Abstract
Neutrophils are the all-terrain vehicle of the innate immune system because of their ability to gain entry into tissues and organs, and thus, play an essential role in host defense. Exactly how this marvel of nature works is still incompletely understood. In the last two to three years, new players and processes have been identified in the endothelial - leukocyte adhesion cascade. Novel signaling pathways have been discovered in both the endothelium and the neutrophil that regulate various steps in the recruitment process. This review focuses on these emerging pathways and the mechanisms that regulate neutrophil recruitment across endothelium.
The multi-step adhesion cascade paradigm
Early studies suggested that chemotactic factors acting on the leukocyte or on endothelial cells caused increased leukocyte adhesion and recruitment into tissues [1]. By the mid 1980s studies had revealed that treatment of cultured vascular endothelium with proinflammatory stimuli including bacterial lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), or interleukin-1β (IL-1β) dramatically altered the endothelial cell phenotype in vitro ([2–4] and see Fig 1). Soon thereafter, investigators observed that leukocytes rolled, arrested and emigrated across the endothelium of post capillary venules in animal models by direct visualization using intravital microscopy techniques. These steps constituted the now accepted multi-step adhesion cascade for leukocyte recruitment (reviewed in [5]; see Box 1 and Figure 1).
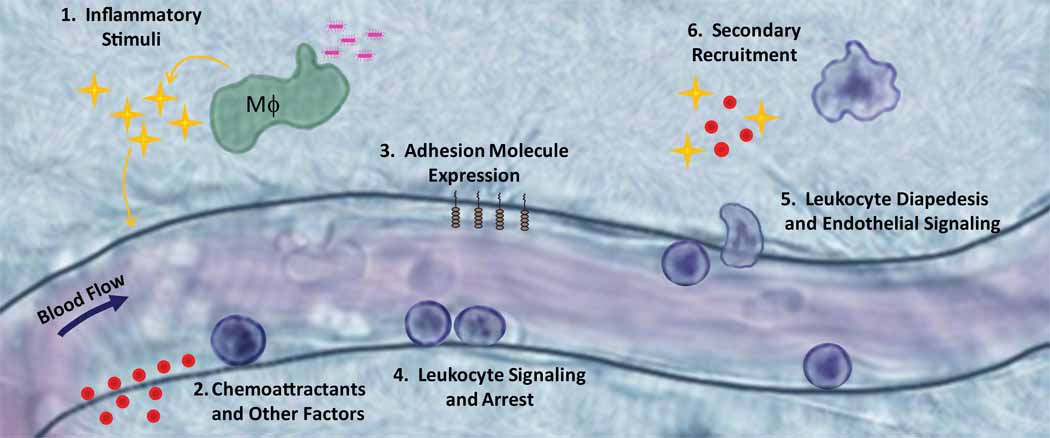
Figure 1. Classic View of Neutrophil Recruitment.
1) In this classical view, neutrophil recruitment is initiated by exposure of macrophages and other innate immune cells to a PAMP or DAMP that induces expression of inflammatory stimuli such as TNF-α or IL-1β. 2, 3) The vascular endothelium expresses chemoattractants and adhesion molecules in response to these proinflammatory stimuli. 4) Neutrophils bind to these adhesion molecules and arrest on the endothelial surface. 5) Upon neutrophil arrest, both endothelial cells and neutrophils undergo intracellular signaling to mediate leukocyte spreading, followed by apical migration and ultimately diapedesis to the site of injury or infection. 6) Neutrophils that enter the tissue space can then play a role in recruitment of a second wave of neutrophils or other leukocyte type through alteration in chemokine production.
BOX 1.
The first leukocyte type recruited to sites of tissue damage or infection are innate immune cells, primarily neutrophils. Several endothelial- and leukocyte-expressed adhesion molecules mediate neutrophil recruitment, and their function in the multi-step adhesion cascade is fairly well understood. For review purposes, these concepts are summarized in Box 1. With the rapid advances now occurring in this field, researchers have moved beyond studying direct interactions between neutrophils and endothelial cells and therefore the complexity of leukocyte recruitment has become more evident. Here we review in vivo evidence for newly identified molecules and molecular mechanisms involved in neutrophil recruitment starting from the initial steps of inflammatory stimuli release, chemoattractant presentation, and adhesion molecule expression, which lead to leukocyte signaling and arrest, endothelial signaling, leukocyte transendothelial cell migration, and finally secondary recruitment of other leukocyte types to the tissue.
DAMPS and PAMPs are key initiators of inflammation
The inflammatory response is initiated mostly by exposure of innate immune cells, primarily macrophages, dendritic cells and neutrophils, to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). This results in the secretion of inflammatory cytokines IL-1β, IL-6 and TNF-α, and many different chemokines. PAMPs and DAMPs are recognized by surface expressed toll-like receptors (TLRs) and C-type lectin receptors (CLRs), as well as by cytoplasmic receptors including NOD-like receptors (NLRs), RIG-I-like receptors (RLRs) and TLR-7,-9 and -10 [6, 7]. Inflammation induced by microbial stimuli through PAMPs has been reviewed in detail elsewhere [7].
Recently, researchers have begun to focus on DAMP derived models of sterile inflammation. Several molecules can act as DAMPs including hyaluronan, heparin sulfate, heat shock proteins, and ATP [8]. The role of ATP as a DAMP has recently been examined in a murine model of sterile inflammation triggered by cellular necrosis upon thermal injury in the liver [9]. Neutrophil recruitment in this model was mediated by interactions between neutrophil expressed Mac-1 (αMβ2, CD11b/CD18) integrin and intercellular adhesion molecule-1 (ICAM-1) expressed on the endothelium. A striking finding was that blood neutrophils did not arrest in liver sinusoids adjacent to necrotic cells, likely because of absence of perfused sinusoids in the necrotic zone, and instead neutrophils adhered firmly a few hundred microns away, and then crawled by an intravascular route to the necrotic area. The crawling neutrophils followed two different and partially overlapping chemotactic gradients. The first was MIP2 (CXCL2), which was maximal 150 µm from necrotic site, while the second stimulus was proximal to the necrotic cells, and its identity is not yet known. Neutrophil migration was reduced by a neutralizing antibody to the formyl-peptide-receptor-1 (FPR1). Evidence from this study indicated that ATP released from damaged hepatocytes acted as a DAMP by activating the Nlrp3 inflammasome within liver resident macrophages (Kupffer cells), which generate inflammatory cytokines and chemokines to recruit neutrophils. Another study reported that the inflammatory cytokines TNF-α or IL1β are required for ATP to act as a DAMP in an in vitro model of sterile inflammation[10]. Similarly, in the thermal injury model, IL1β signaling was required for neutrophil recruitment; however, this was attributed to the downstream effect of IL1β-induced upregulation of ICAM-1 expression in the sinusoidal endothelium, and not due to IL1β and ATP activation of the macrophage inflammasome. This difference may depend on the identity of resident cells (macrophage, endothelial, hepatic epithelium, etc) in different organs and tissues.
Chemoattractants: chemokines and their silent receptors reveal their true complexities in vivo
How chemoattractants recruit leukocytes to sites of inflammation or infection has been studied extensively. Neutrophil receptors for chemokines and chemoattractants (including activated Complement component 5a (C5a), formyl-Met-Leu-Phe (fMLP), platelet-activating factor and leukotriene B4 (LTB4)) are linked to G protein-coupled receptor pathways that initiate inside-out signals that activate neutrophil β2 integrins for directed cell migration. The role of chemoattractants in leukocyte recruitment is complex, as recently demonstrated in a study showing that CXCL5 (epithelial cell-derived neutrophil-activating peptide-78 or ENA-78) is strongly correlated with neutrophil number in the lung fluid of patients with acute respiratory distress syndrome [11]. Duffy Antigen Receptor for Chemokines (DARC) expression on platelets and erythrocytes regulates neutrophil homeostasis and pulmonary inflammation though binding to CXCL5 [12]. DARC is considered a “silent” chemokine receptor because it lacks the motif that triggers G-protein signaling and therefore its expression in erythrocytes is thought to act as a sink or scavenger and regulate bioavailability of several chemokines including CXCL5, CXCL1, CXCL2 and CXCL8. Studies using DARC null mice reported a role for DARC in enhancing neutrophil trafficking into tissues [13]. The mechanisms for DARC function were recently revealed. First, during homeostasis, CXCL5 is produced by platelets and binds to DARC expressed on erythrocytes, and this event is thought to control the egress of neutrophils from the bone marrow. The origin of homeostatic CXCL5 is from platelets because thrombocytopenic mice (Mpl−/− and Fog1ki/ki mice) have very low amounts of plasma and erythrocyte-bound CXCL5 compared to WT mice. In a second model, during self-limited inflammation (LPS inhalation), CXCL5 is produced by alveolar type II epithelial cells (AE II) in the inflamed lung, whereas CXCL1 and CXCL2 are not present at high concentrations in the plasma. The high amounts of CXCL5 in the lung provide a strong chemotactic gradient that recruits neutrophils into the lung air space in WT mice. As one might expect, Cxcl5−/− mice show impaired recruitment of neutrophils in the inflamed lung in this model. And third, during a severe inflammatory response in the lung induced by intratracheal E. coli instillation, high amounts of CXCL5 together with CXCL1 and CXCL2 are produced by AE II cells, leading to high circulating plasma concentrations of all three chemokines. CXCL5 has a higher affinity than CXCL1 and CXCL2 for DARC, limiting the ability of DARC to scavenge CXCL1 and CXCL2. This enhances circulating amounts of CXCL1 and CXCL2, which might desensitize CXCR2 and impair gradient formation between the plasma and alveolar space, and as a consequence reduces neutrophil recruitment into the alveolar space. In contrast to the milder model of inflammation where Cxcl5−/− mice had impaired recruitment, Cxcl5−/− mice showed increased neutrophil recruitment in the lung in response to E.coli, which resulted in better clearance of bacteria and better survival. Thus DARC scavenges proinflammatory chemokines during inflammation and CXCL5 is the key modulating factor. This model illustrates how chemokine gradients in plasma and lung can influence the inflammatory response in organs and tissues. The three different conditions evaluated (homeostasis, mild and strong lung inflammation) illustrate the complexity of the mechanisms that regulate neutrophil recruitment in different inflammatory settings within a single organ.
A new player in leukocyte adhesion under flow
During infection and tissue injury, immune complexes (ICs) are generated. The function of receptors that recognize ICs, the neutrophil FcγRs, have been examined in certain autoimmune diseases that feature deposition of ICs as an underlying pathogenic factor (reviewed in [14]), and a new paradigm in neutrophil adhesion and recruitment may be warranted to explain these recent observations. Under conditions that ICs are accessible to circulating neutrophils, such as in the case of in situ anti-endothelial cell antibodies (AECA), anti-glomerular basement membrane (anti-GBM) antibodies, or soluble ICs deposited in the blood vessels, ICs can support initial adhesion of neutrophils in vivo and in vitro under defined fluid shear flow conditions. Thus, using an in vitro flow model, the neutrophil FcγRIII mediated neutrophil adhesion to immobilized ICs, in isolation, under physiologically relevant levels of shear stress [15], whereas in the context of activated vascular endothelial monolayers and the presence of monomeric AECA, the FcγRIIA in conjunction with apical chemokine enhances neutrophil adhesion [16] (Figure 2A). In vivo studies in which ICs were deposited within the vessel wall revealed that FcγR contributed to a reduced rolling velocity of cells tethering to immobilized P-selectin molecules and greater adhesion and emigration [15]. Taken together, neutrophil FcγRs should be considered as active players in neutrophil recruitment in disease characterized by intravascular IC deposition.
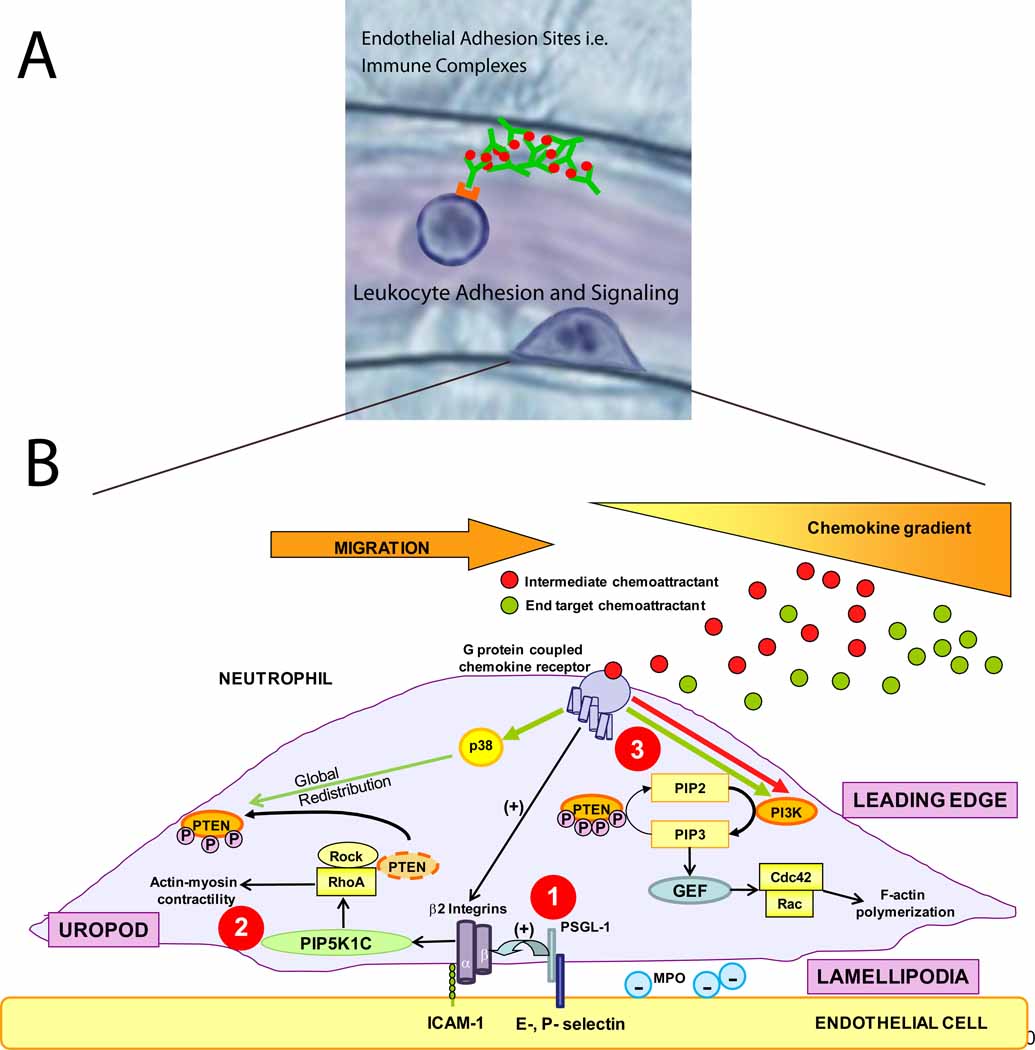
Figure 2. How neutrophils get in the groove.
a) Recent studies have identified new adhesion molecules involved in neutrophil recruitment. Immune complexes bound to the endothelium can support neutrophil capture and rolling via interaction with neutrophil Fcγ receptors [15]. The procession of leukocytes from rolling to firm adhesion, crawling, and finally transmigration is mediated by a number of intracellular signaling events within the leukocyte. b) There are many signaling pathways involved in neutrophil adhesion, polarization and directed transendothelial migration. 1) PSGL-1 and chemokine receptor engagement both trigger β2 integrin activation. 2) The engagement of these integrins with their counter-receptors in endothelial cells (ICAM-1) promotes neutrophil polarization through rapid vesicular trafficking of PIP5K1C kinase to the uropod, which enables activation of RhoA specifically in the uropod [23]. 3) In addition to activating β2 integrins, engagement of G-protein coupled chemokine receptors activate various cell signaling pathways. In the case where both intermediate and end target chemoattractants are present, neutrophils are able to prioritize end target attractants over intermediary attractants [19,46].
Neutrophil signaling in polarization and chemotaxis: getting to where it counts also involves new integrin-dependent signals
Neutrophils exposed to chemoattractants undergo cell shape polarization with a front, or “leading edge”, and a rear trailing end, called the “uropod”. During host responses to bacterial infection or tissue injury, neutrophils respond to multiple chemoattractants secreted from more than one cell source within the tissue as well as neutrophils themselves (see below). The mechanism underlying neutrophil navigation in such conditions is not fully understood. Early in vitro studies reported that during neutrophil migration towards one chemoattractant, neutrophils may lock onto a different chemoattractant and crawl towards this source and totally ignore the initial gradient [17]. An emerging model to explain this step-by-step migration is that neutrophils prioritize chemotactic signals by distinguishing “intermediary” (LTB4, CXCL8, PAF) and “end-target” (fMLP, C5a) chemoattractants with distinctly different intracellular signaling pathways, and hence avoid “distraction” in a complex milieu of chemoattractants [18, 19]. Intermediary chemoattractant receptors signal through the phosphoinositide 3-kinase (PI3K) and phosphatase and tensin homolog (PTEN) pathway (Figure 2B). This pathway converts PI(4,5)P2 to PI(3,4,5)P3, a critical second messenger [20–22], in the plasma membrane at the leading edge, orients neutrophils to the chemokine gradient, and promotes uropod formation by localization of PTEN to the uropod, which dephosphorylates biologically active PI(3,4,5)P3 back to inactive PI(4,5)P2 [19, 23, 24]. In contrast, end-target chemoattractants, like fMLP or C5a, induce migration through a phospholipase A2 and p38 MAPK pathway [19]. A confounding observation was that both end-target and intermediary chemoattractants individually activated the PI3K pathway, but PI3K activity was totally inactivated in neutrophils navigating in an environment of both end-target and intermediary chemoattractants. The decision of neutrophils to migrate toward end-target chemoattractants, while ignoring intermediary chemoattractant gradients, is explained, in part, because p38 MAPK activation either inhibited the PI3K pathway through rapid localization of PTEN to the plasma membrane throughout the cell and its action to convert PI(3,4,5)P3 back into PI(4,5)P2 [19], or the PTEN redistribution impairs localized production of PI(3,4,5)P3. The underlying mechanism that creates this intriguing, hierarchal chemoattractant-induced redistribution of PTEN is not known (Figure 2B), however, recent studies have implicated the RhoA/ROCK1 pathway as key regulator of PTEN [23, 25, 26]. It is not clear how the spatio-temporal activity of PTEN – RhoA/ROCK1- PI3K pathways are controlled in neutrophils during directed migration to sites of inflammation. The role of PTEN in neutrophil navigation requires further investigation. For example, on one hand a recent study reported that PTEN-deficient neutrophils had impaired recruitment in a murine models of S. aureus peritonitis and arthritis that produces IC and C5a dependent joint inflammation [19] whereas a different study using an E. coli or thioglycollate induced peritonitis showed enhanced neutrophil recruitment [27]. One explanation for these different outcomes is that these investigators used different Cre transgenic mice in these studies,
Neutrophil polarization is accompanied by the redistribution of several cytosolic proteins that signal and control the biochemical and mechanical process of cell migration. The small GTPases Rac and Cdc42, and PIP3 localize to the leading edge whereas Rho A, the Rho A guanine nucleotide exchange factor PDZRhoGEF, the ezrin-moesin-radixin (ERM) family and PTEN are enriched in the uropod (Figure 2). The current paradigm is that chemotatic gradients drive both the biochemical and cell polarization responses in neutrophils, even though the molecular mechanisms are not completely understood. This view has been extended in a study [28] demonstrating neutrophil polarization in the absence of a chemotactic gradient. Surprisingly, neutrophil polarization was driven by β2-integrin engagement and its later signaling bestows mouse neutrophil polarity shortly before or just after adhesion (within minutes). The authors reported that this mechanism involves rapid polarization of the PIP5K1C-90 kinase isoform by a clathrin-dependent vesicular trafficking pathway. Once polarized, PIP5K1C enables activation of RhoA specifically in the uropod, thus determining the position of the uropod, independent of a chemoattractant gradient. Deficiency in PIP5K1C does not affect neutrophil chemotaxis because once the neutrophil is polarized, the chemoattractant gradient then determines the direction of migration. Integrin-regulated PIP5K1C-90 polarization is critical for neutrophil infiltration in vivo, and this kinase seems to control both the initial polarization of neutrophils on the endothelium and formation of Rac-dependent lamellipodia in the leading edge required for neutrophil probing of endothelial cell junctions to ferret out sites for transmigration, but its activity is not required for directed migration to chemoattractants. More studies are needed to determine how β2-integrin binding to ligands initiates vesicle-mediated polarization of PIP5K1C-90 and how this kinase, either directly or indirectly, activates RhoA activity in the uropod.
In addition to promoting inflammation, certain proteins secreted by neutrophils can play an inhibitory role in leukocyte transmigration and have important functions in modulating the inflammatory response to prevent collateral tissue damage (reviewed in [29, 30]). An important regulatory role for pentraxin 3 (PTX3), a member of the pentraxin family that is stored in neutrophil granules, was recently reported [31]. In addition to its function as an opsonizing protein and activator of complement, PTX3 binds to P-selectin and impairs neutrophil rolling and recruitment in vivo. Mice lacking PTX3 exhibit increased leukocyte rolling in thrombin-stimulated mesenteric venules and increased leukocyte recruitment in a model of acute lung injury.
Endothelial and leukocyte signaling and transmigration
The process of leukocyte transendothelial migration is a fundamental step in leukocyte recruitment and involves the coordinated function of a large number of molecules (Figure 3). These molecules differ in their cellular localization, their expression levels and in the ability of certain cytokines to influence their expression. Several are enriched at endothelial cell-cell contacts, which may explain why neutrophils transmigrate preferentially at the cell-cell junctions [32]. These molecules include endothelial cell surface adhesion molecules such as PECAM-1, ICAM-1, JAMs, CD99, CD47, and endothelial adherens junction proteins, the VE-cadherin complex (α-,β-, and γ-catenins and p120-catenin). Other molecules are intracellular proteins located adjacent to cell-cell borders or in the plasma membrane and function in signal transduction. These include the Rho GTPases RhoA, RhoG, Rac and Cdc42, the Src family kinases (SFK), PI3K, eNOS, tetraspannins, the Ezrin/Radixin/Moesin (ERMs) family proteins and cytoskeletal adaptor proteins cortactin, filamin, α-actinin. We refer the reader to recent reviews and references therein that cover more extensively the molecules involved in neutrophil recruitment [32–34].
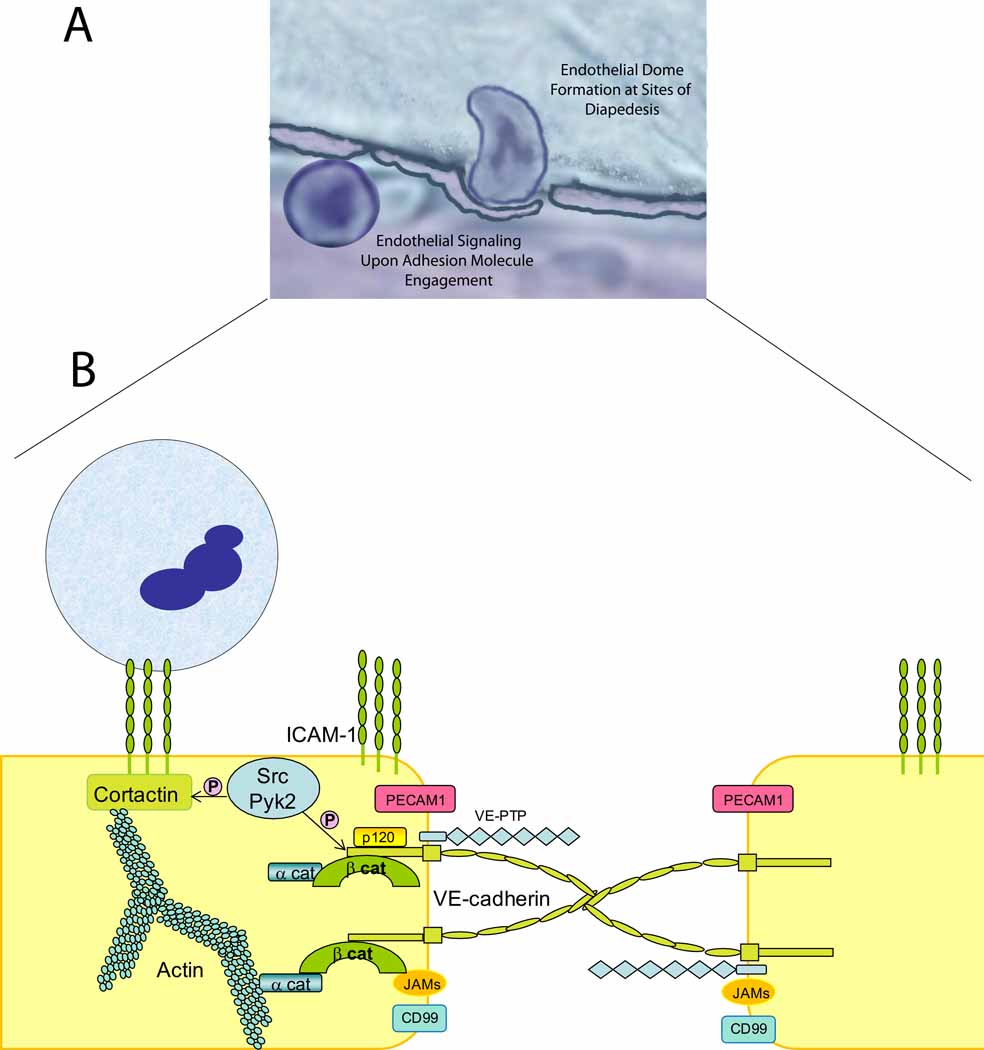
Figure 3. Domes, Rings and Gaps.
a). The vascular endothelium plays an active role in neutrophil recruitment. Recent work has identified new structures that surround actively transmigrating neutrophils, termed endothelial cell domes. b). Activated endothelium forms rings enriched in ICAM-1, VCAM-1 and cytoskeletal adaptor molecules at sites of neutrophil transmigration. Upon engagement of these endothelial adhesion molecules by their neutrophil counterreceptors, cytoskeletal remodeling occurs via Rho GTPases and activation of Src and Pyk-2 kinases. Src kinases phosphorylate VE-cadherin, impairing the binding of p120-catenin and β-catenin leading to disassembly of the VE-cadherin complex and gap formation, allowing neutrophil transmigration [32]. Recent data provides evidence for a new, endothelial specific player in this process. VE-PTP is a phosphatase that interacts with VE-cadherin. Upon neutrophil arrest, VE-PTP disassociates from VE-cadherin facilitating neutrophil transmigration. These reports identify two new mechanisms that regulate VE-cadherin function(s) and neutrophil transmigration.
Biochemical evidence suggests that during neutrophil transmigration signaling and adaptor molecules work in concert with endothelial junctional molecules to disassemble the VE-cadherin complex at cell-cell junctions, forming a gap and allowing neutrophils to squeeze between endothelial cells. There is compelling evidence that the VE-cadherin complex acts as a gatekeeper during transmigration (reviewed in [32, 35]). Neutrophil β2 integrins bind to ICAM-1 and trigger phosphorylation events in endothelium mediated by Src family kinases (Src, Yes, Fyn, Lyn are expressed in endothelium [36]) and pyk-2 kinase resuting in phosphorylation of tyrosine residues in the cytoplasmic tail of VE-cadherin and the cytoskeletal scaffold protein cortactin (for in depth review see [32]). These are opposing events in that phosphorylation of the VE-cadherin tail enable its disassociation from catenins while cortactin phosphorylation secures ICAM-1 attachment to cortactin and hence the cytoskeleton. Taken together, a working model envisions that specific phosphorylation signals in endothelial cells take place upon neutrophil arrest, the cytoskeleton remodels, in part, via Rho GTPase activities and the VE-cadherin complex disassembles at the junctions forming a gap that allows neutrophil passage into the inflamed tissue and reseals once transmigration is completed (Figure 3). The mechanism regulating the disruption and reassembly of the VE-cadherin complex reassembly has yet to be elucidated.
New players have recently been described as important regulators of VE-cadherin disassembly and subsequent transendothelial migration. p120-catenin, a Src kinase substrate that binds to the cytoplasmic tail of VE-cadherin and is essential for VE-cadherin surface expression, when overexpressed in HUVEC or DMVEC inhibits VE-cadherin complex disassembly and leads to a dramatic reduction of neutrophil transmigration [37]. VE-cadherin phosphorylation was significantly reduced under these conditions, indicating that p120-catenin regulates neutrophil transmigration by inhibiting VE-cadherin phosphorylation. Vascular endothelial protein tyrosine phosphatase (VE-PTP) is an additional player in the process [38]. VE-PTP is the only known receptor-type tyrosine phosphatase that is endothelial specific and it associates with VE-cadherin through its extracellular domain (Figure 3) [39]. When either VE-PTP or p120-catenin are knocked out in mouse endothelium, the animals die during development due to the lack of stability of VE-cadherin which results in impaired vessel development. It was recently shown that upon neutrophil adhesion, VE-PTP disassociates from VE-cadherin [38]. More importantly, silencing of VE-PTP enhanced neutrophil transmigration. These data indicate that VE-PTP regulates neutrophil transmigration by regulating VE-cadherin phosphorylation and disassembly. In addition to the above discussed mechanisms of neutrophil recruitment, some other recently described processes can add to the complexity of neutrophil egress from the blood stream into the tissues. This includes NOGO A/B, a protein localized to the endoplasmic reticulum expressed in all cell types that is involved in controlling neutrophil egress in vitro under flow conditions [40]. NOGO A/B−/− (Rtn-4A−/−:Rtn-4B−/−) mice have reduced monocyte and neutrophil recruitment in an in vivo murine air pouch model of carrageenan-induced inflammation, and in vitro siRNA silencing in endothelium reduced ICAM-1 dependent signaling via Src and Pyk-2 kinases and reduced VE-cadherin tyrosine phosphorylation, ultimately reducing neutrophil transmigration. The molecular mechanism remains unknown. Thus these newly identified players involved in transmigration further demonstrate the complexity of neutrophil recruitment during inflammation and illustrate the concept that the endothelium plays a crucial active role in sensing the inflammatory stimulus and controlling neutrophil passage from the blood vessel into the tissue.
Endothelial cells form nanoscale adhesion platforms enriched in ICAM-1, VCAM-1, tetraspannins, actin and cytoskeletal adapter proteins that are thought to contribute to formation of ring or cup-like macromolecular structures at the base of adherent leukocytes [41–43]. In addition to these structures, recent studies have provided evidence for the formation of an endothelial dome-like membrane structure that surrounds transmigrating leukocytes in vivo [44] which is proposed to limit loss of barrier function during leukocyte transmigration [45]. Formation of dome-like structures is dependent, in part, on leukocyte-specific binding protein 1 (LSP1) in endothelium (and not leukocytes) because mice deficient in LSP1 formed many fewer domes, and exhibited reduced leukocyte recruitment in vivo [45]. LSP1 is found mostly in the nuclear and cytoplasm fractions and upon TNF-α activation LSP1 is increased in the cytoskeletal fraction. LSP1 is a substrate for MAPKAP kinase 2 and protein kinase C, although the precise mechanism that links LSP1 to dome formation and barrier preservation has yet to be elucidated. Dome formation during leukocyte transmigration may be important in preventing vascular leakage during leukocyte transendothelial migration by allowing endothelial cells to form a seal around transmigrating cells. Interestingly, dome formation has yet to be observed in in vitro models. One potential explanation for this may be that endothelial cells grown on flat solid surfaces in vitro rather than in a vascular niche in vivo are incapable of forming domes due to limited membrane availability or appropriate cofactors.
Secondary recruitment: Neutrophil secreted products that modulate recruitment
It has also been noted that neutrophils have the capacity to modulate the recruitment and/or adhesion of a subsequent cohort of leukocytes [46, 47]. Recently the potential of myeloperoxidase (MPO) to affect neutrophil locomotion and recruitment through an effect on electrostatic charge was evaluated [48]. MPO is a cationic heme-containing protein with bactericidal and pro-inflammatory properties that is released by recruited neutrophils and accumulates on endothelial cells and in matrix proteoglycans in the abluminal space. MPO has chemotactic properties, remarkably, even if inactivated by a catalytic inhibitor, and the extent of cells migrating in a chemotaxis assay was similar to that seen in response to CXCL8. Surprisingly, neutrophil chemotaxis to MPO did not form a polarized morphology with a leading edge; instead cells remained rounded. These findings were explored in models of hepatic ischemia and reperfusion injury, and by intravital microscopy of TNF-α-activated microvessels of the cremaster muscle. Endothelial deposition of MPO was detected in WT mice but not Mpo−/− mice and Mpo−/− mice exhibited diminished neutrophil infiltration in both models. The authors envision a mechanism in which MPO reduces electrostatic interactions between the cationic MPO and the negatively charged neutrophil surface to enable migration.
Chronic inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease (IBD) and atherosclerosis develop over months to decades and often exhibit relapsing and remitting phases. The molecular mechanisms driving the inflammatory responses in these diseases are multi-factorial and complex and have been difficult to develop effective therapeutic agents. New studies have begun to shed light on certain of the mechanisms. For example, in a murine model of autoantibody-induced rheumatoid arthritis, which parallels the human disease albeit with some important differences, the neutrophil Fcγ receptors play a key role in disease development and the recruitment of neutrophils to the joint, reinforcing the importance of these receptors in leukocyte recruitment, as already discussed. In a recent report using the murine K/BxN disease model a “cascade of chemoattractants” both amplified and propagated continued recruitment of neutrophils, and was required for disease progression [49]. These results are thoroughly discussed in this issue (Sadik et al [50]) and are another example of the complexity and arguably non-redundant chemoattractant networks that evolve in space and time to recruit neutrophils during a chronic inflammatory disease and establish unique roles for chemokines in this disease model.
Concluding remarks
The molecular characterization of leukocyte recruitment began about 25 years ago, and the core group of adhesion molecules and chemoattractants have now been established and vetted in both in vivo and in vitro models. Despite this in depth knowledge, there remain many challenges in understanding the processes controlling leukocyte recruitment and developing rationally designed therapeutics to selectively target inflammation has been difficult. Some recommendations for future goals are creating and characterizing models that better mimic human conditions of acute and chronic inflammatory diseases and to use these models to identify the key cheomoattractants and/or chemokine networks that recruit neutrophils. A second goal should be to develop a systems approach to integrate the endothelial cell signaling pathways that initiate disruption of the endothelial VE-cadherin complex and to gain insight into factors that stabilize this complex. Lastly, an in depth understanding of the signaling pathways that control neutrophil-directed migration could serve as a foundation to develop rationale antiinflammatory therapeutics.
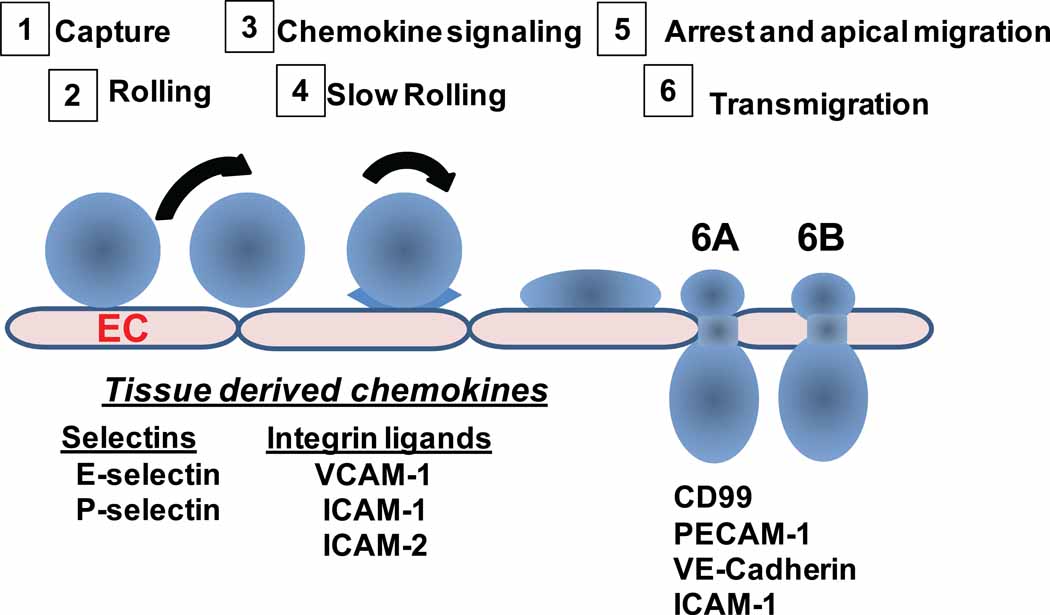
Group BOX 1. Exiting the vasculature
Recruitment of neutrophils involves a sequential, multistep adhesion cascade. The process starts with the initial contact of free flowing leukocytes to the activated vascular endothelium, followed by slow rolling of neutrophils along the vessel wall (steps 1 and 2). Neutrophil capture and rolling are mediated by endothelial expressed E- and P-selectin and neutrophil expressed L-selectins and E- and P-selectin ligands (i.e., PSGL-1, ESL-1, and CD44) [51, 52]. During rolling, neutrophils are in intimate contact with the vascular endothelium and this enables endothelial-bound chemokines to bind to their respective receptors on the neutrophil surface. Chemokine receptor-mediated signaling by G protein coupled receptors triggers activation of leukocyte expressed β2 integrins by inside-outside signaling. Activated integrins (LFA-1 and Mac1) subsequently interact with its endothelium expressed ligand ICAM-1, which leads to a reduction of leukocyte rolling velocity or slow rolling (Step 3 and 4), and eventually to firm leukocyte arrest on the endothelium (Step 5). Following firm adhesion, neutrophils prepare for extravasation into tissues and transmigration across the endothelial cell barrier. Neutrophils can transmigrate at the endothelial cell junctions (step 6A) and also at non-junctional locations (6B) as demonstrated in in vivo and in vitro models ( see [53 for review). Several endothelial transmembrane proteins, including PECAM-1, ICAM-1, VE-Cadherin, JAMs and CD99 have been shown to play a role in regulating transmigration [32, 33, 35]. Recently, other molecules have been identified as important players in the process of transmigration (or diapedesis) as discussed in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoover RL, et al. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978;14:423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- 2.Bevilacqua MP, et al. Interleukin-1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J. Clin. Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamble JR, et al. Stimulation of the adherence of neutrophils to umbilical vein endothelium by recombinant tumor necrosis factor. Proc. Natl. Acad. Sci. USA. 1985;82:8667–8670. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schleimer RP, Rutledge RK. Cultured human vascular endothelial cells acquire adhesiveness for leukocytes following stimulation with interleukin-1, endotoxin and tumor-promoting phorbol-esters. J. Immunol. 1986;136:649–656. [PubMed] [Google Scholar]
- 5.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 10.Franchi L, et al. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J. Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 11.Goodman RB, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care. Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 12.Mei J, et al. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity. 2010;33:106–117. doi: 10.1016/j.immuni.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruenster M, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat. Immunol. 2009;10:101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayadas TN, et al. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coxon A, et al. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 16.Florey OJ, et al. Antiendothelial cell antibodies mediate enhanced leukocyte adhesion to cytokine-activated endothelial cells through a novel mechanism requiring cooperation between Fc gamma RIIa and CXCR1/2. Blood. 2007;109:3881–3889. doi: 10.1182/blood-2006-08-044669. [DOI] [PubMed] [Google Scholar]
- 17.Foxman EF, et al. Multistep Navigation and the Combinatorial Control of Leukocyte Chemotaxis. J. Cell. Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heit B, et al. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell. Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heit B, et al. PTEN functions to 'prioritize' chemotactic cues and prevent 'distraction' in migrating neutrophils. Nat. Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 20.Cantley LC. The Phosphoinositide 3-Kinase Pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 21.Stephens L, et al. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr. Opin. Cell. Biol. 2002;14:203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins PT, et al. PI3K signaling in neutrophils. Curr. Top. Microbiol. Immunol. 2010;346:183–202. doi: 10.1007/82_2010_40. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, et al. Regulation of PTEN by Rho small GTPases. Nat. Cell. Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, et al. A requirement of MAPKAPK2 in the uropod localization of PTEN during FMLP-induced neutrophil chemotaxis. Biochem. Biophys. Res. Commun. 2004;316:666–672. doi: 10.1016/j.bbrc.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez T, et al. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Pro.c Natl. Acad. Sci. U S A. 2005;102:4312–4317. doi: 10.1073/pnas.0409784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vemula S, et al. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;115:1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian KK, et al. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W, et al. Integrin-Induced PIP5K1C Kinase Polarization Regulates Neutrophil Polarization, Directionality, and In Vivo Infiltration. Immunity. 2010;33:340–350. doi: 10.1016/j.immuni.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat. Rev. Immunol. 2009;9:364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 30.Petri B, et al. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 31.Deban L, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 32.Alcaide P, et al. Neutrophil recruitment under shear flow: it's all about endothelial cell rings and gaps. Microcirculation. 2009;16:43–57. doi: 10.1080/10739680802273892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 34.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol. Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 35.Vestweber D, et al. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends in Cell Biology. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Hu G, et al. Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules. Chem. Biol. Interact. 2008;171:177–189. doi: 10.1016/j.cbi.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alcaide P, et al. p120-catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nottebaum AF, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J. Exp. Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nawroth R, et al. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Lorenzo A, et al. Endothelial reticulon-4B (Nogo-B) regulates ICAM-1mediated leukocyte transmigration and acute inflammation. Blood. 2011;117:2284–2295. doi: 10.1182/blood-2010-04-281956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barreiro O, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J. Cell. Biol. 2008;183:527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw SK, et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompanies neutrophil transmigration. J. Exp. Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell. Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillipson M, et al. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS. ONE. 2008;3:e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petri B, et al. Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood. 2011;117:942–952. doi: 10.1182/blood-2010-02-270561. [DOI] [PubMed] [Google Scholar]
- 46.Rao RM, et al. Elastase release by transmigrating neutrophils deactivates endothelial bound SDF-1 alpha and attenuates subsequent T-lymphocyte transendothelial migration. J. Exp. Med. 2004;200:1713–1724. doi: 10.1084/jem.20040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurst SM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 48.Klinke A, et al. Myeloperoxidase attracts neutrophils by physical forces. Blood. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 49.Chou RC, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadik, et al. Neutrophils cascading their way to inflammation. 2011 doi: 10.1016/j.it.2011.06.008. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yago T, et al. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin {alpha}L{beta}2-mediated slow leukocyte rolling. Blood. 2010;116:485–494. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mueller H, et al. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC){gamma}2 and PI3K{gamma} pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur. J. Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]