Abstract
This article analyzes the efficacy of the Together for Empowerment Activities (TEA) intervention in decreasing depressive symptoms and improving social support for persons living with HIV (PLH) and their family members. A total of 79 families, consisting of 88 PLH and 79 family members, were recruited from Anhui province, China, and randomized to the TEA intervention (n = 38) or a control condition (n = 41). The intervention was delivered at three levels: 1) TEA Gathering (small group for PLH and family members); 2) TEA Time (home-based family activities with children that accompany each TEA Gathering); and 3) TEA Garden (community events that build social integration). Face-to-face interviews were administered at baseline, 3, and 6 months. Mixed effects regression models and kernel density estimation were used for data analysis. PLH and their family members in the intervention reported significant improvements in depressive symptoms, social support, and family functioning at the 3-month and 6-month follow-up assessments compared to those in the control condition. Heterogeneous intervention effects on social support and family functioning were indicated at the 6-month follow-up. The intervention could have various effect patterns for different subgroups within the intervention condition. This study provides preliminary data to support the feasibility and efficacy of a multilevel intervention.
Keywords: HIV, family, intervention, depression, China, social support
INTRODUCTION
The China Ministry of Health estimates that there were 740,000 persons living with HIV (PLH) in China by the end of 2009 (Ministry of Health China, 2010). PLH in China face the same challenges as those in other parts of the world: maintaining physical health and treatment adherence, dealing with HIV-related disclosure and stigma, maintaining positive family relationships and social support, and dealing with mental health issues such as depression (Bartlett & Gallant, 2001; Derlega & Barbee, 1998; Hamra et al., 2005; Herek, 1999; Kalichman, 1995; Lee et al., 2002; Parker & Aggleton, 2003; Rotheram-Borus et al., 2001; Silver, E.B. et al., 2003; Thomas et al., 2005). These stressors are interconnected, emphasizing the need for interventions that not only target PLH, but also strengthen the family to adapt to a life living with HIV.
Several studies have reinforced that HIV has an impact on family members (e.g., Bor et al., 1993; Pequegnat et al., 2001; Prachakul et al., 2007; Rotheram-Borus et al., 2005; Schuster et al., 2000). For example, psychological distress, such as symptoms of depression, has been found to impact not only PLH but also their families (Feaster & Szapocznik, 2002; Heckman et al., 2004; Moore et al., 2006). Once HIV serostatus has been disclosed to family members, treatment becomes a challenge for the entire family (Li et al., 2007). Caring for PLH often poses significant challenges, including physical and emotional suffering, socioeconomic burden, and an increased risk of acquiring HIV due to a lack of access to self-protection materials (Lee et al., 2010; Ogden et al., 2004; Palattiyil & Chakrabarti, 2008).
With increased access to antiretroviral therapy (ARV), the focus on immediate survival from acute infection has shifted to coping with HIV as a chronic illness. HIV-related issues are also becoming prolonged challenges faced by PLH and their family members. The Chinese government has guaranteed access to ARV for many PLH, reflecting a shift in policy and opportunities for families coping with HIV (Wu et al., 2007). China's new policies in testing and treatment have resulted in an increased number of families living with HIV in the country, but there are few intervention programs to help families cope with HIV-related challenges. Worldwide, only a limited number of intervention studies have been done in the domain of strengthening families living with HIV (Bhana et al., 2010; Kmita et al., 2002; Li et al., 2010). Together for Empowerment Activities (TEA) is the first family intervention implemented in China, targeting not only PLH but also other family members. The intervention is designed to be implemented at multiple levels involving PLH, seronegative family members, children, and community. The goal of this pilot study was to examine the intervention effect with data collected at baseline, 3-, and 6-month follow-up assessments. This study evaluated potential intervention effects in decreasing depressive symptoms, improving perceived social support, and improving family functioning of PLH and family members participating in the TEA intervention compared to that of a control group.
METHODS
Participants
This study was conducted from August 2009 to April 2010 in Anhui Province, China, a region where over two-thirds of HIV infections were caused by paid plasma donations (Wu et al., 1995; Wu et al., 2001). The following inclusion criteria were used for the initial screenings: 1) confirmation of an AIDS diagnosis or HIV-positive status; 2) having a seronegative family member at home; and 3) having a family member who was aware of the HIV status of the PLH and willingness to participate in the study. Family members who were informed of the PLH's HIV status were eligible to participate in the study. The PLH participants were recruited first and asked to invite an adult family member who was considered their primary caregiver in the family to join the study. All family members were contacted after obtaining permission from the PLH participants. With the PLH's permission and recommendation, the selected family members were approached and recruited with a separate process of informed consent. Of the 81 families who met eligibility criteria, 79 families consented to participate in the study.
Procedures
All procedures and forms were reviewed and approved by the Institutional Review Board of the University of California, Los Angeles, the Institutional Review Board of the Anhui Province Center for Disease Prevention and Control, and the Medical Institution Review Board of Anhui Medical University. With assistance from village health workers, project recruiters approached potential participants by following standardized scripts to ensure all ethical issues were covered and consent was secured. Following informed consent, in-person interviews were conducted either at a family's home or another preferred venue such as a village clinic. During the interview, PLH and family members were asked about their demographics, including age, gender, educational status, marital status, annual income, and HIV status, and about their health, mental health, family relationship, social support, and access to services. Each interview took about 45 to 60 minutes. All participants were paid 50 yuan (equivalent to U.S. $8.00) for the assessment.
Study Design
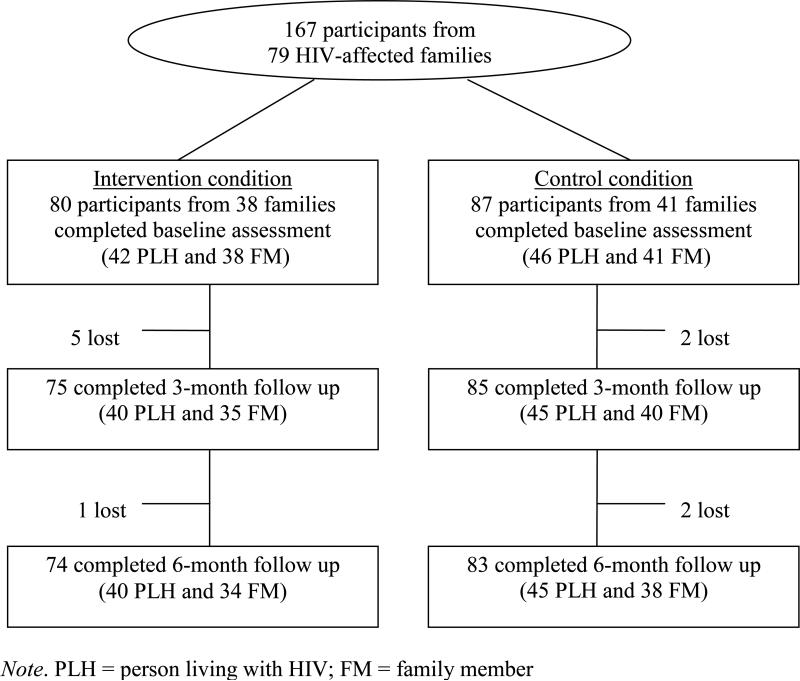
This study is an intervention pilot with a cluster randomization design. A total of 79 families from four villages were recruited into either the intervention or a control group. The village, not the family, was the unit of randomization. All families included one PLH and one seronegative family member, except for eight families where two PLH and one family member were recruited. The study sample contains 167 participants who completed the baseline assessment and were randomized to the intervention (n = 80) or the control (n = 87) condition (Fig. 1). Participants in both conditions were assessed at 3 and 6 months after baseline. The intervention effect was assessed by comparing the different levels of outcome measure between the intervention and the control condition over a period of 6 months.
FIGURE 1.
Flow of study participants.
Intervention Methods
Together for Empowerment Activities (TEA), an intervention conceptualized and developed based on social action theory (Ewart, 1991), emphasizes social interdependence and its connection to personal health. According to social action theory, individual behavior change requires a framework that incorporates contextual influences as well as self-change processes (Ewart, 1991). Family is an important contextual influence in the lives of PLH. The quality of one's relationships and the family's support significantly impact not only the physical and mental health of PLH, but also the well-being of the family as a whole. Other contextual factors (e.g., community stigma, availability of ARV, healthcare support) are also relevant under the social action framework. Based on this framework we considered the family as a dynamic unit and an individual's health as being influenced by close personal relationships and environmental contextual factors. We identified our main outcomes as individual mental health, family functioning and social support.
The TEA intervention underwent three phases of development. Two needs assessments were conducted in 2005 to 2006 to identify particular challenges to the target population (Ji et al., 2007; Li et al., 2009; Lin et al., 2008; Sun et al., 2008). Based on the findings, an intervention feasibility pilot was conducted with 20 HIV-affected families in 2007. The pilot included three prototype sessions, and there were two focus groups for PLH and family members, respectively. Feedback from the groups helped to shape the intervention in a format more easily accepted by the target population and delivered by local health educators. The last phase of development involved refinements during multiple intervention training and team practice sessions. During this phase, the intervention team performed mock sessions to smooth the flow between activities and sessions; local culture elements were added, including local role models and local expression and language. The intervention manual was finalized after incorporating all local feedback.
As shown in Table 1, the TEA intervention includes three modules (Healthy Body & Healthy Mind, Positive Family Interactions, and Quality of Life), with each module containing two TEA Gatherings. Each TEA Gathering is associated with and followed by a TEA Time activity, which is discussed at the beginning of the next TEA Gathering, and each module concludes with a TEA Garden event. The intervention content and topics reflect the identified challenges faced by HIV-affected families. For example, Session 1 of TEA Gathering emphasizes the importance of physical health and healthy lifestyle by maintaining a daily family routine. Session 2 addresses issues related to good mental health and learning coping skills addressing stress. Session 3 aims to enhance family unity to overcome difficulties and address life challenges. Session 4 strengthens positive relationships for family support between parents and children and other family members. Session 5 addresses how parents or primary caregivers in a family play an active role in building confidence and supporting healthy child development while facing adversity. Finally, session 6 emphasizes community integration to deal with stigma and discrimination.
TABLE 1.
Summary of TEA intervention activities.
| Preparation Session | Sign-in and introduction, activities (Pair share, Role play, Relaxation), group rules, IRB, Q&A, distributing cameras and learning how to use for home work |
||
|---|---|---|---|
| TEA Gathering | TEA Time | TEA Garden | |
| Module 1: Healthy body & healthy mind | Session 1: Staying in healthy daily routine | Family kitchen; Family album | Community health fair |
| Session 2: Maintaining good mental health | Family emotion rainbow; Table topics | ||
| Module 2: Positive family interactions | Session 3: Strengthening family unity to overcome difficulties | Fabric of family; My colorful family bags | Community sporting event |
| Session 4: Developing positive parent-child relationships for family support | “I love my family” memory book | ||
| Module 3: Quality of life | Session 5: Building confidence and growing up with adversity | My dream: a children paintings show | Children painting exhibition and Family talent show |
| Section 6: Integrating into community and contributing to society | A tea party for neighbors and friends | ||
The implementation of the TEA intervention included three levels of activities: 1) TEA Gathering (six small group sessions for PLH and family members, after a preparation section); 2) TEA Time (six kinds of home-based family activities with all family members including children after each TEA Gathering session); and 3) TEA Garden (three community events that build social integration). While all TEA aspects are connected, each follows its own schedule. Each group session lasted about two hours, and it took about two and a half months to complete all the intervention activities for a study site.
The TEA intervention was delivered by a team of intervention facilitators recruited from a pool of health educators working at various agencies at provincial, country, and township levels. This collaboration allowed us to combine the merits from different facilitators to strengthen the overall quality of the delivery. Provincial CDC facilitators provided prominent knowledge and policy understanding, while local facilitators provided knowledge of local cultural norms and experience working with local people. All intervention facilitators went through intensive training and numerous mock sessions. Facilitators worked together for all activities. For example, for each group session, each facilitator played a role throughout the session, although the presenters might change from topic to topic. The facilitators were only responsible for the intervention delivery and had no role in the assessment activities in order to avoid potential biases.
Measures
Depressive symptoms were measured using the short version of the Zung Self-Rating Depression Scale (Zung, 1965). This is a 9-item instrument adapted from the original 20-item questionnaire. Participants were asked how often they felt each of the nine situations, including “I feel down-hearted and blue,” “I get tired for no reason,” and “I have trouble sleeping at night.” Response categories were from (1) “a little of the time” to (4) “most of the time.” The overall scale was the sum of the individual items. Some items were reverse coded so that a higher score indicated a higher level of depressive symptoms. Cronbach's alpha value for this scale was 0.81.
Social support was measured using the 19-item item scale developed by Medical Outcome Study (MOS) Social Support Survey (Sherbourne & Stewart, 1991). The scale was used to measure the perceived availability of different types of support and covered four domains: 1) eight items for emotional/informational support, 2) four items for tangible support, 3) three items for affectionate support, and 4) three items for positive social interaction. In addition, there was one item on overall support. For each item, participants were asked how often that kind of support was available. Responses were recorded on a 5-point scale ranging from (1) “none of the time” to (5) “all of the time.” Scores were the sum of ratings from all of the domains, with a higher score indicating a better level of social support. Cronbach's alpha for the overall scale was 0.91.
Family functioning was measured by an adapted version of the family functioning scale (Bloom, 1985; Bloom & Naar, 1994). The original scale has 75-items consisting of 15 sub-scales measuring dimensions of family relationship, system maintenance, and personal growth. For this study, three subscales—family cohesion, family conflict, and family sociability—were selected. Each of the subscales consists of five items. For each item, participants were asked to rate how true each statement was for their own family on a 4-point Likert scale. The score was the sum of all 15 items. Some items were reverse coded so that a higher score indicated better family functioning. Cronbach's alpha was 0.83.
Participant demographic information such as age, gender, education, marital status, occupation, and income was collected. Age was computed by subtracting the reported year of birth from the assessment year. Annual individual income instead of family income was used in this study because of differing definitions of “family”.
Statistical Analysis
An intent-to-treat approach was used to analyze intervention effects. Baseline differences between intervention and control samples were tested using Chi-square and t tests (or Wilcoxon rank tests) for categorical and continuous variables, respectively. Mixed-effects regression models with family- and participant-level random effects were used to assess the intervention effect on the improvement of depressive symptoms, social support, and family functioning measures. Covariates included age, gender, HIV status, group (control vs. intervention), visit (baseline, 3-, or 6-month follow-up), and group-by-visit interaction. The model included two levels of random effects, family- and participant-level random effects, to account for dependence within families and the correlation between repeated observations for each participant. Multilevel modeling allowed for separation of the nested sources of variation, which helped to avoid underestimation of the fixed effect variances and increased efficiency in identifying important sources of variation (Snijders & Bosker, 1999).
We graphically examined the improvement in each of the three outcome measures over time between the control and intervention groups using kernel density estimation (KDE) (Silverman, 1986). KDE is a non-parametric way of estimating the probability density function of a random variable, and can provide valuable features used in comparing data such as different shapes of distribution (i.e., skewness) and multimodality. This may be easily seen from the analysis. In some cases, they will yield conclusions that may be regarded as self-evidently true, while in other cases all they will do is to point the way to further analysis. We calculated the KDE curves of each primary outcome measure and plotted them by group and by time.
From the KDE analyses, we recognized that the intervention effects might be different for PLH and caregivers. Thus, we conducted sensitivity analyses using the model described above, separately for PLH and caregivers, for each of the outcome measures. All statistical analyses were carried out with the SAS System for Windows (Version 9.2) and all of the graphs were generated using the publicly available statistical software R (R Development Core Team, 2011).
RESULTS
Demographic Characteristics at Baseline
Participant demographics and baseline characteristics are summarized in Table 2. Of the 79 families, 38 families (n = 80) were randomized to the intervention condition and 41 families (n = 87) to the control condition (Table 2). About 52% of the participants were women, and slightly more than half (52%) of the participants were HIV-positive. All PLHs were parents. More than half of the family members were the PLH's spouse. The other participants included parents and sisters or brothers. The average age of participants was about 41; over 88% reported being married, and more than 50% of the participants were full-time farmers. Thirty-nine percent of the control participants versus 24% of the intervention participants had an educational level of junior high school or above. Only 15% of the PLH reported no education, whereas 28% of their caregivers reported no education (P = 0.0382). More than half of the sample reported having an annual income of 2,000 yuan (U.S. $310.00) or less per year. Sixty percent of the PLH vs. 34% of the caregivers were male (P = 0.0008). At baseline, no significant differences were observed for HIV status, gender, age, marital status, education, occupation, or annual income. Comparable levels of depressive symptoms, social support, and family functioning across the two intervention conditions were observed.
TABLE 2.
Demographic and background characteristics at baseline, by treatment group.
| Control (n =87) |
Intervention (n=80) |
||
|---|---|---|---|
| Characteristics | Number (%) | Number (%) | Pa |
| HIV Positive | 46 (52.9) | 42 (52.5) | 0.96 |
| Gender | 0.92 | ||
| Male | 42 (48.3) | 38 (47.5) | |
| Female | 45 (51.7) | 42 (52.5) | |
| Age (Year) (M ± SD) | 40.4 ± 8.8 | 41.5 ± 8.2 | 0.43 |
| 35 or younger | 22 (25.3) | 14 (17.5) | |
| 36-40 | 23 (26.4) | 28 (35.0) | |
| 41-45 | 26 (29.9) | 22 (27.5) | |
| 46 or older | 16 (18.4) | 16 (20.0) | |
| Marital status | 0.56 | ||
| Married/living as married | 77 (88.5) | 73 (91.3) | |
| Other | 10 (11.5) | 7 (8.8) | |
| Education (Year) (M ± SD) | 4.8 ± 3.1 | 3.9 ± 3.3 | 0.06 |
| No schooling | 15 (17.2) | 20 (25.0) | |
| Primary school | 38 (43.7) | 41 (51.3) | |
| Junior high or higher | 34 (39.1) | 19 (23.8) | |
| Occupation | 0.13 | ||
| Full-time farmer | 45 (51.7) | 42 (52.5) | |
| Part-time farmer | 17 (19.5) | 24 (30.0) | |
| Other | 25 (28.7) | 14 (17.5) | |
| Annual Individual Income (Yuan) | 0.49 | ||
| ≤2000 | 39 (44.8) | 38 (48.1) | |
| 2001-5000 | 26 (29.9) | 27 (34.2) | |
| 5001 or more | 22 (25.3) | 14 (17.7) | |
| Depressive Symptoms M ± SD | 19.4 ± 5.8 | 18.6 ± 5.1 | 0.35 |
| Social Support M ± SD | 56.2 ± 15.7 | 57.3 ± 13.9 | 0.64 |
| Family Functioning M ± SD | 44.7 ± 5.4 | 44.5 ± 5.4 | 0.79 |
Significance level of difference between intervention and control.
Attrition
As shown in Figure 1, there was little attrition. No significant differences were observed in attrition between the intervention and the control group. Of the 80 participants assigned to the intervention, 93.8% completed the 3-month and 92.5% completed the 6-month follow-ups. Similarly, of the 87 participants in the control group, 97.7% completed the 3-month and 95.4% completed the 6-month assessments. The overall follow-up rates were 95.8% at 3 months and 90.1% at 6 months, respectively.
The intervention attendance was also excellent. Based on the field report, the vast majority of the intervention participants (about 87%) completed all sessions; among the 13% of participants who partially completed the intervention, most missed one session because of the weather or illness.
Intervention Effects on Depressive Symptoms
Table 3 presents the results for depressive symptoms, social support, and family functioning measures from the mixed-effects regression models. Comparing the intervention and the control groups, we observed a significant decrease in depressive symptoms over time. A significant interaction effect, intervention-by-visit, indicates that the reduction of the level of depressive symptoms was significantly larger for the intervention participants compared to the control participants at the 3- and 6-month follow-ups.
TABLE 3.
Estimates, standard errors and p-values from mixed-effects regression model with two-level random effects.
| Depressive Symptoms |
Social Support |
Family Functioning |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P |
| Age | 0.11 | 0.03 | 0.001 | -0.39 | 0.10 | 0.001 | -0.06 | 0.04 | 0.12 |
| Gender (M - F) | -2.06 | 0.53 | 0.0001 | 5.55 | 1.68 | 0.001 | 1.97 | 0.63 | 0.002 |
| HIV Status | 3.40 | 0.53 | <.0001 | 0.29 | 1.67 | 0.86 | -1.70 | 0.62 | 0.006 |
| Intervention Effect at Baseline (Intervention - Control) | -0.87 | 0.84 | 0.30 | 1.72 | 2.71 | 0.53 | -0.13 | 1.14 | 0.91 |
| Time (in month) | <.0001 | <.0001 | 0.001 | ||||||
| Intervention × Time | <.0001 | <.0001 | 0.001 | ||||||
| Estimated Difference in Change from Baseline (Intervention - Control) | |||||||||
| 3-Month | -4.00 | 0.78 | <.0001 | 9.70 | 2.58 | 0.001 | 3.66 | 1.13 | 0.001 |
| 6-Month | -3.01 | 0.79 | 0.0002 | 10.24 | 2.60 | <.0001 | 4.55 | 1.14 | <.0001 |
Age was positively associated with level of depressive symptoms, as were gender and HIV status. Women reported a higher level of depressive symptoms than men, and PLH were more likely than their seronegative family members to have depressive symptoms.
Intervention Effects on Social Support and Family Functioning
Across both the intervention and control groups, we observed a significant increase in both social support and family functioning measures over time (Table 3). Significant interaction effects for both measures were observed, indicating that the improvements of social support and family functioning were significantly higher for the intervention participants compared to the control participants at the 3-month follow-up. At the 6-month follow-up, stronger intervention effects were indicated for both measures.
Gender was significantly associated with levels of social support and family functioning. Male participants were likely to perceive better social support and family functioning than their female counterparts. Age was negatively associated with level of social support; HIV status was significantly associated with lower level of family functioning.
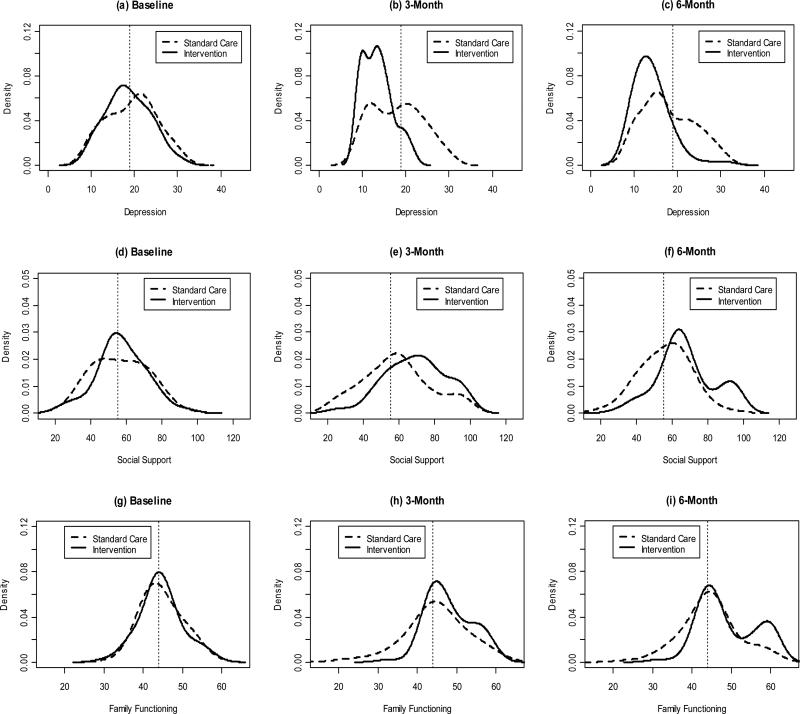
Kernel Density Estimation Curves of Outcome Measures over Time
Figure 2 presents the kernel density estimation (KDE) of depressive symptoms, social support, and family functioning at baseline, 3-, and 6-month follow-ups. For depressive symptoms, Figure 2a shows the control and intervention KDE curves almost completely overlap. The intervention curve clearly shifts toward the left in Figure 2b, implying that the level of depressive symptoms decreased at the 3-month follow-up, whereas some improvement was also observed for the control group. Figure 2c shows the intervention effect preserved at the 6-month follow-up.
FIGURE 2.
Kernel density curves for depression (top row), social support (middle row), and family functioning (bottom row) by group at baseline (first column), 3-month follow-up (second column), and 6-month follow-up (third column). Solid curve represents intervention and dashed curve represents control. The vertical dashed line represents the median score at baseline.
Similarly, the two KDE curves for social support almost completely lie on top of each other at baseline. Figure 2e shows the intervention curve shifts to the right, meaning the level of social support increased after 3 months, whereas almost no change was observed for the control group. At the 6-month follow-up, the intervention curve on the right side of the reference line in Figure 2f appears to split into two modes, one moving slightly to the left and the other one not moving at all. This reveals that after 6 months of the intervention, the improvement of social support was fading for some intervention participants; however, some intervention participants were able to preserve the effect. We observed a similar effect on family functioning, but the effect was slightly weaker.
Stratified Analyses
We conducted stratified analyses to further explore potential intervention effects on outcomes for PLH and family members, respectively. We observed that the results for PLH were very similar to those found in the main analyses, and the results for depression and social support for family members were similar to those found in the main analyses. However, there was no significant intervention effect on family functioning for caregivers. We also observed that the bimodal distribution on family functioning measure was related to HIV status. When we examined the KDE curves separately for PLH and caregivers (data not shown), we clearly observed the bimodal distribution on family functioning for PLH in the intervention group. A subset of PLH in the intervention group (the group with the higher mode) showed a significantly higher rate of improvement on family functioning. Compared to the group with the lower mode, more women were in this group; 87% of them reported primary school or higher education; and they were about 4 years younger on average.
DISCUSSION
This article details a pilot study of the first multilevel intervention for families affected by HIV in China. The main finding from the outcome comparison was that PLH and their family members in the intervention reported significant improvements in depressive symptoms at the 3-month and 6-month follow-ups compared to those in the control condition. The positive outcomes we observed might result from the unique approach of the intervention. Maintaining mental health is one major challenge faced by PLH and their family members. A PLH's psychological states can affect the mental health or well-being of their family members; at the same time, family members’ coping and mental health can also influence a PLH's psychological states. The concept of a multilevel intervention was based on the assumption that individual behavior is interwoven with multiple layers; therefore, interventions that bring strategies to address these levels can simultaneously achieve a greater overall impact. TEA is a case study of such interventions. Chinese society is tight-knit, and the shame felt by families affected by HIV can destroy family pride, and devastate family identity and overall community integration (Li et al., 2008). The results of this study provide evidence that positive outcomes for PLH and family members can be achieved with a multilevel intervention designed and implemented to meet the population needs and complement cultural norms.
This study revealed that women had a higher level of depressive symptoms than men. This finding is not surprising given the burden of the HIV epidemic may impact differentially across gender, perhaps reflecting women's and men's different roles in household and family activities. Chinese families tend to take responsibility for their sick family members and relatives (Yang et al., 2003), and women are often the main caregivers. The situation could become more stressful when the caregiving woman herself is infected, as she struggles to balance her own health concerns with the demands of her families. For women, the gender roles imposed by society may increase their vulnerability to the consequences of HIV. We suggest that future intervention programs give special attention to women's mental health and coping with HIV and the related burden of being a caregiver.
There are several limitations to this study. First, this is an intervention outcome study with a relatively small sample. Second, to be eligible for this study, a PLH had to have at least one family member who knew about their HIV status. It is possible that the outcome measures reported by those who did not disclose their status differ from our study participants. Caution should be exercised when study findings are generalized to PLH who did not disclose their HIV status. Third, although validated in previous studies, all outcome measures used in this study were prone to potential biases associated with self-reports. Furthermore, we assessed depressive symptoms rather than diagnosis for depression. Thus, the findings cannot be generalizable to PLH with clinical depression. Also, we were not able to link the intervention attendance information to the outcomes in this study, which remains an important area to explore in the future. Finally, because of the short study period, we could only assess the intervention outcomes at the 3- and 6-month follow-up assessments; therefore, we cannot determine whether the benefits from the intervention are sustainable in the long-term.
This study has implications for future programs. As HIV infections increase in China, more and more PLH and family members will rely on the strength of their families to cope with psychological stress and other HIV-related challenges. HIV in China is predominantly a rural epidemic. Yet, many rural areas—where most of China's HIV-positive population resides—do not have the capacity to monitor patients’ treatment and care (Gill & Okie, 2007; Wu et al., 2007). Identifying interventions that can be successfully applied in rural settings is critical to combat HIV in China. Our study underscores the importance of intervention programs that address amenable factors such as depressive symptom, social support, and family functioning. For example, mental health issues should be recognized and treated in a timely way. A multidisciplinary approach is necessary to address multifaceted challenges to generate understanding and information for policy development. Program developers and service providers working with PLH may find it useful to address mental health challenges in their programs. There is great potential that the TEA intervention activities can be applied to not only rural families in China, but also families in other countries with limited resources.
We learned from this study that the intervention effects on social support and family functioning sustained for some of the participants while they decreased for others. The graphical examination of the improvement in outcome measures over time revealed a bimodal distribution at 6 months for some of the outcomes (e.g., a significant intervention effect on family functioning for PLH, but not for their caregivers, based on the stratified analysis). This finding implies that the intervention effects were likely to be non-homogeneous, which highlights the need to identify factors that may be related to the group with sustained outcomes and those associated with the diminished effects of the intervention. This information can be used to fine-tune and tailor future interventions. In conclusion, there is an increased recognition that behavioral interventions are more likely to succeed if they address family and community factors (Coates et al., 2008; Schensul et al., 2009). This study provides preliminary data to support the feasibility and efficacy of such interventions.
Acknowledgments
This study was funded by National Institute of Mental Health grant number R01MH080606. We would like to thank the project team members in China and Los Angeles, U.S, for their contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartlett JG, Gallant JE. Medical management of HIV infection (2000-2001) Johns Hopkins University Press, Division of Infectious Diseases; Baltimore, MD: 2001. [Google Scholar]
- Bhana A, McKay MM, Petersen I, Bell C. Family-based HIV prevention and intervention services for youth living in poverty-affected contexts: the CHAMP model of collaborative, evidence-informed programme development. Journal of the International AIDS Society. 2010;13(Suppl 2):S8. doi: 10.1186/1758-2652-13-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. A factor analysis of self-report measures of family functioning. Family Process. 1985;24(2):225–239. doi: 10.1111/j.1545-5300.1985.00225.x. [DOI] [PubMed] [Google Scholar]
- Bloom B, Naar S. Self-report measures of family functioning: extensions of a factorial analysis. Family Process. 1994;33:203–216. doi: 10.1111/j.1545-5300.1994.00203.x. [DOI] [PubMed] [Google Scholar]
- Bor R, Miller R, Goldman E. HIV/AIDS and the family: A review of research in the first decade. Journal of Family Therapy. 1993;15:187–204. [Google Scholar]
- Coates T, Richter L, Caceres C. Behavioral Strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372(9639):669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derlega VJ, Barbee AP. HIV and social interaction. Sage; Thousand Oaks, CA: 1998. [Google Scholar]
- Ewart C. Social action theory for a public health psychology. American Psychology. 1991;46(9):931–946. doi: 10.1037//0003-066x.46.9.931. [DOI] [PubMed] [Google Scholar]
- Feaster D, Szapocznik J. Interdependence of stress processes among African American family members: influence of HIV serostatus and a new infant. Psychological Health. 2002;17(3):339–363. doi: 10.1080/08870440290029584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill B, Okie S. China and HIV—a window of opportunity. The New England Journal of Medicine. 2007;356:1801–1805. doi: 10.1056/NEJMp078010. [DOI] [PubMed] [Google Scholar]
- Hamra M, Ross MW, Karuri K, Orrs M, D'Agostino A. The relationship between expressed HIV/AIDS-related stigma and beliefs and knowledge about care and support of people living with AIDS in families caring for HIV-infected children in Kenya. AIDS Care. 2005;17:911–922. doi: 10.1080/09540120500100593. [DOI] [PubMed] [Google Scholar]
- Heckman TG, Anderson ES, Silkkema KJ, Kochman A, Kalichman SC, Anderson T. Emotional distress in nonmetropolitan persons living with HIV disease enrolled in a telephone-delivered, coping improvement group intervention. Health Psychology. 2004;23(1):94–100. doi: 10.1037/0278-6133.23.1.94. [DOI] [PubMed] [Google Scholar]
- Herek GM. AIDS and stigma. American Behavioral Scientist. 1999;42:1106–1116. [Google Scholar]
- Ji G, Li L, Sun S. The impact of HIV/AIDS on families and children: a study in China. AIDS. 2007;21(suppl 8):S157–S161. doi: 10.1097/01.aids.0000304712.87164.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S. Understanding AIDS: A Guide for Mental Health Professionals. American Psychological Association; Washington, DC: 1995. [Google Scholar]
- Kmita G, Baranska M, Niemiec T. Psychosocial intervention in the process of empowering families with children living with HIV/AIDS-a descriptive study. AIDS Care. 2002;14:279–284. doi: 10.1080/09540120120076959. [DOI] [PubMed] [Google Scholar]
- Lee RS, Kochman A, Sikkema KJ. Internalized stigma among people living with HIV-AIDS. AIDS and Behavior. 2002;6:309–319. [Google Scholar]
- Lee SJ, Li L, Jiraphongsa C, Rotheram-Borus MJ. Caregiver Burden of Family Members of Persons Living with HIV in Thailand. International Journal of Nursing Practice. 2010;16:57–63. doi: 10.1111/j.1440-172X.2009.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Sun S, Wu ZY, Wu S, Lin CQ, Yan ZH. Disclosure is a family matter, Field notes from China. Journal of Family Psychology. 2007;21(2):307–314. doi: 10.1037/0893-3200.21.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wu ZY, Wu S, Jia MH, Lieber E, Lu Y. Impacts of HIV/AIDS stigma on family identity and interactions in China. Family System & Health. 2008;26(4):431–442. doi: 10.1037/1091-7527.26.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lin C, Sun S, Rotheram-Borus MJ, Ji G. Parents living with HIV in China: family functioning and quality of life. Journal of Child and Family Studies. 2009;18(1):93–101. doi: 10.1007/s10826-008-9210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lee SJ, Jiraphongsa C, Khumtong S, Iamsirinthaworn S, Thammawiijaya P, et al. Improving health and mental health for people living with HIV/AIDS: 12-month assessment of a behavioral intervention in Thailand. American Journal of Public Health. 2010 doi: 10.2105/AJPH.2009.185462. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CQ, Li L, Ji GP, Wu S, Semaan A. Children body mass index and nutrition intake in HIV/AIDS affected families in China. Vulnerable Child Youth Studies. 2008;3(1):16–23. doi: 10.1080/17450120701660602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R, Vosvick M, Amey F. Stress, social support and depression in informal caregivers to people with HIV/AIDS in Lomé, Togo. International Journal of Sociology and Social Policy. 2006;26(12):63–73. [Google Scholar]
- Ministry of Health China UNGASS Country Progress Report. 2010 [Google Scholar]
- Ogden J, Esim S, Grown C. Expanding the care continuum for HIV/AIDS: bringing carers into focus (HorizonsReport) Population Council and International Center for Research on Women; Washington, DC: 2004. [Google Scholar]
- Palattiyil G, Chakrabarti M. Coping strategies of families in HIV/AIDS care: some exploratory data from two developmental contexts. AIDS Care. 2008;20(7):881–885. doi: 10.1080/09540120701767166. [DOI] [PubMed] [Google Scholar]
- Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Social Science & Medicine. 2003;57:13–24. doi: 10.1016/s0277-9536(02)00304-0. [DOI] [PubMed] [Google Scholar]
- Pequegnat W, Bauman LJ, Bray JH, DiClemente R, Dillorio C, Hoppe S, et al. Measurement of the role of families in prevention and adaptation to HIV/AIDS. AIDS and Behavior. 2001;5(1):1–19. [Google Scholar]
- Prachakul W, Grant JS, Keltner N. Relationships among functional social support, HIV-related stigma, social problem solving, and depressive symptoms in people living with HIV: a pilot study. The Journal of the Association of Nurses in AIDS Care. 2007;18(6):67–76. doi: 10.1016/j.jana.2007.08.002. [DOI] [PubMed] [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing. 2011 http://www.R-project.org.
- Rotheram-Borus MJ, Lee MB, Gwadz M, Draimin B. An intervention for parents with AIDS and their adolescent children. Am J Public Health. 2001;91:1294–1302. doi: 10.2105/ajph.91.8.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Flannery D, Rice E, Lester P. Families living with HIV. AIDS Care. 2005;17(8):978–987. doi: 10.1080/09540120500101690. [DOI] [PubMed] [Google Scholar]
- Schensul SL, Saggurti N, Singh R, Verma RK, Nastasi BK, Mazumder PG. Multilevel perspectives on community intervention: an example from an Indo-U.S. HIV prevention project in Mumbai, India. American Journal of Community Psychology. 2009;43(3-4):277–291. doi: 10.1007/s10464-009-9241-0. [DOI] [PubMed] [Google Scholar]
- Schuster MA, Kanouse DE, Morton SC, Bozzeette SA, Miu A, Scott GB, et al. HIV-infected parents and their children in the United States. American Journal of Public Health. 2000;90(7):1074–1081. doi: 10.2105/ajph.90.7.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne C, Stewart A. The MOS social support survey. Social Science & Medicine. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Silver EJ, Bauman LJ, Camacho S, Hudis J. Factors associated with psychological distress in urban mothers with late-stage HIV/AIDS. AIDS and Behavior. 2003;7:421–431. doi: 10.1023/b:aibe.0000004734.21864.25. [DOI] [PubMed] [Google Scholar]
- Silverman B. Density Estimation for Statistics and Data Analysis, Monographs on Statistics and Applied Probability. Chapman and Hall; London, United Kingdom: 1986. [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis. Sage; Thousand Oaks, CA: 1999. [Google Scholar]
- Sun S, Li L, Lin C, Semaan A. Child behavior and parenting in HIV/AIDS affected families in China. Vulnerable Child Youth Studies. 2008;3(3):192–202. doi: 10.1080/17450120802241997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BE, Rehman F, Suryanarayanan D, Josephine K, Dilip M, Dorairaj VS, et al. How stigmatizing is stigma in the life of people living with HIV: A Study on HIV positive individuals from Chennai, South India. AIDS Care. 2005;17:795–801. doi: 10.1080/09540120500099936. [DOI] [PubMed] [Google Scholar]
- Wu Z, Liu Z, Detels R. HIV-1 infection in commercial plasma donors in China. Lancet. 1995;346:61–62. doi: 10.1016/s0140-6736(95)92698-4. [DOI] [PubMed] [Google Scholar]
- Wu Z, Rou K, Detels R. Prevalence of HIV infection among former commercial plasma donors in rural eastern China. Health Policy Plan. 2001;16(1):41–46. doi: 10.1093/heapol/16.1.41. [DOI] [PubMed] [Google Scholar]
- Wu Z, Sullivan S, Rotheram-Borus MJ, Detels R. Evolution of China's response to HIV/AIDS. Lancet. 2007;369:679–690. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Chen YM, Kuo BI, Wang KY. Quality of life and related factors for people living with HIV/AIDS in Northern Taiwan. Journal of Nursing Research. 2003;11:217–26. doi: 10.1097/01.jnr.0000347638.41089.20. [DOI] [PubMed] [Google Scholar]
- Zung W. A self-rating depression scale. Archives of General Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]