Abstract
Despite numerous studies on the role of medial temporal lobe structures in Alzheimer's disease (AD), the magnitude and clinical significance of amygdala atrophy has been relatively sparsely investigated. In this study we compared the level of amygdala atrophy to that of the hippocampus in very mild and mild AD subjects in two large samples (Sample 1 n=90; Sample 2 n=174). Using a series of linear regression analyses, we investigated whether amygdala atrophy is related to global cognitive functioning (Clinical Dementia Rating Sum of Boxes: CDR-SB; Mini Mental State Examination: MMSE) and neuropsychiatric status. Results indicated that amygdala atrophy was comparable to hippocampal atrophy in both samples. MMSE and CDR-SB were strongly related to amygdala atrophy, with amygdala atrophy predicting MMSE scores as well as hippocampal atrophy, but predicting CDR-SB scores less robustly. Amygdala atrophy was related to aberrant motor behavior, with potential relationships to anxiety and irritability. These results suggest that the magnitude of amygdala atrophy is comparable to that of the hippocampus in the earliest clinical stages of AD, and is related to global illness severity. There also appear to be specific relationships between the level of amygdala atrophy and neuropsychiatric symptoms that deserve further investigation.
Keywords: Hippocampus, Magnetic resonance imaging, Neuropsychiatric symptoms
1. Introduction
By the time patients exhibit the hallmark amnesic syndrome of Alzheimer's disease (AD), neuropathology has usually decimated medial temporal lobe (MTL) structures (Braak and Braak, 1991). In vivo evidence for this process can be plainly seen by viewing magnetic resonance images. Extensive investigations have demonstrated quantitative morphometric abnormalities of the hippocampal formation, entorhinal cortex, and perirhinal cortex early in the illness (prior to dementia). Furthermore, these abnormalities correlate with the overall severity of clinical impairment and are specifically related to episodic memory deficits (Di Paola et al., 2007). In post-mortem studies, amyloid (senile) plaques, neurofibrillary tangles, and neuronal loss have all been observed in the amygdala (Arriagada et al., 1992; Herzog and Kemper, 1980; Scott et al., 1991; Scott et al., 1992; Tsuchiya and Kosaka, 1990). Although these post-mortem studies have called attention to similar neuropathological abnormalities in the amygdala as are found in the hippocampus, there has been far less in vivo investigation of amygdala atrophy and its clinical correlates in AD.
With respect to amygdala atrophy in early AD, several important anatomic and clinical questions remain incompletely answered. First, across the 13 published studies of amygdala atrophy in AD, findings regarding the magnitude of atrophy have been very inconsistent, with reports of atrophy ranging from 15% to 41% compared to older controls (OC). Furthermore, it is unclear whether the magnitude of amygdala atrophy is greater than (Basso et al., 2006; Cuenod et al., 1993; Krasuski et al., 1998; Lehericy et al., 1994; Mori et al., 1997), less than (Callen et al., 2001; Farrow et al., 2007; Horinek et al., 2006; Jack et al., 1997), or similar to (Barnes et al., 2006; Killiany et al., 1993; Mizuno et al., 2000; Schultz et al., 2009) that of the hippocampus. Given the substantial variability in the frequency and types of socioaffective symptoms in AD, it seems reasonable to hypothesize that the amygdala would be more variably affected within a sample of AD patients than the hippocampus. Second, although amygdala atrophy has been shown to relate to global illness severity in AD (Jack et al., 1997; Mizuno et al., 2000), there has been little investigation comparing the strength of this relationship with that of the hippocampus. Since the size of these structures is collinear, it is important to try to understand which of them is most strongly related to illness severity and whether the amount of atrophy in the other explains additional variance in overall symptom severity. We hypothesized that hippocampal atrophy is most strongly related to illness severity but that the amount of amygdala atrophy present would explain additional variance in illness severity beyond that explained by the hippocampus. Finally, although behavioral (psychiatric) symptoms are a major contributor to patient-family dysfunction and distress in AD, there has been surprisingly little effort to investigate whether amygdala atrophy relates to this domain of symptoms. The only study to specifically examine the relation between amygdala atrophy and psychiatric symptoms in mild AD reported no relationship (Horinek et al., 2006).
In the present study, we used automated MRI-derived measurements of in-vivo human brain volumes to investigate the magnitude and consistency of amygdala atrophy in two large and independent samples of patients with AD (and older controls). The main goal of having a second sample in this study design was to demonstrate the reliability of the findings, supporting their generalizability. Both samples included a large number of patients with very mild (CDR = 0.5) and mild (CDR = 1) AD, allowing for measurement of amygdala atrophy early in the illness. To address the question of whether the amygdala shows comparable atrophy to the hippocampus, the magnitude and variance of atrophy in the two structures were compared.
Second, we explored the clinical significance of amygdala atrophy in mild AD, investigating the relationship between amygdala atrophy and cognitive function using the Mini Mental State Examination (MMSE) and the Clinical Dementia Rating Scale Sum-of-Boxes (CDR-SB). In addition, to try to determine the specificity of the relationship, we performed an additional analysis controlling for hippocampal volume. The goal of these analyses was to determine whether the magnitude of amygdala atrophy is a reflection of global severity of the illness and whether it accounts for illness severity beyond its shared variance with hippocampal atrophy.
Finally, to address questions regarding specific relationships between amygdala atrophy and types and severity of neuropsychiatric symptoms in AD, data from the Neuropsychiatric Inventory (NPI) were analyzed. Animal and human studies have suggested that amygdala lesions are associated with agitation/aggression and irritability (less) (Wright et al., 2007), anxiety (less)(Davidson, 2002), and apathy (more) (Kile et al., 2009). Data are conflicting in regard to depression (Omura et al., 2005). We examined the level of amygdala atrophy in AD patients with either no, mild or moderate/severe impairment using the NPI items reflecting these symptoms, testing hypotheses based on prior findings as well as exploring the current data de novo.
2. Method
2.1. Participants
Sample 1
This sample consisted of one hundred eighty AD and OC participants in a longitudinal study at the Washington University Alzheimer's Disease Research Center, conducted in accordance with guidelines of the Washington University Human Studies Committee. There were 177 subjects (60 males and 117 females; mean age 77.4±7.3; mean education 14.0±2.2). Data from subsets of these subjects have been published in previous studies (Buckner et al., 2004; Fotenos et al., 2005; Salat et al., 2004). At study enrollment, subjects with non-AD disorders that could potentially cause dementia, active neurologic or psychiatric illness, serious head injury, clinical history of stroke, gross anatomical abnormalities on MRI and use of psychoactive drugs were excluded (Marcus et al., 2007). Participants underwent detailed structured evaluations, including a health history, depression inventory, aphasia battery and medication inventory, MMSE, CDR. Diagnostic criteria for AD required the gradual onset and progression of impairment in memory and in at least one other cognitive and functional domain, comparable to standard diagnostic criteria for probable AD (McKhann et al., 1984).
Sample 2
This sample consisted of three hundred sixty-nine AD and OC participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) (www.loni.ucla.edu/ADNI). There were 367 subjects (192 males and 175 females; mean age 75.5±6.2; mean education 15.5±3.0). The ADNI was launched in 2003 by the NIA, the NIBIB, the FDA, private pharmaceutical companies and non-profit organizations, as a $60-million, 5-year public-private partnership. The primary goal of ADNI has been to test whether imaging measures, biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
All subjects underwent thorough clinical and cognitive assessment, including MMSE, CDR, and NPI. The diagnosis of AD was made if the subject had a MMSE score between 20 and 26, CDR score of 0.5 or 1, and met NINCDS/ADRDA criteria for probable Alzheimer's disease. The OC had MMSE scores between 28 and 30, a global CDR of 0, and no symptoms of depression, MCI, or other forms of dementia. Subjects were excluded if they had any serious neurological disease other than incipient AD, any history of brain lesions or head trauma, or psychoactive medication use. The study was conducted with written informed consent according to Good Clinical Practice, the Declaration of Helsinki and U.S. 21 CFR Part 50-Protection of Human Subjects, and Part 56-Institutional Review Boards.
2.2. Magnetic resonance imaging data acquisition and analysis
Sample 1
For each subject, multiple (3 or 4) high-resolution structural T1-weighted magnetization-prepared rapid gradient echo (MP–RAGE) images were acquired entry on a 1.5T Siemens Medical Systems scanner with the following parameters: repetition time (TR) 9.7 ms, echo time (TE) 4 ms, flip angle (FA) 10°, inversion time (TI) 20 ms, voxel size 1 × 1 × 1.25 mm. These data have been made openly available to the scientific community (http://www.oasis-brains.org/).
Sample 2
For each subject, 2 high-resolution structural T1-weighted MP-RAGE images were acquired either on a 1.5T General Electric Healthcare, a 1.5T Siemens Medical Solutions or a 1.5T Phillips Medical System scanner. Acquisition parameters were as follows: TR 2400 ms, TE minimum full time excitation, FA 8°, TI 1000 ms, voxel size 1.25×1.25×1.2 mm. These data have been made available to the scientific community (http://www.loni.ucla.edu/ADNI/).
Volumetric analysis was performed using Freesurfer software (http://surfer.nmr.mgh.harvard.edu) as previously described in detail (Fischl et al., 2002). Briefly, for each subject, the multiple structural scans were motion corrected and averaged to create a single volume. The resulting averaged volume was used to segment cerebral white matter and deep gray matter structures (including hippocampus and amygdala). This segmentation procedure automatically assigned a neuroanatomical label to each voxel in an MRI volume based on probabilistic information automatically estimated from a manually labeled training set. This probability is based on the voxel's location in the volume, the neighboring voxels' tissue classes, and the intensity value in each voxel. The technique has previously been shown to be comparable in accuracy to manual labeling (Fischl et al., 2002). For each subject, we visually inspected the segmentation of the amygdala and determined whether it was acceptable according to standard anatomic criteria as we have employed in prior studies (Wright et al., Biol Psych, 2007). This process resulted in the exclusion of 3 subjects (AD patients) from Sample 1 and 2 subjects (AD patients) from Sample 2, all of whom had poor segmentations that underestimated the volume of the amygdala. For the purposes of optimal reproducibility, we chose to exclude the scans of these individuals rather than manually edit them.
MRI data were used to calculate adjusted amygdala and hippocampus volumes as follows. Right and left amygdala (r=0.849) and right and left hippocampi (r=0.926) were averaged into one single amygdala and one single hippocampal volumetric measure per subject. To adjust for differences in head size, each subject's average amygdala and hippocampal volumes were divided by the subject's total intracranial volume (TIV)(Jenkins et al., 2000) and then multiplied by the sample's mean TIV (Callen et al., 2001).
2.3. Statistical analysis
The magnitude of amygdala atrophy was calculated as [(1 - AD subject adjusted amygdala)/OC mean adjusted amygdala] and then averaged for all AD subjects (a similar computation was performed for the hippocampus). We used the t statistic for the various group comparisons of the magnitude of amygdala and hippocampal atrophy. To estimate the effect size of AD on amygdala and hippocampus, Cohen's d was calculated for each sample. In addition, we calculated a coefficient of variation for amygdala and hippocampal volumes of AD subjects by dividing the standard deviation of the structure's volume by the mean volume for each sample.
Both cognitive and neuropsychiatric data were available for Sample 2 only: statistical analyses were performed on this sample. Using a series of linear regressions, we then examined whether amygdala atrophy predicted degree of cognitive function (MMSE and CDR-SB). We performed the analyses twice, first controlling for the demographic variables age, education and gender, and then also for hippocampal volume. In addition, we also selected 5 neuropsychiatric items from the NPI for hypothesis-driven analysis of select symptoms: 1) agitation/aggression, 2) depression/dysthymia, 3) anxiety, 4) apathy/indifference, 5) irritability, and we performed exploratory analyses of the other NPI variables. In these regression analyses, amygdala volumes served as the dependant variable and NPI items the predictors. Because the predictors are categorical (ordinal), NPI items were converted to dummy variables, allowing us to compare amygdala volumes at three levels of symptom severity: 0 (absent), 1 (mild), and 2 (moderate). There were too few patients with NPI scores of 3 to perform specific analyses.
Analyses of variance and chi-square analyses were used to compare groups on quantitative continuous and on categorical demographic variables respectively. All tests of statistical significance were 2-tailed, and were considered significant at P<0.05 and trend-level at P<0.1.
3. Results
In Sample 1, AD and OC groups were equivalent in age (P=0.653) but there were more men in the AD (41%) versus the OC (26%) groups (χ2=4.2, df=2, P=0.041) and the education level was lower in the AD (13.7±2.2) versus the OC (14.4±2.2) groups (t=2.12, df=178, P=0.035). In Sample 2, AD and OC groups did not differ in age (P=0.945) or sex (P>0.93) but the education level was lower in the AD (14.8±3.1) versus the OC (16.2±2.8) groups (t=4.65, df=367, P<0.001). Demographic and clinical data are presented in Table 1.
Table 1. Demographic characteristics and amygdala and hippocampus volumes for Alzheimer's disease and older controls for samples 1 and 2.
| Subjects | ||||||
|---|---|---|---|---|---|---|
| Sample 1 (n=177) | Sample 2 (n=367) | |||||
| Demographics | OC (n=87) | AD (n=90) | p | OC (n=193) | AD (n=174) | p |
| Sex (m/f) | 23/64 | 37/53 | 0.041 | 100/93 | 92/82 | 0.930 |
| Age (years) | 77.7±7.9 | 77.2±6.7 | 0.653 | 75.6±5.1 | 75.5±7.3 | 0.945 |
| Education (years) | 14.4±2.2 | 13.7±2.2 | 0.035 | 16.2±2.8 | 14.8±3.1 | <0.001 |
| MMSE (/30) | 28.9±1.2 | 24.6±3.9 | <0.001 | 29.1±1.0 | 23.3±2.0 | <0.001 |
| CDR 0/0.5/1 | 87/0/0 | 0/65/28 | <0.001 | 193/0/0 | 0/92/84 | <0.001 |
| CDR-SB | 0.23±0.12 | 4.25±1.63 | <0.001 | |||
| NPI global score | 0.30±0.76 | 3.43±3.33 | <0.001 | |||
Abbreviations: AD = Alzheimer's disease; CDR = Clinical Dementia Rating Scale; CDR-SB = Clinical Dementia Rating Scale - Sum of Boxes; MMSE = Mini Mental State Examination; NPI = Neuropsychiatric Inventory; OC = older controls
For continuous variables, data shown are mean ± standard deviation.
Table 2 presents volumetric data for amygdala and hippocampus for AD and OC. Amygdala atrophy was substantial in subjects with very mild to mild AD. In Sample 1, the magnitudes of amygdala and hippocampal atrophy (compared to OC) were equivalent (P=0.3); in Sample 2, amygdala atrophy was slightly less prominent than hippocampal atrophy (t=3.40, df=175, P=0.0008). The effect size (Cohen's d) of AD on amygdala and hippocampus was respectively 1.0 and 1.0 for Sample 1 and 1.5 and 1.7 for Sample 2. The coefficients of variation for adjusted amygdala volume (Sample 1 = 19.3%; Sample 2 = 18.5%) were comparable to the coefficients of variation of adjusted hippocampus volume (Sample 1 = 18.3%; Sample 2 = 19.1%), indicating that there was similar variance in both structures in these patients. Figure 2 provides details on amygdala and hippocampal atrophy (cross-sectionally to clinical data acquisition).
Table 2. Amygdala and hippocampus volumes and atrophy in Alzheimer's disease n comparison to older controls for samples 1 and 2.
| Subjects | ||||||
|---|---|---|---|---|---|---|
| Sample 1 (n=177) | Sample 2 (n=367) | |||||
| OC (n=87) | AD (n=90) | % OC volume | OC (n=193) | AD (n=174) | % OC volume | |
| Adjusted volumes (mm3) | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||
| Amygdala | 1459±238 | 1215±230* | 83.2 | 1380±187 | 1095±200* | 79.3 |
| CDR 0.5 | 1250±195 | 85.7 | 1130±185 | 81.9 | ||
| CDR 1.0 | 1133±290 | 77.5 | 1053±210 | 76.3 | ||
| Hippocampus | 3641±549 | 3075±564* | 84.5 | 3731±479 | 2838±542* | 76.1 |
| CDR 0.5 | 3167±531 | 87.0 | 2980±558 | 79.9 | ||
| CDR 1.0 | 2861±589 | 78.6 | 2681±480 | 71.9 | ||
Abbreviations: AD = Alzheimer's disease; CDR = Clinical Dementia Rating Scale; OC = older controls
Significant t-test p < 0.001
Figure 2. The magnitude of amygdala atrophy is similar to that of hippocampal atrophy in early Alzheimer's disease.
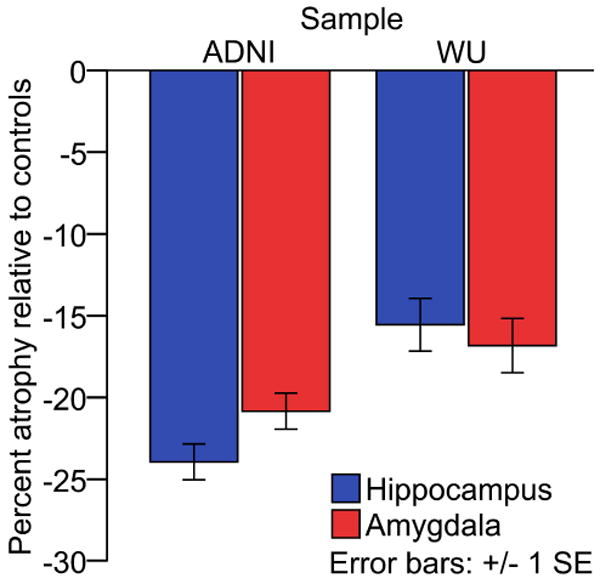
ADNI: Alzheimer's disease neuroimaging initiative; WU: Washington University
Amygdala atrophy and hippocampal atrophy strongly paralleled each other at different levels of dementia severity and were essentially comparable at every level of the very mild to mild severity spectrum of the disease. These relationships are illustrated in Figures 3a and 3b. At CDR 0.5 (n=157, combining across samples), amygdala atrophy (-16.5%) was comparable to hippocampal atrophy (-17.2%) (P=0.5). At CDR 1 (n=107), amygdala atrophy (-23.5%) was slightly less prominent than hippocampal atrophy (-26.5%) (t=2.4, df=106, P=0.01).
Figure 3. Amygdala and hippocampal atrophy relate to MMSE scores and CDR-SB in very mild to mild AD.
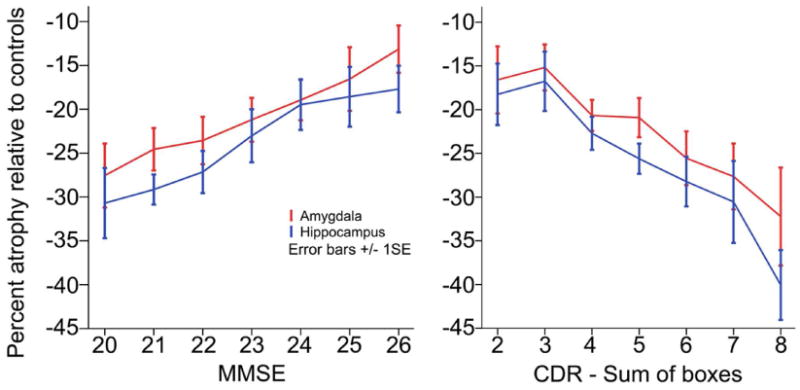
In linear regression analyses, amygdala volumes predicted scores on the MMSE (r=0.24, P=0.001) with a similar magnitude of correlation as hippocampal volumes (r=0.25, P<0.001). In contrast, amygdala volume was less strongly associated with CDR-SB (r=0.27, P<0.0003) than was the hippocampus (r=0.37, P<0.0000006). In linear regression analyses, amygdala volumes did not explain additional variance in the MMSE (regression equation r=0.27, P=0.001; β=0.135, delta r2=0.01, P=0.163) or in CDR-SB scores (regression equation r=0.37, P<0.000003; β=-0.059, delta r2=0.002, P=0.531) over and above hippocampal volumes.
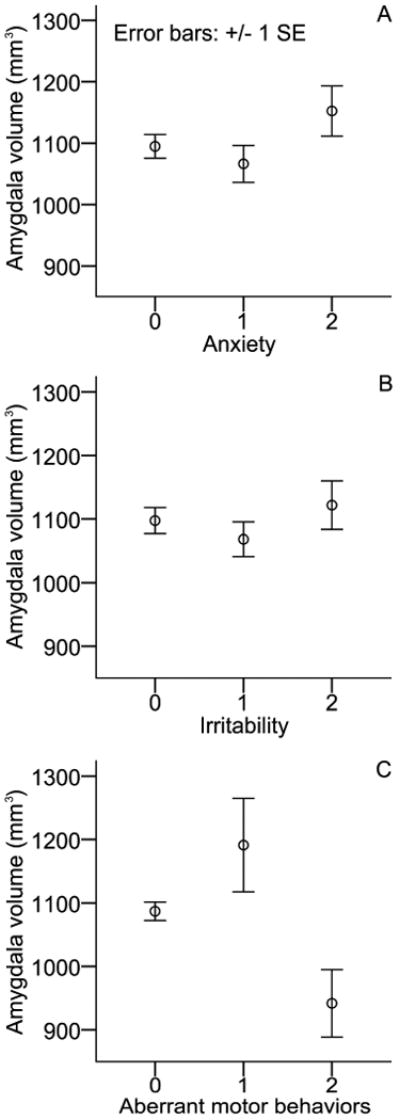
There was no association between amygdala and any of the 5 targeted neuropsychiatric symptom domains (agitation/aggression, depression/dysthymia, anxiety, apathy/indifference, irritability), although exploratory analyses suggested several possible relationships worthy of future exploration. In post hoc exploratory analyses, greater amygdala atrophy was associated with more prominent aberrant motor behavior (AMB) (F=4.45, df=175, P=0.01; see Figure 4c), but not with scores on other NPI items (delusions, hallucinations, euphoria/elation, disinhibition, sleep and appetite; all P values>0.1). Figures 4a and 4b illustrates the interesting potential relationships with anxiety and irritability, suggesting that subjects with greater anxiety (OC=1379±187; AD NPI anxiety: 2=1152±158, 1=1066±199, 0=1095±208) and irritability (OC=1379±187; AD NPI anxiety: 2=1122±162, 1=1068±177, 0=1098±218) might have less amygdala atrophy. Although these relations did not reach conventional levels of statistical significance, AD with NPI anxiety = 2 showed trend level significantly higher amygdala volume compared to NPI anxiety = 1 (one-sided t=1.382, df=173, P=0.085). Finally, neither the amygdala (r=0.080, P=0.292) nor the hippocampal volumes (r=0.020, P=0.796) were related to the NPI global score.
Figure 4. Relationships between amygdala volume and specific neuropsychiatric symptoms in AD, including irritability, anxiety, and aberrant motor behavior.
4. Discussion
To date, the magnitude and consistency of amygdala atrophy early in the course of AD dementia has been unclear, with conflicting reports in the literature. The results of this study help to resolve this issue by showing that amygdala atrophy is comparable to hippocampal atrophy in two very large, independent samples of very mild and mild AD dementia patients. Furthermore, the magnitude of amygdala atrophy is related to the severity of cognitive impairment (as measured by MMSE and CDR-SB), even at these early stages of dementia. With regard to neuropsychiatric symptoms, greater amygdala atrophy is seen in patients with more aberrant motor behavior, and there are also possible relationships with anxiety and irritability (both being suggestively associated with a lesser degree of amygdala atrophy).
The literature comparing the magnitude of amygdala vs. hippocampal atrophy in AD contains heterogeneous results. The variability in findings is likely due to small samples, the inclusion of AD patients at varying stages of the illness (not just mild), and varying measurement techniques and anatomic boundary criteria (Barnes et al., 2006; Horinek et al., 2006; Schultz et al., 2009). The large sample sizes and similar characteristics of subjects in the present investigation, as well as the uniformity of volumetric measurement techniques, allow for a more definitive comparison of amygdala and hippocampal atrophy, and indicate that both of these MTL structures are similarly and consistently affected in the mildest clinical stages of AD dementia. Of note, Figure 2 suggests less hippocampal atrophy and amygdala atrophy in AD subjects from sample 1 (WU) in comparison to AD subjects from sample 2 (ADNI). The most likely explanation for this is that AD subjects from sample 2 may be slightly more clinically impaired (in Sample 1, mean MMSE = 24.6 and 30% were CDR 1 (as opposed to CDR 0.5); in Sample 2, mean MMSE = 23.3 and 48% were CDR 1).
Post-mortem studies have shown that amygdala atrophy in AD is prominent (Scott et al., 1991) and is associated with substantial neuronal loss (Scott et al., 1992). Both plaque and tangle pathology affect the amygdala in cases who die at mild stages of the illness, and to a generally similar degree as that of the hippocampus (Arriagada et al., 1992; Price et al., 1991; Price and Morris, 1999). Significant decreases in cell packing density have been observed in all divisions of the amygdala (Herzog and Kemper, 1980) although the basolateral complex (lateral, basal and accessory basal nuclei) seems more particularly affected by neurofibrillary tangles and neuropil threads as early as neuropathological stage II (Braak and Braak, 1991). This region is reciprocally connected to the hippocampus and frontotemporal cortical regions (Scott et al., 1991).
The consistency of amygdala and hippocampal atrophy, along with their known connectivity, suggests that pathology in both of these MTL structures could contribute to the characteristic cognitive and behavioral changes of mild AD. Several studies have reported on the relation between amygdala and measures of global cognitive functioning such as the MMSE (Basso et al., 2006; Cuenod et al., 1993; Deweer et al., 1995; Horinek et al., 2006; Laakso et al., 1995) and the CDR (Jack et al., 1997; Mizuno et al., 2000) in AD and its prodromal phase (mild cognitive impairment)(Kovacevic et al., 2009). Since the level of hippocampal atrophy has been shown in many studies to be strongly and consistently related to the severity of impairment in AD, and since the level of amygdala atrophy is correlated with that of the hippocampus, it would be of interest to know which explains the most variance in impairment and to what degree the other explains additional, unique variance. None of the prior studies have made these direct comparisons. Our results indicate that, although amygdala volume alone correlates with cognitive impairment, the magnitude of amygdala atrophy does not account for additional variance in measures of illness severity (MMSE; CDR-SB) beyond its shared variance with the hippocampus. Further support for this finding was provided by a prior longitudinal study of non-demented elderly which demonstrated that, while amygdala atrophy predicts conversion to AD, it is a less powerful predictor than hippocampal atrophy (den Heijer et al., 2006).
The present results also indicate that amygdala atrophy relates to certain aspects of neuropsychiatric status in mild AD. Mild aberrant motor behaviors were associated with relatively preserved amygdala volume and moderate/severe AMB were associated with significant amygdala atrophy. AMB represents repetitive purposeless behaviors such as fidgeting, wandering, pacing or rummaging. Although AMB are relatively frequent in AD (Mega et al., 1996), there has been no report of their neuroanatomical correlates in AD. In a mixed dementia group including AD and frontotemporal dementia, AMB were associated with reduced grey matter density in the dorsal anterior cingulate and supplementary motor area, but these effects were mostly driven by the frontotemporal dementia patients in the group and there were no findings associated with grey matter density in the amygdala (Rosen et al., 2005). In addition to Kluver-Bucy syndrome, bilateral lesions of the amygdala in primates have been related to stereotyped behaviors (repetitive and purposeless behaviors) such as rocking or self-biting (Bauman et al., 2008), which may be broadly similar to some types of AMB in AD. Future studies would benefit from more detailed characterization of the types and severity of AMB in AD, and from studies of other anatomic structures that may be involved (e.g., basal ganglia).
Finally, our study provides preliminary results suggesting that anxiety and irritability could be associated with relatively preserved amygdala volume in AD. Of note, no hypertrophy was evidenced as the mean amygdala volume at every anxiety and irritability severity level was well below the mean amygdala volume in OC. This possible association between NPS and relatively preserved amygdala volume deserves additional investigation. Structural and functional neuroimaging studies have indicated that there is a relationship between the amygdala and anxiety-related behaviors in healthy individuals in those with primary anxiety disorders. Increased amygdala activation has been observed in several anxiety-provoking paradigms in these populations (Rauch et al., 2003). In normal young girls, larger amygdala volume correlates with greater fearfulness (van der Plas et al.). Generalized anxiety disorder has been associated with amygdala hypertrophy (Schienle et al.). Human amygdala lesions are, at times, associated with a reduction in the experience of fear (Tranel et al., 2006) and lack of emotional arousal to negative stimuli (Berntson et al., 2007). The amygdala appears to play a crucial role in learning of new stimulus-threat contingencies and in the expression of cue-specific fear (Davidson, 2002). It is intriguing to speculate that, in the setting of reduced ability to interpret the environment and regulate emotional responses (due to memory, executive, and other cognitive impairment), AD patients with relatively preserved amygdala function may exhibit heightened and possibly less differentiated emotional responses that seem inappropriate to caregivers, such as anxiety and irritability.
The main limitation in the present investigation concerns the assessment of neuropsychiatric symptoms with the NPIQ. Ascertainment and quantification of behavioral disturbances are more reliably performed using diagnostic interviews, and many other more refined questionnaire-based instruments are also available for psychiatric symptoms. Although such approaches are difficult to implement in a large study like ADNI, smaller prospective studies could be designed to follow up on preliminary observations reported here between the amygdala and AMB, anxiety, and irritability. A second limitation concerns the volumetric measurement with automated segmentation techniques, which although highly reliable may not have been quite as accurate as detailed manual tracing, which is still considered the gold standard. However, this method is highly time consuming and would have been difficult to perform in samples as large as those in this study. Finally, we focused specifically on the amygdala and hippocampus in this study but future studies should investigate the possible relationships of amygdala atrophy with atrophy in cortical and/or subcortical structures and their collective contributions to cognitive and behavioral status in AD.
Figure 1. Example brain segmentations from a subject with Alzheimer's disease and an older control subject.
AD: Alzheimer's disease; OC: older control
Acknowledgments
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI investigators include (complete listing available at http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Authorship_List.pdf)
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. This work was also supported by R01-AG029411, R21-AG029840, P50-AG05134, P50-AG05681, and P01-AG003991, U24-RR021382, R01-MH56584, R01-AG030311, and DP1OD003312. This work was supported in part by the McLaughlin Dean's grant, Laval University, Quebec City, Canada.
Footnotes
Disclosures:
Dr. Poulin reports no disclosure.
Ms. Dautoff reports no disclosure.
Dr. Morris reports no disclosure
Dr. Barrett reports no disclosure.
Dr. Dickerson reports no disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stéphane P. Poulin, Email: spoulin@nmr.mgh.harvard.edu.
Rebecca Dautoff, Email: dautoff@nmr.mgh.harvard.edu.
John C. Morris, Email: morrisj@abraxas.wustl.edu.
Lisa Feldman Barrett, Email: lisa@affective-science.org.
References
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Archives of Neurology. 2006;63:1434–9. doi: 10.1001/archneur.63.10.1434. [DOI] [PubMed] [Google Scholar]
- Basso M, Yang J, Warren L, MacAvoy MG, Varma P, Bronen RA, van Dyck CH. Volumetry of amygdala and hippocampus and memory performance in Alzheimer's disease. Psychiatry Research. 2006;146:251–61. doi: 10.1016/j.pscychresns.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behavioral Neuroscience. 2008;122:1005–15. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bechara A, Damasio H, Tranel D, Cacioppo JT. Amygdala contribution to selective dimensions of emotion. Social Cognitive Affective Neuroscience. 2007;2:123–9. doi: 10.1093/scan/nsm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Callen DJ, Black SE, Gao F, Caldwell CB, Szalai JP. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–74. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- Cuenod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Syrota A, Boller F. Amygdala atrophy in Alzheimer's disease. An in vivo magnetic resonance imaging study. Archives of Neurology. 1993;50:941–5. doi: 10.1001/archneur.1993.00540090046009. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Archives of General Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Deweer B, Lehericy S, Pillon B, Baulac M, Chiras J, Marsault C, Agid Y, Dubois B. Memory disorders in probable Alzheimer's disease: the role of hippocampal atrophy as shown with MRI. Journal of Neurology, Neurosurgery and Psychiatry. 1995;58:590–7. doi: 10.1136/jnnp.58.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M, Macaluso E, Carlesimo GA, Tomaiuolo F, Worsley KJ, Fadda L, Caltagirone C. Episodic memory impairment in patients with Alzheimer's disease is correlated with entorhinal cortex atrophy. A voxel-based morphometry study. Journal of Neurology. 2007;254:774–81. doi: 10.1007/s00415-006-0435-1. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Thiyagesh SN, Wilkinson ID, Parks RW, Ingram L, Woodruff PW. Fronto-temporal-lobe atrophy in early-stage Alzheimer's disease identified using an improved detection methodology. Psychiatry Research. 2007;155:11–9. doi: 10.1016/j.pscychresns.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–9. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Kemper TL. Amygdaloid changes in aging and dementia. Archives of Neurology. 1980;37:625–9. doi: 10.1001/archneur.1980.00500590049006. [DOI] [PubMed] [Google Scholar]
- Horinek D, Petrovicky P, Hort J, Krasensky J, Brabec J, Bojar M, Vaneckova M, Seidl Z. Amygdalar volume and psychiatric symptoms in Alzheimer's disease: an MRI analysis. Acta Neurologica Scandinavica. 2006;113:40–5. doi: 10.1111/j.1600-0404.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, Fox NC, Rossor AM, Harvey RJ, Rossor MN. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Archives of Neurology. 2000;57:220–4. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- Kile SJ, Ellis WG, Olichney JM, Farias S, DeCarli C. Alzheimer abnormalities of the amygdala with Kluver-Bucy syndrome symptoms: an amygdaloid variant of Alzheimer disease. Archives of Neurology. 2009;66:125–9. doi: 10.1001/archneurol.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Albert MS, Sandor T, Tieman J, Jolesz F. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Archives of Neurology. 1993;50:949–54. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- Kovacevic S, Rafii MS, Brewer JB. High-throughput, fully automated volumetry for prediction of MMSE and CDR decline in mild cognitive impairment. Alzheimer Disease and Associated Disorders. 2009;23:139–45. doi: 10.1097/WAD.0b013e318192e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasuski JS, Alexander GE, Horwitz B, Daly EM, Murphy DG, Rapoport SI, Schapiro MB. Volumes of medial temporal lobe structures in patients with Alzheimer's disease and mild cognitive impairment (and in healthy controls) Biological Psychiatry. 1998;43:60–8. doi: 10.1016/s0006-3223(97)00013-9. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Partanen K, Lehtovirta M, Hallikainen M, Hanninen T, Vainio P, Riekkinen P, Sr, Soininen H. MRI of amygdala fails to diagnose early Alzheimer's disease. Neuroreport. 1995;6:2414–8. doi: 10.1097/00001756-199511270-00032. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Baulac M, Chiras J, Pierot L, Martin N, Pillon B, Deweer B, Dubois B, Marsault C. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. American Journal of Neuroradiology. 1994;15:929–37. [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. Journal of Cognitive Neuroscience. 2007;19:1498–507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer's disease. Neurology. 1996;46:130–5. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Wakai M, Takeda A, Sobue G. Medial temporal atrophy and memory impairment in early stage of Alzheimer's disease: an MRI volumetric and memory assessment study. Journal of the Neurological Sciences. 2000;173:18–24. doi: 10.1016/s0022-510x(99)00289-0. [DOI] [PubMed] [Google Scholar]
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer's disease: an MRI volumetric study. Journal of Neurology, Neurosurgery and Psychiatry. 1997;63:214–21. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura K, Todd Constable R, Canli T. Amygdala gray matter concentration is associated with extraversion and neuroticism. Neuroreport. 2005;16:1905–8. doi: 10.1097/01.wnr.0000186596.64458.76. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiology of Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Annals of Neurology. 1999;45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schienle A, Ebner F, Schafer A. Localized gray matter volume abnormalities in generalized anxiety disorder. European Archives of Psychiatry and Clinical Neuroscience. doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- Schultz RR, de Castro CC, Bertolucci PH. Memory with emotional content, brain amygdala and Alzheimer's disease. Acta Neurologica Scandinavica. 2009;120:101–10. doi: 10.1111/j.1600-0404.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- Scott SA, DeKosky ST, Scheff SW. Volumetric atrophy of the amygdala in Alzheimer's disease: quantitative serial reconstruction. Neurology. 1991;41:351–6. doi: 10.1212/wnl.41.3.351. [DOI] [PubMed] [Google Scholar]
- Scott SA, DeKosky ST, Sparks DL, Knox CA, Scheff SW. Amygdala cell loss and atrophy in Alzheimer's disease. Annals of Neurology. 1992;32:555–63. doi: 10.1002/ana.410320412. [DOI] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cognitive Neuropsychiatry. 2006;11:219–32. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Kosaka K. Neuropathological study of the amygdala in presenile Alzheimer's disease. Journal of the Neurological Sciences. 1990;100:165–73. doi: 10.1016/0022-510x(90)90029-m. [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Boes AD, Wemmie JA, Tranel D, Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer's disease. Biological Psychiatry. 2007;62:1388–95. doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]


