicate lesion border.
A) Longitudinal section of spinal cord from OP-Control rat. The central lesion consisted cyst formation and cavitation.
B) Longitudinal section of spinal cord in Nissl-Myelin staining shows survival of NRP/GRP transplanted into the injured spinal cord at 8 weeks post-transplantation. Dotted line indicates graft-host interface. The cavity was largely filled with transplanted cells, and few cysts were apparent.
C) Longitudinal section of spinal cord in Nissl-Myelin staining shows that transplanted NRP/GRP survived and combined treatment of NRP/GRP and NBQX protected more host tissue than single treatment of NRP/GRP.
Table 2.
Spinal cord lesion volume
| OP-Controls | NRP/GRP alone | NBQX&NRP/GRP | |
|---|---|---|---|
| Spared spinal cord volume (mm3) | 12.1 ± 0.7 | 16.6 ± 1.5†† | 22.6 ± 0.9‡‡,## |
| Spared spinal cord volume /Total spinal cord volume (%) | 58.8 ± 1.5 | 71.1 ± 2.1†† | 75.1 ± 1.6‡‡,# |
p<0.01
p<0.01
p<0.05,NRP/GRP alone vs. NBQX&NRP/GRP
p<0.01
†NRP/GRP alone vs. OP-Controls
‡NBQX&NRP/GRP vs. OP-Controls
Reorganization of descending modulatory pathways
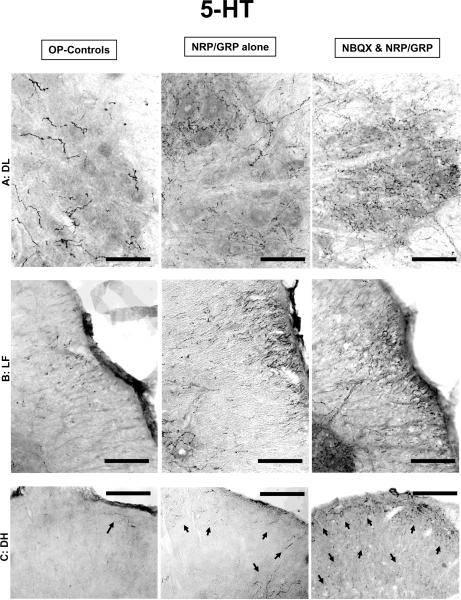
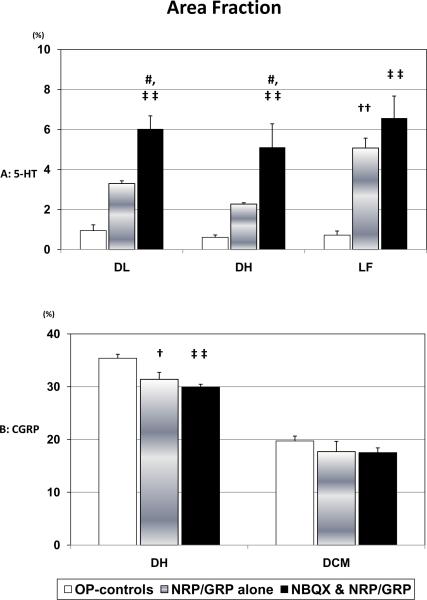
Several sites in the brainstem project serotonergic (5-HT) axons into the lumbosacral spinal cord. These pathways, particularly those projecting to the dorsolateral nucleus (DL), have been implicated in the central control of bladder and urethra and recovery of bladder-external urethral sphincter coordination in SCI rats [Ding, et.al., 1995, Marson, 1997, Nadelhaft and Vera, 1996, Pikov and Wrathall, 2001, Vizzard, et.al., 1995]. 5-HT-positive fibers in the DL were significantly denser in NBQX&NRP/GRP rats compared to NRP/GRP alone or OP-Controls (Fig. 4-A, 6-A). Rats with NRP/GRP alone also had higher 5-HT fiber density in the DL than OP-controls, but it was not statistically significant (Fig. 6-A). In NRP/GRP recipients, but not OP-Controls, numerous 5-HT fibers were identified in the lateral funiculus (LF) adjacent to the DL. 5-HT fiber density in the LF was higher in rats with combined treatment of NBQX and NRP/GRP (Fig. 4-B, 6-A). Serotonergic fibers in the dorsal horn (DH), which are associated with modulation of pain reception [Suzuki, et.al., 2002], were observed following the same trend as the DL (Fig. 4-C, 6-A). Thus, combined treatment of NBQX&NRP/GRP can induce more spouting, regeneration or sparing of 5-HT projections to the lumbosacral spinal cord.
Fig. 4.
Descending modulatory pathways. Bars indicate 100μm.
A) Serotonergic fibers in the DL were more densely distributed with significant difference in NBQX&NRP/GRP rats compared to NRP/GRP alone or OP-Controls, which close to unoperated normal controls
B) In NRP/GRP recipients, but not Unoperated Controls or OP-Controls, 5-HT fibers were identified in the LF adjacent to the DL nucleus, which was much denser in rats with combined treatment of NRP/GRP and NBQX.
C) In the DH, 5-HT-positive fibers in the DH were much denser in NBQX&NRP/GRP rats compared to NRP/GRP alone or OP-Controls (arrow).
Fig. 6.
Area fraction at the L6 spinal cord
A) Serotonergic fibers in the DL were more densely distributed with significant difference in NBQX&NRP/GRP (p<0.01). In NRP/GRP recipients, but not OP-Controls, 5-HT fibers were identified in the LF, which was more dense in rats with combined treatment of NRP/GRP and NBQX (p<0.01). Serotonergic fibers in the DH (p<0.01), were observed with the same trend as the DL.
B) CGRP-immunoreactivity in the DH was significantly denser in OP-Controls than in NRP/GRP alone (p<0.05) or NBQX&NRP/GRP (p<0.01), which was similar to Unoperated Controls. Meanwhile, there were no differences in DCM among groups.
†: NRP/GRP alone vs. OP-Controls
‡: NBQX & NRP/GRP vs. OP-Controls
#: NRP/GRP alone vs. NBQX & NRP/GRP
Reorganization of primary afferent pathways
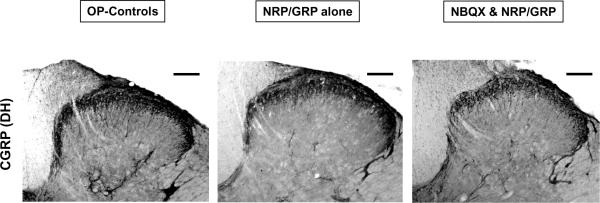
Sprouting of the small diameter dorsal root afferents, such as CGRP-positive fibers, that convey pain and visceral information to the spinal cord has been implicated in autonomic dysfunction. CGRP-immunoreactivity in the DH was denser in OP-Controls than in NRP/GRP alone or NBQX&NRP/GRP (Fig.5, 6-A, OP-Controls vs. NRP/GRP alone: p<0.05, OP-Controls vs. NBQX&NRP/GRP p<0.01), although there were no differences in CGRP projections to the dorsal commissure (DCM) among the groups (Fig.5, 6-B). These results suggest increased sprouting of small caliber dorsal root axons to the DH in OP-Controls, compared to NRP/GRP alone and NBQX&NRP/GRP, which resembled unoperated normal rats in the previous study [Mitsui, et.al., 2005b].
Fig. 5.
Primary afferent targets in the DH. Bars indicate 100μm.
Distribution of immunoreactivity of CGRP appeared denser in OP-Controls than NRP/GRP alone and NBQX&NRP/GRP, which was similar in Unoperated normal rats.
4. DISCUSSION
Lower urinary tract dysfunction that frequently accompanies SCI, results from damage to descending pathways and alterations in primary afferent pathways. It is difficult to manage and contributes to poor quality of life [Liu, et.al., 2010, Widerstrom-Noga, et.al., 2004]. SCI produces a dyssynergia between bladder and urethral sphincter, leading to functional bladder outlet obstruction, identified by urinary retention and increased micturition pressure. NVC, manifested as phasic bladder contractions during urine storage, results in urinary incontinence and high intravesical pressures, leading to bladder hypertrophy and deterioration of the upper urinary tract. Even modest increases in bladder pressure can elicit episodes of autonomic dysreflexia that can be life-threatening [Hagen, et.al., 2011, Santajuliana, et.al., 1995]. While there are some treatments for NVC, dyssynergia between the bladder and urethral sphincter remains difficult to manage without catheterization or surgical interventions. We have shown in previous studies that NRP/GRP could form a neuronal relay by extending active axons across the injured spinal cord to the intended target [Bonner, et.al., 2011] and delayed transplantation NRP/GRP resulted in increased tissue sparing, modified intraspinal lumbosacral circuitry and improved bladder function and locomotion [Mitsui, et.al., 2005b, Neuhuber, et.al., 2008]. However, recovery of bladder control was limited, suggesting that additional treatments are necessary to achieve greater functional recovery.
Secondary injury is initiated by the excessive release of excitatory aminoacids, activation of their receptors and damaging tissue processes such as free-radical release. Therefore, a potential target for acute treatment is the AMPA/kainite receptor, which has been implicated in the excitotoxicity-mediated tissue damage that ensues within minutes following traumatic SCI. Administration of NBQX, a AMPA receptor antagonist has been shown to significantly decrease tissue loss following spinal cord contusion, accompanied by a significant increase in serotonin immunoreactivity caudal to the lesion [Rosenberg, et.al., 1999, Wrathall, et.al., 1994]. The beneficial effects of NBQX on tissue sparing and functional recovery are believed to be the result of its ability to mitigate oligodendrocyte cell death post-SCI, and to alleviate the associated white matter pathology [Rosenberg, et.al., 1999]. Actually, NBQX alone decreased lesion volume and improved hinlimb function as the same level as NRP/GRP alone in the pilot study (data not shown).
In the present study, we show that combined treatments of NBQX at the acute stage of SCI and delayed transplantation of NRP/GRP produce further improvement of lower urinary tract function. Micturition pressures were lower in NRP/GRP recipients, indicating amelioration of dyssynergia between bladder and urethral sphincter [Mitsui, et.al., 2003, Mitsui, et.al., 2005a, Mitsui, et.al., 2005b]. We suggest that dyssynergia in SCI is associated with the loss of supraspinal projections into the lumbosacral spinal cord that normally modulate function. NRP/GRP transplanted into an incomplete injury effectively reduced the damage potentially by reducing secondary injury to these modulatory systems. There were fewer episodes of NVC in NRP/GRP recipients compared to OP-Controls. The reduction of NVC suggests an attenuation of hyperactive bladder reflexes that normally follow SCI, perhaps because sprouting from pathological bladder afferents was diminished. Bladder capacity in NBQX&NRP/GRP resembled unoperated normal rats [Mitsui, et.al., 2005b], suggesting that bladder sensation could be recovered.
More sprouting or sparing of descending pathways (5-HT) was observed in NBQX&NRP/GRP close to normal values in the lumbosacral spinal cord [Mitsui, et.al., 2005b]. Also, the volume of spared host spinal tissue was significantly greater in rats with NBQX&NRP/GRP compared to single treatment of NRP/GRP or NBQX (data no shown). Thus, the potential for greater descending control over spinal targets mediating control of locomotor function and coordination between bladder and urethral sphincter function could be increased in animals with combined treatment. In addition, NRP/GRP transplants inhibited dorsal root sprouting (CGRP) resembling normal values [Mitsui, et.al., 2005b], such as c-fibers, which has been implicated in NVC. NRP/GRP transplants may favor sprouting by spared descending pathways and thus inhibit sprouting of afferent axons.
5. CONCLUSIONS
Transplants of NRP/GRP combined with NBQX further promote recovery of bladder function following spinal cord injury, through increased protection and plasticity of the spinal cord. The combination of pharmacotherapy for inhibition of secondary injury with cellular transplants thus offers a rich potential in the search for treatments of lower urinary tract dysfunction in SCI.
Highlights
NBQX&NRP/GRP accelerated recovery of bladder contraction from spinal shock.
NBQX and neural precursors improved cystometric parameters in SCI.
NBQX and neural precursors protected host spinal tissue in SCI.
NBQX and NRP/GRP induced descending projections to the lumbosacral cord.
NBQX&NRP/GRP inhibited sprouting of primary afferents in lumbosacral cord.
ACKNOWLEDGMENTS
This study was supported by NIH grant NS055976 and funds provided by the Spinal Cord Research Center of Drexel University College of Medicine. We would like to thank Drs. Amanda Conta, Jed Shumsky and Marion Murray for thoughtful comments and careful reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- [2].Bonner JF, Connors TM, Silverman WF, et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4675–86. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ding YQ, Takada M, Tokuno H, et al. Direct projections from the dorsolateral pontine tegmentum to pudendal motoneurons innervating the external urethral sphincter muscle in the rat. J Comp Neurol. 1995;357:318–30. doi: 10.1002/cne.903570210. [DOI] [PubMed] [Google Scholar]
- [4].Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–6. doi: 10.1089/neu.1992.9.123. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- [5].Hagen EM, Faerestrand S, Hoff JM, et al. Cardiovascular and urological dysfunction in spinal cord injury. Acta Neurol Scand Suppl. 2011:71–8. doi: 10.1111/j.1600-0404.2011.01547.x. [DOI] [PubMed] [Google Scholar]
- [6].Han SS, Kang DY, Mujtaba T, et al. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol. 2002;177:360–75. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- [7].Han SS, Liu Y, Tyler-Polsz C, et al. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- [8].Lepore AC, Han SS, Tyler-Polsz CJ, et al. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–26. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu CW, Attar KH, Gall A, et al. The relationship between bladder management and health-related quality of life in patients with spinal cord injury in the UK. Spinal Cord. 2010;48:319–24. doi: 10.1038/sc.2009.132. [DOI] [PubMed] [Google Scholar]
- [10].Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- [11].Mitsui T, Kakizaki H, Tanaka H, et al. Immortalized neural stem cells transplanted into the injured spinal cord promote recovery of voiding function in the rat. J Urol. 2003;170:1421–5. doi: 10.1097/01.ju.0000075501.05758.33. [DOI] [PubMed] [Google Scholar]
- [12].Mitsui T, Fischer I, Shumsky JS, et al. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol. 2005a;194:410–31. doi: 10.1016/j.expneurol.2005.02.022. [DOI] [PubMed] [Google Scholar]
- [13].Mitsui T, Shumsky JS, Lepore AC, et al. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005b;25:9624–36. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol. 1996;375:502–17. doi: 10.1002/(SICI)1096-9861(19961118)375:3<502::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [15].Neuhuber B, Barshinger AL, Paul C, et al. Stem cell delivery by lumbar puncture as a therapeutic alternative to direct injection into injured spinal cord. J Neurosurg Spine. 2008;9:390–9. doi: 10.3171/SPI.2008.9.10.390. [DOI] [PubMed] [Google Scholar]
- [16].Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–99. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- [17].Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci. 2001;21:559–69. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19:464–75. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Santajuliana D, Zukowska-Grojec Z, Osborn JW. Contribution of alpha- and beta-adrenoceptors and neuropeptide-Y to autonomic dysreflexia. Clin Auton Res. 1995;5:91–7. doi: 10.1007/BF01827469. [DOI] [PubMed] [Google Scholar]
- [20].Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–31. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- [21].Suzuki R, Morcuende S, Webber M, et al. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–26. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- [22].Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- [23].Vizzard MA, Erickson VL, Card JP, et al. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol. 1995;355:629–40. doi: 10.1002/cne.903550411. [DOI] [PubMed] [Google Scholar]
- [24].Widerstrom-Noga E, Cruz-Almeida Y, Krassioukov A. Is there a relationship between chronic pain and autonomic dysreflexia in persons with cervical spinal cord injury? J Neurotrauma. 2004;21:195–204. doi: 10.1089/089771504322778659. [DOI] [PubMed] [Google Scholar]
- [25].Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J Neurosci. 1994;14:6598–607. doi: 10.1523/JNEUROSCI.14-11-06598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wrathall JR, Teng YD, Marriott R. Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol. 1997;145:565–73. doi: 10.1006/exnr.1997.6506. [DOI] [PubMed] [Google Scholar]