Abstract
Poly(ethylenimine) (PEI) and PEI-based systems have been widely studied for use as nucleic acid delivery vehicles. However, many of these vehicles display high cytotoxicity, rendering them unfit for therapeutic use. By exploring the mechanisms that cause cytotoxicity, and through understanding structure-function relationships between polymers and intracellular interactions, nucleic acid delivery vehicles with precise intracellular properties can be tailored for specific function. Previous research has shown that PEI is able to depolarize mitochondria, but the exact mechanism as to how depolarization is induced remains elusive and therefore is the focus of the current study. Potential mechanisms for mitochondrial depolarization include direct mitochondrial membrane permeabilization by PEI or PEI polyplexes, activation of the mitochondrial permeability transition pore, and interference with mitochondrial membrane proton pumps, specifically Complex I of the electron transport chain and F0F1-ATPase. Herein, confocal microscopy and live cell imaging showed that PEI polyplexes do colocalize to some degree with mitochondria early in transfection, and the degree of colocalization increases over time. Cyclosporine a was used to prevent activation of the mitochondrial membrane permeability transition pore, and it was found that early in transfection cyclosporine a was unable to prevent the loss of mitochondrial membrane potential. Further studies done using rotenone and oligomycin to inhibit Complex I of the electron transport chain and F0F1-ATPase, respectively, indicate that both of these mitochondrial proton pumps are functioning during PEI transfection. Overall, we conclude that direct interaction between polyplexes and mitochondria may be the reason why mitochondrial function is impaired during PEI transfection.
Keywords: apoptosis, mitochondria, membrane potential, caspase activation
INTRODUCTION
The delivery of nucleic acids has great potential for advancing biomedical and therapeutic research; however, the field has faced several obstacles that are mostly related to the lack of effective delivery methods. In response to the problems associated with viral vectors,1 several research groups have developed polycation-based delivery vehicles. While some systems are effective, generally, the vehicles with the highest efficacy have been shown to display a cytotoxic response, and the mechanisms that cause cytotoxicity remain unclear. In particular, polyethylenimine (PEI) has been widely studied over the last decade as it is very efficient at delivering various types of nucleic acids into a wide variety of cell types.2–8 Yet, the high cytotoxicity associated with this material has prevented it from being used clinically.1, 9–12 Gaining a better understanding of the mechanisms associated with toxicity and the role that polymer structure plays in transfection will enhance the ability of researchers to design vehicles with the ideal combination of low toxicity and high delivery efficiency. Herein, we aim to further advance the knowledge of how synthetic materials interact with and adversely affect cellular function by examining the specific effect of linear PEI on mitochondrial function, which is responsible for regulating cell cycle, differentiation, energy, signaling, growth, and cell death. To this end, we chose to use a commercially available linear PEI (JetPEI, Polyplus-transfection Inc.) due to its widespread use as a transfection reagent.
Currently, the mechanisms involved in the cytotoxic response of common delivery agents such as PEI are beginning to be uncovered but currently remain unclear. One study indicated altered gene expression in cells treated with branched 25 kDa PEI, with an increase in genes specific for oxidative stress, inflammation, and cytotoxicity evident six hours after transfection.13 Further studies by Godbey et al. on branched PEI indicate that cell death appears to occur in two phases; one at 2 hours characterized by altered cell morphology and cell detachment and a later phase occurring between seven and nine hours after transfection.2 The later phase was characterized by increased secretion of the gene products tissue-type plasminogen activator (tPA), plasminogen activator inhibitor type 1 (PAI-1), and von Willebrand Factor (vWF). Increased levels of these gene products are linked to endothelial cellular stress responses.14–17 Another study has found that 25 kDa branched PEI and 750 kDa linear PEI both induce toxicity in two phases; a necrotic-like cell death at 30 minutes of transfection and a mitochondrial-mediated apoptotic cell death at 24 hours after transfection.12 The first involves an early necrotic-like toxicity due to membrane damage, characterized by lactate dehydrogenase (LDH) release in cells treated with PEI. It is interesting to note that in that study, phosphatidylserine exposure, a sign of apoptosis, was evident in cells treated with high concentrations of PEI within 30 minutes of transfection. However, the authors mention that the early phosphatidylserine exposure could be due to necrotic-like damage as well as apoptosis, since the phosphatidlyserine exposure occurred at the same time as LDH release.12 The second mechanism involves a late, apoptotic-like cell death 24 hours after transfection.12 Moghimi et al. concluded that the apoptotic-like cell death was a result of mitochondrial-mediated cell death pathways due to an increase in caspase 3 activation and loss of mitochondrial membrane potential 24 hours after transfection. Furthermore, Moghimi et. al. conducted additional studies to determine the mechanism of mitochondrial depolarization; isolated mitochondria were treated with either branched or linear PEI and cytochrome c release was measured, and it was found that branched PEI was able to induce more cytochrome c release than linear PEI. It was also found that there was no mitochondrial swelling or change in respiration during cytochrome c release, indicating that the mitochondrial permeability transition pore was not involved in PEI-induced cytochrome c release from isolated mitochondria.12 The means by which mitochondrial membrane potential (MMP) is reduced during PEI transfection remains unknown; therefore, we chose to further investigate the interaction between polyplexes formed with linear PEI and mitochondria by performing several in vitro studies.
The effects of inhibiting the formation of the mitochondrial permeability transition pore were studied during PEI transfection to determine if our studies done within intact cells support the conclusions from the previous studies done in isolated mitochondria. In times of intracellular stress, the voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT), and cyclophilin D come together to form a pore in the mitochondrial membrane known as the mitochondrial permeability transition pore (MPTP). The opening of this pore results in the release of pro-apoptotic factors from the intermembrane space and loss of mitochondrial membrane potential.18 Cyclosporine a (CsA) is able to stop this pore from opening by preventing cyclophilin D from complexing with the other proteins to form the pore;19–20 this inhibits the loss of MMP and can prevent apoptosis in certain situations.21–23
In addition to MPTP activation, another possible reason for the loss of MMP could potentially be interference with membrane pumps.10 Polyamines found in nature, such as spermine, putrescine, and spermidine,24–26 along with polyamine analogues27 such as pentaethylenehexamine, a small 232 Da linear PEI,28 have also been found to bind to mitochondria and subsequently interfere with normal mitochondrial function. Natural polyamines and polyamine analogues have been shown to decrease mitochondrial protein synthesis,29 induce the release of cytochrome c,30 and interfere with calcium transport across the mitochondrial membrane.24, 27–28 Given how a variety of polyamines are able to induce mitochondrial cytotoxicity,31 it was of interest to us to study mitochondrial membrane pump disruption as a potential mechanism for how mitochondrial membrane potential is lost during PEI transfection. MMP is normally maintained by proton pumping via the electron transport chain (ETC) and F0F1-ATPase.32 Complex I of the ETC can be inhibited by rotenone, and F0F1-ATPase can be inhibited by oligomycin, which are useful tools to examine the effects of these signaling pathways on toxicity mechanisms.32 These inhibitors were used during PEI transfection to determine the contribution to MMP by these two mitochondrial membrane pumps.
Another possible reason for the loss of MMP during PEI transfection is that the PEI polyplexes could directly disrupt the mitochondrial membrane, allowing for release of pro-apoptotic factors and eventual cell death. There has been work showing that branched 78 kDa PEI is capable of disrupting lipid membranes.33–35 Indeed, if PEI is able to disrupt lipid membranes, it is possible that the observed loss of MMP is due to PEI disrupting the mitochondrial membrane. Researchers have previously suggested that one route to mitochondrial depolarization is the direct permeabilization of the outer mitochondrial membrane, allowing cytochrome c to escape the intermembrane space, leading to the activation of caspases and apoptosis.12, 36 Since polyamines are capable of binding to mitochondria,24 and given the ability of PEI to negatively impact MMP, it was of interest to us to further study these effects and, more specifically, examine the related outcomes of PEI polyplex interactions with the mitochondria as a function of time. Due to the negative impact of PEI on isolated mitochondrial function found in previous studies, it was of interest to us to study PEI polyplex-mitochondria interactions in vitro. Activation of the MPTP, inhibition of mitochondrial membrane pumps, and direct polyplex interaction with the mitochondrial membrane along with certain inhibitors used in the current study are illustrated in Figure 1.
Figure 1.
Potential mechanisms as to how mitochondrial membrane potential (MMP) could be disrupted during PEI transfection (inhibitors of normal mitochondrial function are shown in boxes on the diagram). PEI could potentially interact with the electron transport chain or F0F1-ATPase, whose contributions to MMP can be observed using rotenone or oligomycin, respectively. The mitochondrial membrane permeability transition pore is formed during times of intracellular stress by the complexation of several proteins, including voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT), and cyclophilin D. Cyclosporine a (CsA) is able to prevent the pore from opening and save the cell from apoptosis in certain cases. Additionally, PEI polyplexes could be directly permeabilizing the outer mitochondrial membrane, resulting in a loss of mitochondrial function.
Based on our results, we propose that the early toxicity of linear PEI is neither due to the opening of the mitochondrial permeability transition pore, nor because of direct interference with mitochondrial pumps. We also show that PEI is able to induce apoptosis early during transfection. In fact, we observe an increase in caspase-9 activity and mitochondrial membrane depolarization within one hour. Our data also indicate increasing colocalization between polyplexes and mitochondria over time. Thus, our data indicates the possibility of linear PEI initiating caspase activation by direct mitochondrial permeabilization, which ultimately leads to toxicity and cell death.
MATERIALS AND METHODS
Cell culture media and supplements were purchased from Gibco®, Invitrogen Corp. (Carlsbad, CA). HeLa cells were purchased from ATCC (Rockville, MD). Cyclosporine a and oligomycin were obtained from CalBioChem, EMD Biosciences Inc. (San Diego, CA). The mitochondrial membrane potential probe DiIC(1)5, phosphatidylserine exposure probe Annexin V, secondary goat anti-rabbit antibody, and viability probe propidium iodide were purchased from Molecular Probes®, Invitrogen Corp., (Eugene, OR) and used according to manufacturer’s protocol. Linear PEI (JetPEI) was purchased from PolyPlus-transfection Inc. (Illkirch, France) and used according to manufacturer’s protocol. Rotenone was purchased from Sigma-Aldrich (St. Louis, MO). Plasmid DNA (pCMV-luc) was purchased from PlasmidFactory GmbH & Co. KG (Bielefeld, Germany).
Polyplex Formation
JetPEI was added to a .02ug/uL solution of plasmid DNA (pDNA) according to manufacturer’s protocol to make a solution with a nitrogen-to-phosphate (N/P) ratio of 5, the N/P ratio recommended by the manufacturer for transfections. Polyplexes were allowed to incubate at room temperature for one hour prior to transfection. For the confocal microscopy studies, pDNA was labeled with Cy5 using a Cy5 LabelIT® Nucleic Acid Labeling Kit from Mirus Bio, LLC (Madison, WI) according to manufacturer’s protocol, which covalently links Cy5 to pDNA, and the Cy5-labeled pDNA was used to form polyplexes.
Mitochondrial Membrane Potential
HeLa cells were seeded at a density of 250,000 cells/well in a 6-well plate in Dulbecco’s Modified Eagle Medium (DMEM) substituted with 10% fetal bovine serum, 100µg/mL streptomycin, 0.25µg/mL amphotericin, and 100 units/mg penicillin. The cells were incubated at 37°C and 5% CO2 for 24 hours. After 23 hours, polyplexes were formed at an N/P of 5 and using 5 µg of plasmid DNA per well. The final volume of each N/P 5 solution was 500 µL (250 µof JetPEI solution added to 250 µL of plasmid DNA at a concentration of 0.02 µg/mL). Polyplexes were incubated for one hour at room temperature, then 1 mL of serum-free OptiMEM was added to each 500 µL polyplex solution to form the transfection solution. Twenty-four hours after the cells were plated, the DMEM was aspirated from the cells, the cells were washed with 1mL of phosphate-buffered saline per well, and 1.5mL of transfection solution was added to each well. Transfections were performed in duplicate. Cells were allowed to transfect for indicated time periods, then transfection media was aspirated off, cells were washed twice with 1 mL of phosphate-buffered saline per well and pelleted. The mitochondrial membrane potential-sensitive dye DiIC(1)5 was used according to manufacturer’s protocol. The dye Annexin V was added to cells where indicated according to manufacturer’s protocol to detect phosphatidyl serine exposure. The viability dye, propidium iodide, was used according to manufacturer’s protocol. Flow cytometry was completed on a BD FACSCanto II (Becton Dickinson Biosciences, San Jose, CA). A minimum of 50,000 events was collected for each sample.
Mitochondrial permeability transition pore inhibition
In order to study the role that the mitochondrial membrane permeability transition pore (MPTP) has on maintaining mitochondrial membrane potential during PEI transfection, the opening of the pore was inhibited using the drug cyclosporin a. HeLa cells were plated in 6 well plates and transfected as described above. At the indicated time points, cyclosporine a was dissolved in dimethyl sulfoxide (DMSO), then added to DMEM substituted with 10% FBS to give a final concentration of 5 µM cyclosporine a. Then, 4 mL of DMEM with or without cyclosporine a were added to each well. The cells in which DMEM containing cyclosporine a was added, were allowed to incubate for one hour, after which MMP was measured using flow cytometry as described above.
Complex I and F0F1-ATPase inhibition
In order to determine whether the respiratory chain in the mitochondrial membrane was contributing to MMP in PEI-treated cells, inhibitors of these pumps were used on PEI-transfected cells to see if MMP would be affected. To inhibit Complex I of the electron transport chain, 2 µM of rotenone was used as described previously.37 To inhibit F0F1-ATPase, 5 µg/mL (6.3 µM) of oligomycin was used as described previously.38 Briefly, the inhibitors were added at the indicated concentrations at 4 hours after transfection or 6 hours after transfection in order to determine if respiratory chain inhibition was the cause of the sudden loss of MMP observed at these time points. The inhibitors were allowed to incubate on the cells for one hour, then MMP was analyzed using flow cytometry as described above.
Confocal Microscopy
Previous work done on isolated mitochondria show that polyplexes formed with 25 kDa PEI at an PEI/DNA weight ratio of 0.8/1 (roughly N/P ratio ~3) did not have strong mitochondrial interactions.39 For our studies, we wanted to determine if linear PEI used at the typical and recommended transfection conditions (N/P=5) interacted with mitochondria in whole cells during transfection. HeLa cells were seeded onto PLL-coated 12 mm diameter round thickness No. 1 coverslips (Fisher Scientific, Pittsburgh, PA) in 12-well plates at a density of 15,000 cells per well. The cells were grown on coverslips for 48 hours in conditions described above (37°C, 5% CO2, supplemented DMEM). Polyplexes were formed by adding 50 µL of PEI N/P=5 solution to 50 µL of 0.02 mg/mL Cy5-labeled pDNA solution and allowing the polyplex solution to incubate for 1 hour at room temperature. Cells were transfected with 100 µL of polyplex solution diluted with 1 mL of OptiMEM for a total of 1 µg of pDNA per well. Cells were fixed at the indicated time points after transfection and immunolabeling of the mitochondria was performed using a 1:3000 dilution of rabbit monoclonal COX IV primary antibody (Cell Signaling Technologies Inc., Danvers, MA) and 5 µg/mL goat anti-rabbit secondary antibody conjugated to Alexa Fluor 488 or Alexa Fluor 555 (Molecular Probes, Eugene, OR) as previously described.40 Cells were imaged on a Zeiss LSM 510 META confocal microscope (Carl Zeiss Inc., Thornwood, NY) and colocalization analyses on fixed cells was performed using the software ImageJ.41
Live Cell Microscopy
For the live cell microscopy work, HeLa cells that stably expressed green fluorescent protein (GFP)-labeled mitochondria (HeLa-TurboGreen-Mito, MARINPHARM GmbH, Luckenwalde, Germany) were used to visualize colocalization of polyplexes with this organelle. The cells were plated with a density of 15,000 cells/well in a 35 mm glass bottom petri dish ( MatTek Corporation, Ashland, MA) 24 hours prior to transfection. The glass diameter was 14 mm (thickness no.1.5). Polyplex formation and transfection were performed as described above except 300 µL of transfection solution (150 µL of JetPEI at an N/P ratio of 5 added to 150 µL of .02 mg/mL solution of Cy5 pDNA) diluted with 900 uL of OptiMEM were used. Imaging was completed 3 hours after transfection on confocal microscope (LSM 510 Meta, Carl Zeiss MicroImaging Inc, Thornwood, NY). Additionally, for this colocalization study, the optical slice thickness was maintained at 0.9 µm for all channels. The time series images (8-bit; 512 × 512 pixels) were acquired using Zeiss Zen 2009 software with a pixel dwell time of 1.60 µs. The time series imaging consisted of 50 scans with a total scan time of 3 minutes and 13 seconds. The deconvolution (Classical Maximum Likelihood Estimation, CMLE algorithm) and colocalization analysis (Mander’s coefficient) of the images were performed using the software ‘Huygens Essential’ (Scientific Volume Imaging B. V., The Netherlands). The brightness and contrast of the video were changed for viewing only.
Statistical Analysis
All data are presented as mean ± standard deviation. For statistical analysis of data, the JMP software was used (S.A.S. Institute Inc., Cary, NC) and means were compared using a Student’s t-test, with P<0.05 being considered as statistically significant.
RESULTS AND DISCUSSION
Due to the integral role of the mitochondria in cytotoxicity and apoptosis, we have explored in detail the mitochondrial interaction of a linear, 22kDa PEI (JetPEI, Polyplus-transfection Inc.), due to its widespread use in the literature and its utility as a transfection reagent.42–43 To study PEI polyplex-mitochondrial interactions, flow cytometry assays, confocal microscopy, and live cell imaging techniques were used to study the interaction of polyplexes and mitochondria over time to further delineate the role of this organelle in PEI-induced cytoxicity and cell death. The effects of cyclosporine a (mitochondrial permeability transition pore inhibitor), rotenone (inhibitor of Complex I), and oligomycin, (F0F1-ATPase inhibitor) on the mitochondrial membrane potential of transfected cells were also studied to better understand mechanisms of mitochondrial depolarization during PEI transfection.
Onset of apoptosis
There are several distinct steps in apoptosis, including a decrease in mitochondrial membrane potential (mitochondrial depolarization) and phosphatidyl serine exposure, two early signs of apoptosis.44–47 To gauge the approximate time points when mitochondria start to become depolarized, studies probing the effect of PEI polyplex exposure early on in the transfection time course were performed using the mitochondrial membrane potential-sensitive cyanine dye DiIC(1)5 (Molecular Probes®, Invitrogen Corp., Eugene, OR). Here, we found about a 20% decrease in DiIC1(5) fluorescence in PEI polyplex-treated cells one hour after transfection when compared to the cells only control, indicating a decrease in MMP (Figure 2, a). To monitor phosphatidyl serine exposure, the dye Annexin V-Alexa Fluor 488 was used. Annexin V is able to bind to exposed phosphatidylserine, then fluorescence from the conjugated fluorescent dye Alexa Fluor 488 can be measured using flow cytometry to distinguish cells with exposed phosphatidylserine (apoptotic cells). A 75% increase in Annexin V-Alexa Fluor 488 fluorescence was found in cells transfected with PEI polyplexes compared to the cells only control after 30 minutes (Figure 2, b). Increased Annexin V fluorescence was also observed in PEI polyplex-treated cells compared to cells only one hour after transfection (Figure 2, b). The results confirmed that PEI polyplexes are able to induce an appreciable loss of mitochondrial membrane potential and increased phosphatidyl serine exposure within one hour post-transfection, signifying that cells are experiencing apoptosis signals at early time points in transfection process.
Figure 2.
PEI induces apoptotic events at early timepoints after transfection. HeLa cells were allowed to transfect for the indicated timepoints before being analyzed by flow cytometry. (a) A loss in (mitochondrial membrane potential (MMP) observed by a decrease in the fluorescence of DiIC1(5) and (b) an increase in phosphatidylserine exposure observed by an increase in Annexin V-Alexa Fluor 488 (AF488) fluorescence. Data were normalized to cells only control. AF488 fluorescence is presented as a % increase from cells only.
Caspase-9 activation
To further understand and determine the point during the time course of transfection when cells are committed to apoptosis, the activation of the caspase cascade was studied. It should be noted that Moghimi et al. have studied the activation of caspase 3, an executioner caspase, 24 hours after treatment with free 25 kDa branched PEI or 750 kDa linear PEI.12 To further elucidate when apoptosis is first initiated during transfection with plasmid DNA, we have chosen to examine the time point of caspase-9 activation, an initiator caspase, early in linear PEI transfection. Caspase-9 is linked to mitochondrial-mediated apoptosis, and is activated upon release of cytochrome c; after which, apoptotic protease activating factor 1 (Apaf-1) is released from the mitochondria into the cytosol.48–51 Upon release, cytochrome c and Apaf-1 combine to form a complex known as the apoptosome. The apoptosome then recruits procaspase-9, activating it into its proteolytic form of caspase-9.48, 52–54 Caspase-9 then activates caspase-3, which goes on to cleave a variety of substrates and hastens the cell on its course to death.55 To study caspase-9 activation, we utilized a carboxyfluorescein (FAM) linked fluorochrome inhibitor of caspases (FLICA) specific for caspase-9 (Neuromics, Inc., Edina, MN). The FAM-FLICA reagent only binds to active caspases, any unbound reagent diffuses out of the cells. Once bound to active caspases, the fluorescence from the fluorochrome on the caspase inhibitor can be measured and used to observe caspase activation; in this case, an increase in fluorescence indicates an increase in caspase-9 activation. As shown in Figure 3, cells were transfected with linear PEI for the indicated time points. Our results reveal a 300% increase in caspase-9 activation as early as one hour after transfection (Figure 3 a), which corresponds to the observed loss of mitochondrial membrane potential and phosphatidyl serine exposure previously shown (Figure 2). These results confirm that apoptosis is induced early during linear PEI polyplex transfection, although the exact trigger for caspase-9 release remains unclear. It is possible that direct interaction of linear PEI with the mitochondria triggers this signaling cascade. Likewise, linear PEI could also initiate apoptosis elsewhere in the cell and that the release of caspase-9 is downstream from that event. Propidium iodide (PI) (Molecular Probes®, Invitrogen Corp., Eugene, OR) was used as a viability dye according to manufacturer’s instructions; increased PI fluorescence indicates an increase in plasma membrane disruption. In this experiment, we found a 250% increase in PI fluorescence in PEI polyplex-treated cells one hour after transfection (Figure 3 b), indicating the plasma membrane integrity of these cells is also compromised at this early time point.
Figure 3.
Cells were transfected for the indicated time points with PEI at an N/P of 5 and then analyzed using flow cytometry to study increases in (a) caspase-9 activity and (b) plasma membrane permeability as measured by increases in FITC and propidium iodide (PI) fluorescence, respectively. Results were normalized to a cells only control and data is presented as % increased fluorescence from cells only.
Colocalization analysis between polyplexes and mitochondria over time
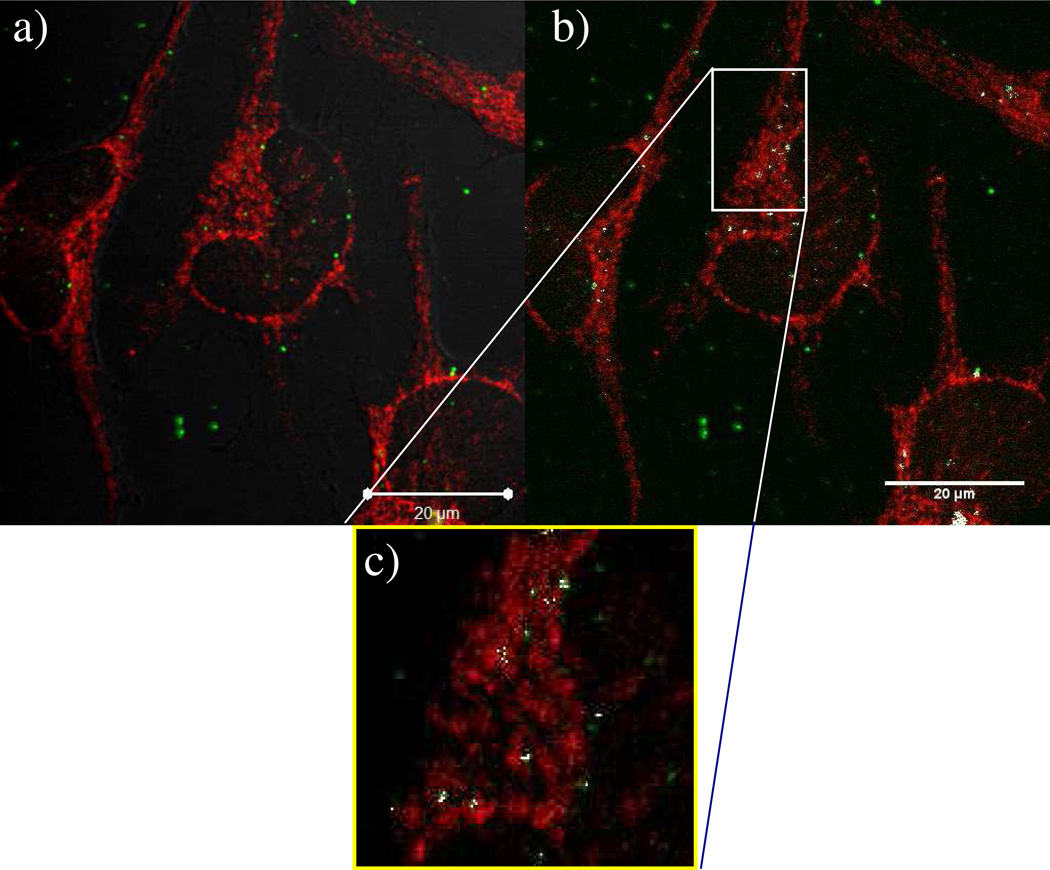
Data in Figures 2 and 3 indicate that apoptosis is signaled early on in the transfection process with linear PEI, which has further prompted us to investigate possible direct interaction between PEI polyplexes and the mitochondria. Colocalization between mitochondria and Cy5-pDNA were observed in fixed HeLa cells at one hour and two hours post-transfection. Microscopy images were taken using a slice thickness of 0.8 µM using a Zeiss LSM 510 META microscope and Zeiss ZEN 2009 software, and images were processed using ImageJ software to calculate the degree of colocalization between polyplexes and mitochondria. Specifically, we wanted to determine the amount of colocalization of the Cy5-labeled pDNA with antibody-labeled mitochondria. The Mander’s coefficients were calculated at one hour post-transfection (Figure 4) and at two hours post-transfection (Figure 5); colocalized pixels are shown as white in both of these figures. At one hour after transfection, the Mander’s coefficient M1 (overlap of Cy5 fluorescence with secondary antibody fluorescence) was 0.349, indicating low colocalization (Figure 4). The degree of colocalization increased with time, as evidenced by the fact that the M1 result was 0.4690 two hours after transfection (Figure 5). To further study the polyplexes’ interactions with mitochondria, studies on live cells were performed.
Figure 4.
Confocal microscopy images of HeLa cells transfected with PEI polyplexes (shown as green from labeled pDNA) showing overlap with mitochondria (red). The cells were fixed one hour after transfection. (a) Image of whole cells transfected with PEI polyplexes. (b) Highlighting colocalized pixels; mitochondria are shown as red, polyplexes are shown as green, and colocalized pixels are shown as white. The Mander’s coefficient (M1) for the amount of green overlapping red was found to be 0.349. (c) Close up view from inside the box in (b) to further highlight colocalization between polyplexes and mitochondria. Scale bar = 20 µm.
Figure 5.
(a) Confocal microscopy image of HeLa cells transfected with PEI polyplexes two hours after transfection. Mitochondria are shown as red, polyplexes are shown as green. (b) Highlighting colocalized points, which are shown as white. The Mander’s coefficient (M1) for the amount of green overlapping red is 0.4690 at this time point. (c) Zoomed-in image of the area indicated by the box in (b) to highlight colocalized pixels. Scale bar = 20 µm.
Live cell imaging
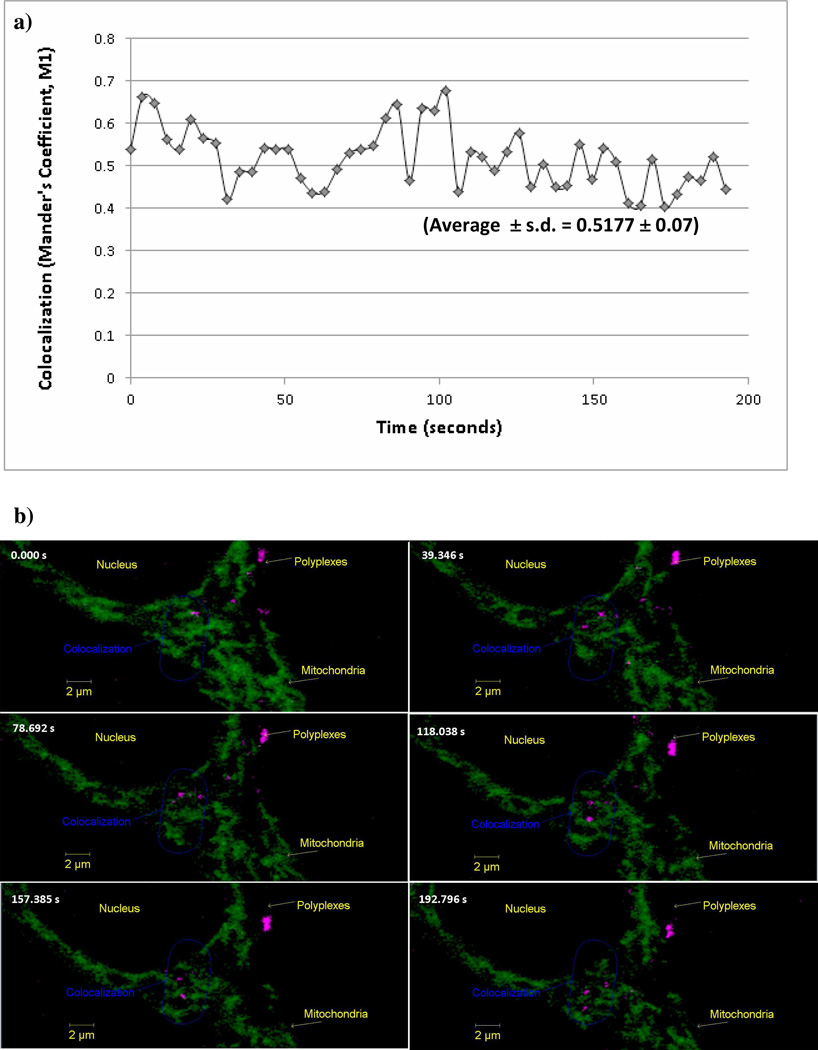
Live cell imaging was performed to understand the temporal colocalization of PEI polyplexes with HeLa cells that stably express a GFP-tagged mitochondrial targeting sequence of human cytochrome c oxidase subunit VIII precursor, which enables visualization of mitochondria via fluorescence in live cells. Mander’s coefficient (M1) for the overlap of Cy5 channel and the GFP channel was calculated for a series of 50 images taken at an interval of 3.93 seconds (3 hours post transfection). Figure 6 a showed that the colocalization values of the PEI polyplexes with GFP-labeled mitochondria varied and an average value of 0.5177 ± 0.07 was found. Figure 6 b shows six images from the total 50 images taken over the period of 3 minutes and 13 seconds.
Figure 6.
(a) Colocalization of PEI polyplexes with GFP-labeled mitochondria in live HeLa cells. Each point indicates one image taken at 3.93 second intervals (a total of 50 images were taken). The average Mander’s coefficient (M1) in this time period was 0.5177 ± 0.07 (average ± standard deviation). (b) Timed sequence of images using live cell confocal microscopy. The HeLa cells used in this study stably express GFP-tagged mitochondrial targeting sequence of human cytochrome c oxidase subunit VIII precursor to enable mitochondrial fluorescence in live cells. Mitochondria are represented as green, polyplexes are represented as magenta. Images were captured at three hours after transfection (0.00 seconds) and continued for (3 minutes and 13 seconds) with images captured every 3.93 seconds to capture a total of 50 images. Only images at six time points are shown here.
This indicates that the Cy5-pDNA (pCMV-luc) from the linear PEI polyplexes (magenta) partially colocalized with mitochondria (green). Since these images were taken 3 hours after transfection, polyplexes were seen to colocalize with mitochondria at a distance closer to the nucleus of the cell (Video-1 in the Supporting Information). Further, as shown in Video-2 (in the Supporting Information), a closer view of a group of polyplexes confirmed their colocalization with mitochondria since they appear to move along with the mitochondria with time. This suggested a partial physical adherence of the polyplexes with the mitochondrial membrane.
The apparent overlap of linear PEI-delivered pDNA with the mitochondria (Figures 4, 5, 6) prompted us to examine the possibility that the interaction of linear PEI with the mitochondria was responsible for the reduced mitochondrial function observed in transfected cells. Specifically, studies were carried out to understand the involvement of linear PEI with the mitochondrial permeability transition pore, Complex I of the electron transport chain, and the F0F1-ATPase pump and determine the roles of these pathways in the loss of mitochondrial membrane potential during PEI-mediated pDNA delivery.
Effect of cyclosporine a on PEI toxicity
One important factor in some forms of mitochondrial-induced apoptosis, as well as necrosis, is the mitochondrial permeability transition pore (MPTP), which is a high conductance channel that opens in times of cellular stress.56 Opening of this pore causes mitochondrial swelling and loss of mitochondrial membrane potential.57–58 The addition of the immunosuppressive drug, cyclosporine a, however, has been shown to prevent MPTP induction and cell death in cases of oxidative stress, hypoxia and the addition of chemical toxins.57,32
To investigate whether prohibiting the MPTP from opening would impede the loss of mitochondrial membrane potential and cell death, the drug cyclosporine a (CsA) was added to cells at various instances after PEI transfection. In each case, the CsA was allowed to incubate on the cells for 1 hour, after which MMP was measured using flow cytometry. The data revealed that there was no effect of MPTP inhibition on the loss of mitochondrial membrane potential two hours after transfection (Figure 7 a). Next, in order to determine whether the MPTP contributed to loss of mitochondrial membrane potential at later timepoints, CsA was added to PEI-treated cells at indicated timepoints after transfection, allowed to incubate on cells for 1 hour, and then mitochondrial membrane potential was analyzed using flow cytometry. When measuring mitochondrial membrane potential 3 hours after transfection, we observed a sharp decrease in MMP in PEI polyplex-treated cells. PEI polyplex-treated cells with cyclosporine a added two hours after transfection were able to retain slightly higher mitochondrial membrane potential, but the loss of MMP was not completely prevented (Figure 7 a). Again, similar results were obtained when CsA was added 4 hours after transfection and MMP was measured 5 hours after transfection (Figure 7 a). Although there was some slight recovery of MMP in PEI-treated cells in the presence of CsA, inhibiting the mitochondrial membrane permeability transition pore did not completely prevent or restore the loss of MMP.
Figure 7.
Flow cytometry results for PEI-transfected HeLa cells. Cells were analyzed at the indicated timepoints to determine the effect of cyclosporine a on (a) mitochondrial membrane potential and (b) propidium iodide (PI) uptake.
Our results suggest that after two hours, even though mitochondrial membrane potential is lowered in PEI polyplex-treated cells, inhibiting the MPTP does not prohibit the decrease in MMP. However, at three hours post-transfection, a sharp decrease in MMP was observed, which is the point at which CsA begins to have an effect on MMP (Figure 7 a). It is possible that the early lowering (within 2 hours of transfection) of MMP is due to PEI polyplexes’ direct mitochondrial interaction or interaction with other organelles, thus initiating apoptosis and stimulating the MPTP to form. As we noted, MMP was slightly restored at the three-hour point in the presence of CsA, indicating that some of the loss of MMP can be attributed to the opening of the MPTP. A similar result occurred when CsA was added 4 hours after transfection and MMP was measured 5 hours post-transfection; there was a slight increase in MMP when cells were treated with cyclosporine a (Figure 7 a). Cyclosporine a had no dramatic effect on cell death, as indicated by propidium iodide fluorescence (Figure 7 b). From these data, we concluded that the MPTP was being induced at 2 hours post-transfection, which should not be considered the principal reason for the loss of mitochondrial membrane potential because MMP was already lowered previous to this time point. While some MMP was recovered in the presence of CsA, cell death was not prevented as shown in Figure 7. This outcome further substantiates that MMP may be a result, rather than the cause, of PEI polyplex-induced cytotoxicity. Rather, the observed reduction in MMP was more likely induced in response to cellular stress at these time points. Also, adding CsA with polyplexes and maintaining 5 µM CsA in transfected HeLa cells for two hours had no effect on MMP (Supporting Information). Instead, we believe this is a downstream occurrence of apoptosis that was initiated elsewhere in the cell. This conclusion is also supported by the caspase-9 data, which indicated that caspase-9 was being activated before the MPTP during PEI transfection (Figure 3). It is possible, therefore, that apoptotic signals such as caspase activation are responsible for inducing the opening of the mitochondrial permeability transition pore, as opposed to it being directly impacted by PEI. This data supports previous research done using 25 kDa branched and 750 kDa linear PEI on isolated mitochondria, which has also found little involvement of the MPTP in PEI-induced mitochondrial depolarization.12
Effect of PEI on mitochondrial respiratory chain function
Another possible mechanism associated with the loss of mitochondrial membrane potential loss is the possibility that PEI and/or PEI polyplexes interact directly with mitochondrial membrane pumps. Normally, proton pumps found in the mitochondrial electron transport chain maintain membrane potential. However, if the pumps are inhibited, the electron transport chain will be inhibited, decreasing mitochondrial membrane potential. In order to maintain homeostasis, F0F1-ATPase pumps in the mitochondrial membrane can pump protons to maintain MMP in the presence of ATP.37, 59 To study this further, we chose to inhibit Complex I of the electron transport chain, which is able to maintain MMP by pumping protons into the intermembrane space of mitochondria and forming a proton gradient. In the current study, rotenone was used as an inhibitor for Complex I.37, 60 If Complex I is inhibited, there should be no observable change in mitochondrial membrane potential for the cells only control, since the F0F1-ATPase pump will be activated and be able to maintain MMP as long as ATP is present. However, if a decrease in MMP is observed in rotenone-treated cells, it would indicate that the F0F1-ATPase is inhibited, either though direct pump interference or via lack of ATP. Similarly, adding oligomycin, an F0F1-ATPase inhibitor,37–38, 61 to cells should not affect MMP as long as the respiratory chain is functioning; if MMP is lowered in the presence of oligomycin it is indicative of an impaired respiratory chain or that the mitochondrial inner membrane is leaking protons.62 We observed that neither rotenone nor oligomycin had an effect on mitochondrial membrane potential in the cells only controls (Figure 8). This is to be expected, since in the case of rotenone addition F0F1-ATPase would maintain MMP as long as ATP is present and in the case of oligomycin addition Complex I and the electron transport chain would still be functional and able to maintain MMP.37 However, when rotenone was added to PEI polyplex-treated cells four hours after transfection, a decrease in MMP was observed compared to the cells only control (Figure 8 a). This indicates that the F0F1-ATPase is not properly functioning and is unable to compensate for the decrease in MMP for cells transfected with PEI, and by inhibiting Complex I it hastens the depolarization of mitochondria. The results also show that Complex I is still contributing to mitochondrial membrane potential at this timepoint; if it was dysfunctional prior to rotenone treatment, then the rotenone should have no further effect on the loss of MMP. Interestingly, a decrease in oligomycin-treated cells also results in a further decrease of MMP than in cells treated with PEI polyplexes only at this timepoint (Figure 8 a). The results indicate that F0F1-ATPase is contributing to mitochondrial membrane potential, but one would expect this to be occurring only if the electron transport chain is inhibited or if the mitochondrial membrane is leaking protons.37, 62 However, since the rotenone results suggest that the electron transport chain is functional at this timepoint, these results could indicate that PEI is inducing changes in MMP in other ways not involving mitochondrial respiratory chain inhibition, such as by inserting a channel into the mitochondrial membrane thus creating a “leaky proton” scenario. At 7 hours after transfection, neither rotenone nor oligomycin had an effect on MMP in PEI-polyplex treated cells (Figure 8 b), indicating that neither of these pumps were functional at this timepoint in PEI polyplex-treated cells. This is likely a result of activation of executioner caspases at this time, which disrupt mitochondrial function by cleaving Complex I of the electron transport chain.1
Figure 8.
The effect of different respiratory chain inhibitors on mitochondrial membrane potential (MMP) at different timepoints during transfection. Rotenone (2 µM) was used to inhibit Complex I of the electron transport chain, and oligomycin (5 µg/mL) was used to inhibit F0F1-ATPase. Inhibitors were added either (a) 4 hours or (b) 6 hours after transfection with PEI polyplexes at an N/P ratio of 5. Cells were incubated with inhibitors for one hour, after which MMP was measured at either (a) 5 hours or (b) 7 hours after transfection.
CONCLUSIONS
There are multiple toxicity pathways present in cells, and PEI has been implicated in several of these.13 Indeed, there are likely multiple interactions between PEI and various intracellular components that could result in an array of cytotoxic events. Another consideration associated with cytotoxicity is the intracellular trafficking of free polymer versus polyplex. In our present study we focused on polyplexes; however, the free PEI polymer could also be initiating a wide variety of cytotoxic cell responses. The research described herein confirmed that PEI polyplexes do colocalize with mitochondria within one hour of transfection. We also showed increased caspase-9 activity and phosphatidylserine exposure at one hour post-transfection, indicating that apoptosis was initiated early in transfection.
We have also observed increased colocalization of PEI polyplexes with mitochondria over time. Polyamines found in nature have been shown to bind to mitochondria and influence their activity. As discussed, previous research done on PEI has shown that mitochondrial membrane potential is lowered during transfection—our goal was to elucidate the mechanisms that contribute to this reduction. Some possible explanations for mitochondrial depolarization include activation of the MPTP and interference with mitochondrial membrane pumps, neither of which proved to be factors early in transfection. However, our results do indicate some involvement of the MPTP at later timepoints in transfection (after 3 hours), supporting the notion of a multiphase mechanism involved in the cytotoxic response with PEI-based polyplexes.12–13 When performing studies on the contribution of Complex I and F0F1-ATPase to mitochondrial membrane potential, we have found that inhibition of either of these pumps results in a decrease in MMP in PEI polyplex-treated cells, indicating that these pumps are still functional during PEI transfection. Had PEI or PEI polyplexes inhibited these pumps, the addition of rotenone or oligomycin should have no further effect on mitochondrial membrane potential. This outcome is similar to the one found when studying the mechanism of mitochondrial depolarization induced by P24, a cytotoxic adenine dinucleotide.37
Our results show increased caspase-9 activity, decreased mitochondrial membrane potential, and increased phosphatidyl serine exposure as early as one hour after treatments with PEI polyplexes at an N/P ratio of 5. Taken together, these data indicate that apoptosis is signaled very early on by polyplexes containing PEI. Even though we do show colocalization between PEI polyplexes with the mitochondria one hour after transfection, it is possible that PEI polyplexes or free PEI are interacting with other organelles in the cell and initiating apoptosis elsewhere, and that the decrease in MMP over time is a result of apoptosis initiation and the caspase cascade activation. We also observed a decrease in MMP over time, which correlated with an increase in PEI polyplex-mitochondria colocalization over time. While direct interaction and permeabilization of mitochondria with PEI remains a possibility, our results suggest that MTP activation and mitochondrial membrane pump dysfunction are not implicated as reasons for early MMP loss. Investigation into PEI and PEI polyplex interactions with other organelles resulting in various mechanisms of cytotoxicity is ongoing in our lab and will be reported in due course.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Dr. Katye Fichter, Dr. Patrick McLendon, Dr. Yemin Liu, and Dr. Kristi DeCourcy for their valuable insight and discussion on this work. We also thank NIH (1DP2OD006669-01) for funding of this project.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Supporting information includes live cell imaging videos depicting colocalization of polyplexes with mitochondria and the effect of maintaining 5 µM CsA on cells throughout PEI transfection. This information is available free of charge via the internet at http://pubs/acs.org/
REFERENCES
- 1.Hunter CA. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Delivery Rev. 2006;58:1523–1531. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph C, Oritz A, Schillinger U, Jauernig J, Plank C, Rosenecker J. Methodological optimization of polyethylenimine (PEI)-based gene delivery to the lungs of mice via aerosol applications. The Journal of Gene Medicine. 2005;7:59–66. doi: 10.1002/jgm.646. [DOI] [PubMed] [Google Scholar]
- 4.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. PNAS. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wightman L, Kircheis R, Rossler V, Carotta S, Ruzicka R, Kursa M, Wagner E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. The Journal of Gene Medicine. 2001;3:362–372. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- 6.DiGioia S, Conese M. Polyethylenimine-mediated gene delivery to the lung and therapeutic applications. Drug Design, Development and Therapy. 2008;2:163–188. doi: 10.2147/dddt.s2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godbey WT, Wu KK, Mikos AG. Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999;45:268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. The Journal of Gene Medicine. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 9.Godbey WT, Wu KK, Mikos AG. Poly(ethyelimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 10.Ira Y, Mely Y, Krishnamoorthy G. DNA vector polyethyleneimine affects cell pH and membrane potential: A time-resolved fluorescence microscopy study. Journal of Fluorescence. 2003;13:339–347. [Google Scholar]
- 11.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Controlled Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethyleneimine)-mediated cytotoxicity: implications for gene transfer/therapy. Molecular Therapy. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Beyerle A, Irmler M, Beckers J, Kissel T, Stoeger T. Toxicity pathway focused gene expression profiling of PEI-based polymers for pulmonary applications. Mol. Pharmaceutics. 2010;7:727–737. doi: 10.1021/mp900278x. [DOI] [PubMed] [Google Scholar]
- 14.Diamond SL, Sharefkin JB, Dieffenbach C, Frasier-Scott K, McIntire LV, Eskin SG. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. Journal of Cellular Phsyiology. 1990;143:364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- 15.Wojta J, Holzer M, Hufnagl P, Christ G, Hoover RL, Binder BR. Hyperthermia stimulates plasminogen activator inhibitor type 1 expression in human umbilical vein endothelial cells in vitro. American Journal of Pathology. 1991;139:911–919. [PMC free article] [PubMed] [Google Scholar]
- 16.Huber D, Cramer EM, Kaufmann JE, Meda P, Masse J-M, Kruithof EKO, Vischer UM. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood. 2002;99:3637–3645. doi: 10.1182/blood.v99.10.3637. [DOI] [PubMed] [Google Scholar]
- 17.Cines DB, Pollack ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt A-M, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 18.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 19.Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 20.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase: Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem. J. 1991;274:611–614. doi: 10.1042/bj2740611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 23.Weissig V, Cheng S-M, D'Souza GGM. Mitochondrial pharmaceutics. Mitochondrion. 2004;3:229–244. doi: 10.1016/j.mito.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Salvi M, Toninello A. Effects of polyamines on Ca2+ transport. Biochimica et Biophysica Acta. 2004;1661:113–124. doi: 10.1016/j.bbamem.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Schuber F. Influence of polyamines on membrane functions. Biochem. J. 1989;260:1–10. doi: 10.1042/bj2600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toninello A, Via LD, Siliprandi D, Garlid KD. Evidence that spermine, spermidine and putrescine are transported electrophoretically in mitochondria by a specific polyamine uniporter. J. of Biol. Chem. 1992;267:18393–18397. [PubMed] [Google Scholar]
- 27.Rustenbeck I, Loptein D, Fricke K, Lenzen S, Reiter H. Polyamine modulation of mitochondrial calcium transport. II. Inhibition of mitochondrial permeability transition by aliphatic polyamines but not by aminoglucosides. Biochem. Pharmacol. 1998;56:987–995. doi: 10.1016/s0006-2952(98)00233-0. [DOI] [PubMed] [Google Scholar]
- 28.Rustenbeck I, Eggers G, Reitzer H, Munster W, Lenzen S. Polyamine modulation of mitochondrial calcium transport I: Stimulatory and inhibitory effects of aliphatic polyamines, aminoglucosides and other polyamine analogues on mitochondrial calcium uptake. Biochem. Pharmacol. 1998;56 doi: 10.1016/s0006-2952(98)00232-9. 977-885. [DOI] [PubMed] [Google Scholar]
- 29.He Y, Suzuki T, Kashiwagi K, Kusama-Eguchi K, Shirahata A, Igarashi K. Correlation between the inhibition of cell growth by bis(ethyl)polyamine analogues and the decrease in the function of mitochondria. Eur. J. Biochem. 1994;221:391–398. doi: 10.1111/j.1432-1033.1994.tb18751.x. [DOI] [PubMed] [Google Scholar]
- 30.Stefanelli C, Stanic I, Zini M, Bonavita F, Flamigni F, Zambonin L, Landi L, Pignatti C, Guarnieri C, Caldarera CM. Polyamines directly induce release of cytochrome c from heart mitochondria. Biochem. J. 2000;347:875–880. [PMC free article] [PubMed] [Google Scholar]
- 31.Seiler N. Pharmacological aspects of cytotoxic polyamine analogues and derivatives for cancer therapy. Pharmacology and Therapeutics. 2005;107:99–119. doi: 10.1016/j.pharmthera.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Bruzzone S, Dodini G, Kaludercic N, Basile G, Millo E, DeFlora A, DiLisa F, Zocchi E. Mitochondrial dysfunction induced by cytotoxic adenine dinucleotide produced by ADP-ribosoyl cyclases from cADPR. J. of Biol. Chem. 2007;282:5045–5052. doi: 10.1074/jbc.M609802200. [DOI] [PubMed] [Google Scholar]
- 33.Hong S, Hessler JA, Banaszak Holl MM, Leroueil P, Mecke A, Orr BG. Physical interactions of nanoparticles with biological membranes: The observation of nanoscale hole formation. Bioconjugate Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 34.Prevette LE, Mullen DG, Banaszak Holl MM. Polycation-induced cell membrane permeability does not enhance cellular uptake or expression efficiency of delivered DNA. Mol. Pharmaceutics. 2010;7:870–883. doi: 10.1021/mp100027g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong S, Leroueil PR, Janus EK, Peters JL, Kober M-M, Islam MT, Orr BG, Baker JR, Holl MMB. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjugate Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 36.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nature Cell Biol. 2001;3:255–263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 37.Bruzzone S, Dodini G, Kaludercic N, Basile G, Millo E, DeFlora A, DiLisa F, Zocchi E. Mitochondrial dysfunction induced by cytotoxic adenine dinucleotide produced by ADP-ribosoyl cyclases from cADPR. J. of Biol. Chem. 2007;282:5045–5052. doi: 10.1074/jbc.M609802200. [DOI] [PubMed] [Google Scholar]
- 38.Shchepina LA, Pletjushkina OY, Avetisyan AV, Bakeeva LE, Fetisova EK, Izyumov DS, Saprunova VB, Vyssokikh MY, Chernyak BV, Skulachev VP. Oligomycin, inhibitor of the F0 part of H+-ATP-synthase, suppres the TNF-induced apoptosis. Oncogene. 2002;21:8149–8157. doi: 10.1038/sj.onc.1206053. [DOI] [PubMed] [Google Scholar]
- 39.Choi JS, Choi MJ, Ko KS, Rhee BD, Pak YK, Bang IS, Lee M. Low molecular weight polyethylenimine-mitochondrial leader peptide conjugate for DNA delivery to mitochondria. Bull. Korean Chem. Soc. 2006;27:1335–13340. [Google Scholar]
- 40.McLendon PM, Fichter KM, Reineke TM. Poly(glycoamidoamine) vehicles promote pDNA uptake through multiple routes and efficient gene expression via caveolae-mediated endocytosis. Mol. Pharmaceutics. 2010;7:738–750. doi: 10.1021/mp900282e. [DOI] [PubMed] [Google Scholar]
- 41.Rasband WS. ImageJ. Bethseda, MA: U.S. National Institutes of Health; pp. 1997–2009. [Google Scholar]
- 42.Beyerle A, Hobel S, Czubayko F, Shulz H, Kissel T, Aigner A, Stoeger T. In vitro cytotoxic and immunomodulatory profiling of low molecular weight polyethylenimines for pulmonary application. Toxicology in Vitro. 2009;23:500–508. doi: 10.1016/j.tiv.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Ohana P, Gofrit O, Ayesh S, Al-Sharef W, Mizrahi A, Birman T, Schneider T, Matouk I, Groot Nd, Tavdy E, Sidi AA, Hochberg A. Regulatory sequences of the H19 gene in DNA based therapy of bladder cancer. Gene Ther Mol Biol. 2004;8:181–192. [Google Scholar]
- 44.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, Schie RCv, LaFace DM, Green DR. Early redistrubution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. The Journal of Experimental Medicine. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fabbri F, Carloni S, Brigliadori G, Zoli W, Lapalombella R, Marini M. Sequential events of apoptosis involving docetaxel, a microtubule-interfering agent: A cytometric study. BMC Cell Biology. 2006;7:6. doi: 10.1186/1471-2121-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engeland Mv, Nieland LJW, Ramaekers FCS, Shutte B, Reutelingsperger CPM. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 47.Engeland Mv, Nieland LJW, Ramaekers FCS, Shutte B, Reutelingsperger CPM. Annexin V-affinity assay: a review on apoptosis detection based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y. A structural view of mitochondria-mediated apoptosis. Nature Structural Biology. 2001;8:394–401. doi: 10.1038/87548. [DOI] [PubMed] [Google Scholar]
- 49.Schimmer AD, Hedley DW, Penn LZ, Minden MD. Receptor- and mitochondrial-mediated apoptosis in acute leukemia: a translational view. Blood. 2001;98:3541–3553. doi: 10.1182/blood.v98.13.3541. [DOI] [PubMed] [Google Scholar]
- 50.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 51.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J-C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1998;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y. Apoptosome: The cellular engine for the activation of caspase-9. Structure. 2002;10:285–288. doi: 10.1016/s0969-2126(02)00732-3. [DOI] [PubMed] [Google Scholar]
- 53.Pop C, Timmer J, Sperandio S, Salvesen GS. The apoptosome activates caspase-9 by dimerization. Molecular Cell. 2006;22:269–275. doi: 10.1016/j.molcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Renatus M, Stennicke HR, Scott FL, Liddington RC, Salvesen GS. Dimer formation drives the activation of the cell death protease caspase 9. PNAS. 2001;98:14250–14255. doi: 10.1073/pnas.231465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang H-G, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchichal activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annual Reviews in Physiology. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 57.Lemasters JJ, Nieminen A, Qian T, Trost LC, Herman B. The mitochondrial permeability transition in toxic, hypoxic and repurfustion injury. Mol. Cell. Biochem. 1997;174:159–165. [PubMed] [Google Scholar]
- 58.Zamzami N, Marchetti P, Castedo M, Hirsch T, Susin SA, Masse B, Kroemer G. Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett. 1996;384:53–57. doi: 10.1016/0014-5793(96)00280-3. [DOI] [PubMed] [Google Scholar]
- 59.Rego AC, Vesce S, Nicholls DG. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death and Differentiation. 2001;8:995–1003. doi: 10.1038/sj.cdd.4400916. [DOI] [PubMed] [Google Scholar]
- 60.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. of Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 61.Penefsky HS. Mechanism of inhibition of mitochondrial adenosine triphosphatase by dicyclohexylcarbodiimide and oligomycin: Relationship to ATP synthesis. PNAS. 1985;82:1589–1593. doi: 10.1073/pnas.82.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rego AC, Vesce S, Nicholls DG. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death and Differentiation. 2001;8:995–1003. doi: 10.1038/sj.cdd.4400916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.