Abstract
The migration of hematopoietic stem cells (HSCs) is essential during embryonic development and throughout adult life. During embryogenesis, trafficking of HSCs is responsible for the sequential colonization of different hematopoietic organs by blood-producing cells. In adulthood, circulation of HSCs maintains homeostasis of the hematopoietic system and participates in innate immune responses. HSC trafficking is also crucial in clinical settings such as bone marrow and stem cell transplantation. This review will provide an overview of the molecular and cellular signals that control and fine-tune trafficking of HSCs and hematopoietic progenitor cells (HSPCs) in embryogenesis and during post-natal life. We will also discuss the potential clinical utility of therapeutic approaches to modulate HSC trafficking in patients.
Hematopoietic stem cells: characteristic features
In the hematopoietic system, self-renewal capacity is the privilege of rare multipotent cells named hematopoietic stem cells (HSCs). Their closest progeny, hematopoietic progenitor cells (HPCs), may be multipotent, oligopotent or unipotent. While HPCs lack significant self-renewing capacity, they are capable of further differentiation into mature blood cells of all hematopoietic lineages. HSCs are responsible for the development, maintenance and regeneration of all blood forming tissues in the body. They are also critical for long-term engraftment and reconstitution in the setting of bone marrow transplantation (BMT) 1.
HSCs and HPCs are not only critical to maintain hematopoiesis, but also may contribute to tissue leukocyte homeostasis. Thus, knowing how the cells migrate between BM, blood and peripheral tissues is of great significance. In particular, clinical applications such as bone marrow transplantation and regenerative medicine could benefit from strategies that enhance, inhibit or modulate migration. Here we examine emerging techniques that can be used to study HSCs and HPCs migration and review current knowledge of the mechanisms that control HSCs and HPCs trafficking throughout the body. A number of in vitro and in vivo engraftment assays are available by which HSCs and HPCs can be discriminated and characterized both at a phenotypic and a functional level 2. However, when it comes to the analysis of in vivo HSC migration and the underlying molecular mechanisms, relatively large numbers (several million) of cells are usually required, which are difficult obtain, especially in mice which each harbor only a few thousand bona fide HSCs 3. As a consequence, the vast majority of studies that address trafficking do not differentiate between true HSCs and HPCs, but rather analyze cell populations enriched in both stem and progenitor cells. Thus, this review will focus on the combined population of hematopoietic stem/progenitor cells (HSPC).
Techniques to dissect the mechanisms of HSPC migration
Various experimental tools are currently available to provide information on how HSPCs find their way in the body (Table 1). Using a combination of several techniques, one can obtain a detailed in-depth description of where, when and how HSPC seed various tissues. Some approaches (such as adoptive transfer experiments, engraftment studies and studies in parabiotic mice) treat the mouse as a “black box”, and answer questions about which cell populations target a specific organ. Other techniques based on single cell visualization, address the dynamics of cell movement and enlighten us of the mechanism utilized by migrating cells to seed a particular organ. The latter utilize intravital microscopy (IVM), which involves microscope-based imaging of a micro-surgically prepared tissue in a live anesthetized animal. A traditional IVM approach employs epifluorescence illumination and video technology, which is useful to study molecular and biophysical mechanisms of HSPC adhesion to endothelial cells and to characterize intravascular cell behavior. IVM strategies that incorporate a laser light source for multiphoton (MP) excitation, provide three-dimensional imaging of single cells in living tissue and analyze cell-cell interactions in extravascular space (reviewed in 4,5).
Table 1.
Techniques to dissect the mechanisms of HSPC migration
| Approach | Principle | Advantage | Disadvantage |
|---|---|---|---|
| Adoptive transfer (homing) experiments | Genetically marked or fluorescently labeled cells are transfused into the circulation of recipient mice. After a relatively short time (up to 72h), cells are identified by fluorescence activated cell sorting (FACS) or immunohistology. | Generate information on cell trafficking and retention at the cell population level and identify multiple organs of preferential HSPC accumulation. Provide information on short-term kinetics of HSPC migration into different tissues. | Cannot distinguish between intra- and extravascular events and do not resolve the dynamics of cell movement. Because cells are transfused i.v., many of them are trapped in lungs, so relatively large numbers of cells have to be injected. |
| Engraftment studies | Genetically marked cells are transfused into the circulation of recipient mice. The read-out is performed at later time points (after several weeks) by FACS-based identification of transfused HSPC progeny. | Generate information on cell ability to proliferate and differentiate in a host. Indirectly, provides information on “niche” availability and functional capacity. | Cannot distinguish between intra- and extravascular events and do not resolve the dynamics of cell movement. |
| Parabiosis | Congenic mice are surgically joined so that their circulatory systems fuse and allow exchange of blood- borne cells between partners. Partner- derived cells are analyzed by FACS. | Can analyze physiological trafficking and engraftment of physiologically low numbers of partner- derived HSCP. Kinetics of HSCP presence and differentiation can be studied in the peripheral blood of same animal. | Cannot distinguish between intra- and extravascular events and does not resolve the dynamics of cell movement. |
| Conventional (epifluorescence illumination) intravital microscopy (IVM) of BM | A microsurgically prepared mouse is placed on a suitable stage under a microscope objective, and BM within long or flat bones is visualized with the help of epifluorescence illumination. Real-time video recording is performed. | Provides information on a single cell level. The setup allows to study rapid intravascular events at high temporal (i.e. video-rate) resolution. Useful to dissect molecular and biophysiological mechanisms of HSPC adhesion to endothelial cells (EC) | Limited ability to observe extravascular events because: (a) cannot provide accurate spatial information in three dimensions; (b) its ability to image fluorescent events that occur below the surface of a solid organ is restricted by scattering and absorption of excitation and emission light. Visible areas of BM might not represent the state of affairs in the total organ. |
| Multiphoton (infra-red laser light source for excitation) intravital microscopy (MP-IVM) of BM | A microsurgically prepared mouse is placed on a suitable stage under a microscope objective, and skull or femur BM is visualized by laser scanning microscopy. | Provides information on a single cell level. Characterizes extravascular events, such as cell behavior and cell-cell interactions in the interstitial compartment. Generates quantitative information for multiple parameters (e.g. space, time, fluorescence and fluorescence intensity etc). | Limited ability to observe intravascular events and limited temporal resolution. Analysis is restricted to anatomically accessible BM areas, which might not represent the state of affairs in the total organ. |
The anatomical inaccessibility of bone marrow (BM) cavities in long bones has long made IVM imaging of undisturbed BM challenging. Early attempts date back decades, when several IVM models in long bones of rabbits were developed. Later on, BM windows were also placed into the mouse femur 6. The surgical procedures required to gain access to femur BM in long bones 6,7 are associated with considerable trauma raising the possibility that observations are skewed by a local inflammatory response that may not represent physiological conditions. An alternative IVM technique that maintains tissue integrity visualizes BM in flat bones of the mouse skull calvarium, which is sufficiently transparent to allow observation of BM cavities without requiring surgical manipulation, except for a small skin incision 8,9. The approach has proved useful to observe cell behavior both within BM vessels 8–10 and in the extravascular space 11,12.
Characteristic features of HSPC migration at different developmental stages
HSPC circulation between tissues is intimately connected with the establishment and maintenance of hematopoiesis, which starts early in embryonic development and continues after birth. Table 2 summarizes trafficking molecules expressed on HSPC during different developmental stages and their role in HSPC migration.
Table 2.
Trafficking molecules expressed by fetal and adult murine HSPC
| Trafficking molecule | Role in HSPC migration | Developmental stage of expression |
|---|---|---|
| Adhesion receptor | ||
| N-cadherin (CD325) | Retention of quiescent HSPC within the BM niche | Quiescent adult HSPCs28 |
| VE-Cadherin (CD144) | HSPC trafficking to and from fetal hematopoietic tissues | Fetal HSPC (yolk sac, AGM and placenta)44,122 |
| α2β integrin (CD41) | HSPC trafficking to and from fetal hematopoietic tissues, trafficking of adult HSPC to the BM compartment | Fetal HSPC (yolk sac, AGM and placenta)47,123 Subset of adult HSPC |
| α4 integrin (CD49d) | Retention of adult HSPC within BM (α4β1), homing to BM microvessels via a4b1 and a4b7 | Adult BM-derived HSPC8,54,86 |
| β1 integrin (CD29) | Colonization of fetal liver, retention of adult HSPC within BM (α4β1) | Fetal and adult HSPC48 |
| β2 integrin (CD18) | Retention of adult HSPC within BM | Adult BM-derived HSPC60,61 |
| CD44 & HCELL (hematopoietic cell E- and L-selectin ligand) | Homing to BM microvessels | Adult BM-derived HSPC88,124 |
| PSGL-1 | Homing to BM microvessels and to peripheral tissues (skin) | Adult BM-derived HSPC8 |
| Chemoattractant receptors | ||
| Ca2+ receptor (CaR) | Receptor for calcium, lodgement of BM HSPC in endosteal niche | Adult HSPC72,73 |
| cKit (Tyrosine kinase receptor) | Receptor for stem cell factor (SCF), retention in the fetal liver, interaction with stromal cells in adult BM | Fetal and adult HSPC63–65 |
| CXCR4 | Receptor for CXCL12 (SDF-1), Major guidance signal for BM colonization, retention in BM hematopoietic niche, migration to peripheral organs in response to tissue injury | Fetal liver HSPC during late fetal development50 Adult HSPC53 |
| S1P receptor 1 (S1P1) | Receptor for the signalling lipid sphingosine-1-phosphate (S1P), exit of HSPC from peripheral non- hematopoietic tissues | Fetal and adult HSPC12 |
HSPC migration in fetal life
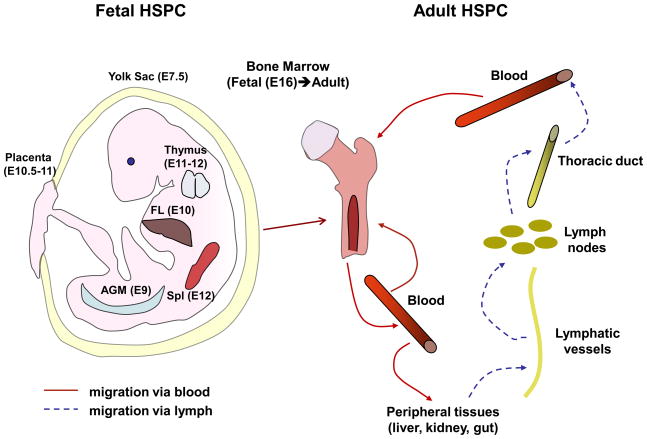
The development of embryonic hematopoiesis and the multiple tissues that demonstrate hematopoietic activity at early stages of gestation has recently be reviewed 13. In brief, mammalian hematopoiesis in mice is first represented by nucleated erythrocytes in the yolk sac (Fig. 1). This “primitive” hematopoiesis is then replaced by adult-type “definitive” hematopoiesis and HSPCs capable of engrafting upon transfer to another recipient are first found in the yolk sac and the aorta-gonad-mesonephros (AGM) region. The placenta is another source of HSPC in the developing mouse embryo. However, it is not clear whether placental HSPCs colonize the organ via the circulation or arise through de novo generation 14, or both. Also, the exact contribution of HSPCs from the above tissues to definite hematopoiesis remains a matter of debate. HSPCs are first found in the fetal liver (FL), the major site of fetal hematopoiesis at E10, where they expand and differentiate. Soon after fetal liver colonization, more differentiated HSPC colonize thymus 15 and spleen 16. Shortly before birth, HSPCs eventually start to seed the bone marrow (BM) 17, the site of permanent adult hematopoiesis (Fig. 1). At that point, FL HSPCs become relatively quiescent and exhibit only limited proliferative activity 18.
Figure 1. Trafficking of hematopoietic stem and progenitor cells in fetal life and during adulthood.
Left panel: fetal HSPCs are originated in yolk sac and migrate to AGM region and placenta. Alternatively, placental HSPCs are originated de novo. Very soon after that, HSPCs from early embryonic hematopoietic sites colonize fetal liver, which becomes the main hematopoietic organ during fetal development. Subsequently, fetal liver emigrants inhabit thymus, spleen and BM. BM becomes the main organ of adult HSPC development (right panel). The majority of HSPCs reside in the BM where they undergo self-renewal and give rise to differentiated hematopoietic cells. However, some HSPCs continuously leave the marrow and enter the blood. Circulating HSPCs either return to the BM or migrate to peripheral organs, which they exit via lymphatics. The major lymph vessel in the body, thoracic duct, drains into venous circulation, therefore HSPCs can reach BM from periphery via blood. Spl – spleen, numbers in parenthesis – day of gestation when a fetal organ is colonized with HSPC.
HSPC trafficking in adulthood
Lodging in the BM by no means indicates the final stop in the journey of HSPCs. In fact, at least some HSPCs maintain a migratory phenotype throughout postnatal life. Several studies show a constant exchange of HSPCs between BM and peripheral blood 12,19 (Fig. 1). It has been estimated that up to 400 HSPCs circulate in the blood of a mouse at any one time. These cells constantly re-engraft the BM and are continuously replenished by HSPCs newly mobilized from the BM 19. Such BM re-engraftment occurs in physiological conditions in the absence of myelosuppression and does not involve or require inflammation. In fact, the recirculation of HSPCs between BM and blood is believed to be important for the maintenance of hematopoietic homeostasis 19.
The efficiency of BM re-engraftment by HSPCs depends on the availability of certain anatomical/morphological structures in the BM known as ‘niches’ 20 (Fig. 2). The highly specialized niche microenvironment controls self-renewal and lineage differentiation of HSPCs (reviewed in 21,22). In un-manipulated animals, the number of available niches is very low (50–500 per mouse) 23. To empty niches for newly arriving HSPCs, hematoablative treatments (such as irradiation, myelosuppressive drugs, and cytokines) are routinely used. Alternatively, hematopoietic engraftment in un-irradiated mice can be enhanced by antibody-mediated depletion of the recipient’s endogenous HSPCs 24.
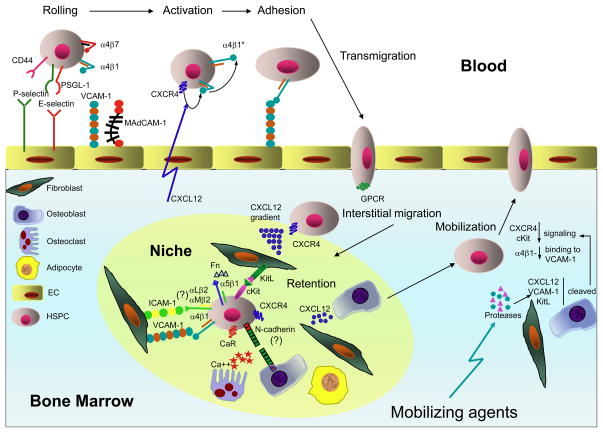
Figure 2. Migration of hematopoietic stem and progenitor cells within BM.
HSPCs enter the BM through sinusoids, which constitutively express traffic molecules that support unique a multistep cascade for HSPC homing. First, circulating HSPCs tether to the vessel wall by engaging vascular selectins, P- and E-selectin, which bind to carbohydrate ligands that are associated with PSGL-1 and/or CD44 (HCELL) on HSPCs Tethered cells then roll slowly engaging both endothelial selectins and the integrin α4β1-VCAM-1 pathway. Some studies have also implicated α4β7 –MAdCAM-1 in the rolling step. The rolling HSPCs are then activated by the chemokine CXCL12, which signals through CXCR4. The chemokine signal induces a conformational change in α4β1 (α4β1*) resulting in increased affinity for VCAM-1, which mediates firm arrest. Next, the adherent HSPCs transmigrate through the vessel wall following extracellular chemoattractants (possibly CXCL12) that signal through G-protein-coupled receptors. Extravascular trafficking allows HSPCs to lodge in specific niches, represented by various stromal cells (fibroblasts, osteoblasts, osteoclasts and adipocytes). Stromal cells maintain the prerequisite conditions for HSPC survival and function in the BM. HSPC retention in niches is mediated by interactions of α4β1 with VCAM-1 and fibronectin (Fn) (the later also interacts with another β1 integrin – α5β1), and β2 integrins with ICAM-1, CXCR4 with CXCL12 and cKit with its ligand (KitL). Retention also involves the Ca2+ receptor (CaR), a member of the large G-protein-coupled receptor family, and homotypic adhesion via N-cadherin. In both homeostatic and stress-induced conditions, some BM-resident HSPCs undergo de-adhere and leave the BM (a process known as “mobilization”). HSPC mobilization involves up-regulation of proteolytic enzymes (matrix metalloptroteinase 9, cysteine protease cathepsin K, dipeptidase CD26), which leads to cleavage of CXCL12, KitL and VCAM-1. As a result, HSPCs can detach from the BM stromal cells and enter the circulation.
Migration of adult HSPCs occurs not only to and from the BM, but also within the BM 25. Most primitive HSPCs are located in the subendosteal region of the BM cavity of long bones where osteoblasts are thought to produce essential growth factors 26,27 and provide regulatory signals for primitive stem cells 28–30. In addition to osteoblasts, nestin+ mesenchymal stem cells have been recently identified to contribute another essential component of the HSC niche in the BM 31. Upon maturation, primitive HSPCs lose their quiescence and migrate towards the center of the BM cavity to proliferative niches 32–34. The existence of specialized niches to which HSPCs migrate during their differentiation may explain the differential distribution of myeloid and lymphoid populations in the BM. For example, one of the cellular components of the niche, endothelial cells, appear to play key roles in megakaryopoiesis since mature megakaryocytes are almost exclusively localized near thin-walled sinusoids 35. In contrast, lymphoid progenitors are found in close contact with osteoblasts 32. In addition, even phenotypically identical HSCs obtained from different anatomical regions of the BM have recently been found to differ with regards to their biological potential 36.
While it had been known for some time that HSPCs travel within the BM and from the BM into the blood and vice versa, an additional route of HSPC recirculation was recently identified 12. BM-derived HSPCs travel from the blood into multiple peripheral tissues, from the tissues into the lymph, and from the lymph via the thoracic duct, the main draining lymph vessel in the body, back into the blood. Once in the circulation, HSPCs can either re-enter the BM or repeat the “peripheral” migration cycle (Fig. 1). It is still unclear, whether the subset of HSPCs that embark on this voyage are somehow different from the bulk of HSPCs, which are found in the BM-blood compartment, or whether all circulating HSPCs have the choice and potential to patrol peripheral tissues. The recirculating HSPCs can differentiate into immune and inflammatory effector cells under certain conditions, such as distress signals that activate HSPC-expressed Toll-like receptors (TLRs), during infection or tissue damage. This differentiation can occur in peripheral tissues and is thought to provide a local source of needed immune cells, such as dendritic cells (DCs) and other myeloid cell types (Fig. 3). Recent evidence suggests a role for TLR2 and TLR4 in the differentiation of mouse HSPCs into myeloid lineage cells 37. In addition, it has been demonstrated that TLR7 and TLR8 are expressed on human BM CD34+ progenitors, and the activation of these TLRs causes differentiation of HSPCs into macrophages and monocytic DC precursors 38. The locally generated HSPC-derived leukocytes can participate in classical innate immune responses and contribute to early eradication of local infection, elimination of dead cells and replenishing of tissue-resident DCs lost during infection due to cell death or migration into draining lymph nodes (reviewed in 39). HSPCs have also been claimed to give rise to hepatocytes in a liver injury model 40. While this so-called transdifferentiation of HSPCs is controversial, the finding raises the intriguing possibility that migratory HSPCs may have a role during the repair of hematopoietic, as well as non-hematopoietic tissues.
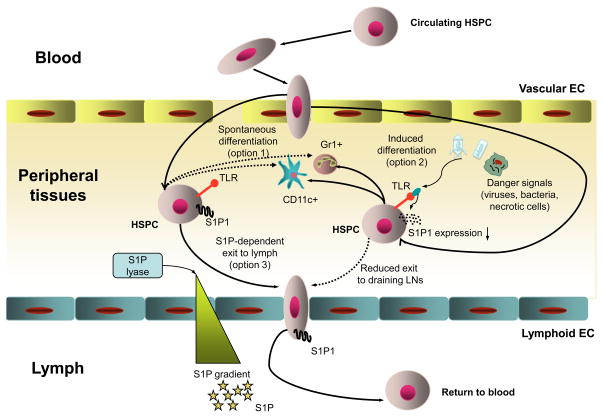
Figure 3. Migration of HSPCs within peripheral tissues.
Blood-borne HSPCs have the capacity to enter peripheral non-hematopoietic tissues from the blood. The mechanisms that control this process are still poorly understood. In the organs, some HSPCs can spontaneously differentiate into immune cells (option 1). This differentiation dramatically increases under stress conditions (such as infection or tissue necrosis), since HSPCs receive signals via toll-like receptors (TLRs) that promote myeloid differentiation (option 2). HSPCs that do not undergo differentiation spend ~48h within peripheral tissues before they access local draining lymphatics (option 3), a migration step that depends on sphingosine-1-phosphate (S1P) and S1P receptor 1 (S1P1). The free S1P level is high in the lymph but low in tissues owing to constant degradation of S1P in the latter by S1P lyase. When HSPCs follow the S1P gradient and enter the lymph, they return to the circulation to can either access the BM or home into another peripheral tissue.
How HSPC migration is controlled in fetal life
Migration of fetal HSPCs between tissues is essential to establish the hematopoietic system during embryonic development. Due to limitations in the intrauterine accessibility of embryos, it is technically challenging to directly visualize fetal HSPC in their native environment, so only a few studies have managed to directly observe and track fetal HSPCs in vivo 41–43. Moreover, the placenta poses a barrier hindering the passage of cells, antibodies, fluorescent dyes and other compounds to dissect the molecular mechanisms of cell movement in the fetal circulation. Thus, our knowledge regarding the molecular mechanisms that orchestrate fetal HSPC migration is currently based mostly on differential expression of trafficking molecules on HSPCs isolated from various embryonic organs. In addition, analysis of fetal hematopoiesis in genetically modified mice as well as in vitro migration assays have provided valuable information on how HSPCs find their way within the fetus
In murine fetal yolk sac, AGM and placenta HSPCs express CD144, also known as vascular endothelium (VE) cadherin 44, a cell-surface glycoprotein that mediates Ca2+-dependent homophilic cell-cell adhesion. Upon transition to the FL, HSPCs down-regulate CD144 expression and become CD144– by E16.5, at which point they leave the FL and colonize the BM 45. The surface expression of CD144 is a unique feature of fetal HSPCs, which is not shared by their adult counterparts. However, whether CD144 directly controls fetal HSPC trafficking remains unclear.
Integrin-mediated adhesion is important for HSPC movement in both embryogenesis and adulthood (see later discussion). HSPCs in the yolk sac, AGM and placenta express CD41 (GPIIb integrin encoded by the gene Itga2b). Expression gradually decreases during development, and adult HSPCs express little or no CD41 (reviewed in 46) 47. It has been shown that inactivation of the Itga2b allele results in increased numbers of HSPCs in various embryonic sites, an effect that may be explained, at least in part, by loss of binding to fibronectin 47, suggesting a potential role of CD41 in retention of HSPCs in their niche (reviewed in 46). In addition to CD41, β1 integrins tune the migration of fetal HSPCs. The use of chimeric mice generated with β1 integrin-deficient fetal HSPCs has revealed that fetal HSPCs lacking β1 integrins form and differentiate but they cannot colonize the FL 48, suggesting an essential role of β1 integrins in fetal HSPC trafficking.
Chemokines and other chemoattractants are also important in fetal HSPC trafficking. Among them, the role of stem cell factor (SCF), also known as Kit ligand (KitL), which binds to the tyrosine kinase receptor cKit, is well established. Fetal HSPCs express high levels of cKit from early stages throughout embryonic development 49. SCF exerts chemoattractant effects on early fetal HSPCs17 and thus promotes their retention and controls their dwell time in the FL. Unlike adult HSPCs, early FL HSPCs do not respond to the chemokine CXCL12, a major guidance signal for BM colonization 50. However, at later stages of embryonic development, FL HSPCs acquire the ability to migrate toward CXCL12 and to colonize the BM 17. The observation that embryos with a genetic deficiency in either CXCL12 or the major HSPC-expressed G-protein-coupled receptor for CXCL12, CXCR4, develop severe hematopoietic defects underscores the pivotal role of the CXCL12-CXCR4 axis in fetal hematopoiesis 51–53.
How HSPC migration from and into the adult BM is controlled
Mechanisms for HSPC exit from the BM
Anchoring of HSPCs within the BM is mediated by interactions of multiple HSPC-expressed receptors with their respective stroma-expressed ligands or soluble factors (Fig. 2). Extensive studies with blocking antibodies 54, functional antagonists 55 and genetically deficient mice 56–58 have shown the particular importance of two molecular pathways for the retention of HSPCs within BM at steady-state: the adhesion molecules α4β1 integrin and VCAM-1, and the CXCR4-CXCL12 chemokine pathway. Additional pathways very likely participate in a cooperative or synergistic fashion (reviewed in 59). The role of the so-called leukocyte integrins that share the common β2 (CD18) chain, αLβ2 (LFA-1 or CD11a/CD18) and αMβ2 (Mac-1, CD11b/CD18), is still under debate. While some studies report their involvement in HSPC retention 60,61, others indicate that the effect of β2 integrins becomes apparent only in synergy with α4β1 62.
Signaling via the cKit-KitL pathway regulates adhesion of HSPCs to stroma by inducing activation of β1 integrins 63–65 and by promoting chemotactic and chemokinetic responses in HSPCs 66. Moreover, several studies demonstrated that the transmembrane form of KitL displayed on stromal cells can confer mechanically stable interactions with HSPC-expressed cKit, thus playing a direct role in cell-cell adhesion 67,68. Another potential player in HSPC trafficking is N-cadherin, which is present on both osteoblasts and quiescent HSPCs 28. Based on expression studies, it has been suggested that N-cadherin facilitates HSPC retention in the niche, however, the role of N-cadherin in HSPC biology has been challenged by recent observations that N-cadherin+ BM cells do not possess stem cell properties 69. Thus, there may be other molecular mechanisms used by stroma cells to retain HSPCS in the BM. Indeed, BM resident osteoblasts are a rich source of CXCL12 in humans and mice 29,70,71, thus providing an abundant local source for this potent HSPCs-attracting chemokine in the BM.
Osteoblasts and osteoclasts are thought to form a functional unit tasked with physiological bone remodeling at the BM-bone interface, which is characteristically associated with a local increase in free Ca2+. HSPCs can respond to variations in extracellular Ca2+ through the Ca2+ receptor (CaR), a G-protein-coupled receptor that is expressed on their surface. In the absence of CaR, HSPCs are defective in their ability to lodge in the endosteal niche, and thus do not engraft after transplantation 72. Correspondingly, pharmacological stimulation of CaR has recently been demonstrated to enhance CXCR4-mediated lodgment of HSPCs at the endosteal niche 73.
Despite the continuous BM retention signals that are received by HSPCs through the pathways discussed, there is always a small but significant fraction of HSPCs in the blood circulation and in peripheral tissues. Experimental evidence indicates that these extramyeloid HSPCs underwent a de-adhesion step and managed to “escape” from the BM by migrating into the blood, a phenomenon known as “mobilization”. Certain drugs can greatly enhance HSPC mobilization, and effect that has been exploited clinically to collect circulating donor HSPCs for transplantation (reviewed in 39,59). A variety of cytokines (G-CSF is the most commonly used among them), chemokines (such as IL-8 and Gro-β) and small molecule drugs have used as mobilizing agents. HSPC mobilization from the niche involves up-regulation of proteolitic enzymes expressed either by stromal elements (such as matrix metalloproteinase 9 (MMP-9) and cysteine protease cathepsin K)34,74 or by HSPCs (e.g. the dipeptidase CD26) 75. Activation of these enzymes leads to proteolitic cleavage of CXCL12, KitL, and VCAM-1 and thus loosens the adhesive contacts between stromal cells and HSPCs.
In recent years, the role of the sympathetic nervous system (SNS) in HSPC traffic has received increasing attention 76. Because the SNS innervates bone and BM stromal cells, and both hematopoietic and stromal cells express neurotransmitters and neuropeptides as well as their receptors in the BM (reviewed in 77 and 76), it has been proposed that the SNS could play a role in BM homeostasis 78. Indeed, SNS signals contribute to G-CSF-induced HSPC mobilization via β-adrenergic signals 79. In homeostatic conditions, the number of blood-borne progenitor cells in both humans and mice is subject to circadian oscillations 80,81. Studies in mice demonstrated that circadian noradrenalin secretion leads to down-regulation of CXCL12 and thus promotes HSPC egress from the BM 82.
Mechanisms for migration of HSPCs into the BM
HSPC ‘homing’, that is migration from blood into the BM, is critical for the clinical success of BM transplantation. Furthermore, homing is integral to the postnatal process during which HSPC are in constant exchange between BM and blood to ensure homeostasis of hematopoietic activity throughout the blood-forming skeletal system 19. To home to the BM, HSPCs must “recognize” endothelial cells in BM microvessels and adhere to them to resist the constant hydrodynamic shear exerted by the flowing blood. This recognition is achieved by interactions of HSPC-expressed adhesion molecules and chemokine receptors with endothelium-expressed binding partners, whose composition is unique to the BM microvasculature.
As a rule, the recruitment of circulating hematopoietic cells, including HSPCs, involves a sequence of at least three intravascular adhesion steps before the cells can egress into a target tissue 83,84. The initial step, which allows the fast flowing cells to tether and slowly roll along the vascular lining, is mediated by primary adhesion molecules, which engage their respective ligands with fast binding kinetics, but a short bond lifetime. To adhere firmly, the rolling cells must receive an activation signal, provided by soluble or surface-bound chemoattractants. In most cases, this activation signal depends on Gαi protein-coupled receptors (GPCRs) and can be blocked by pertussis toxin (PTX) treatment. In addition, chemoattractant signals involving Gαs have been implicated in BM homing and engraftment 27. The activation signals triggers a conformational change in secondary receptors, namely leukocyte expressed integrins, into a high affinity conformation, which is required for their binding to endothelial ligands of the immunoglobulin superfamily. This secondary, activation-dependent step mediates firm adhesion. Subsequently, the adherent cells transmigrate along chemoattractant gradients into and within the target tissue (reviewed in 85).
IVM experiments have provided definitive information on the molecular pathways that mediate HSPC interactions with BM sinusoids during each consecutive step in the homing cascade (Fig. 2). BM microvessels express the endothelial selectins, P- and E-selectin as well as VCAM-1 50. In vascular beds outside the BM, these trafficking molecules are rarely present on endothelial cells without inflammatory stimulation. In fact, the BM is the only organ in which constitutive side-by-side expression of these three adhesion molecules has been demonstrated 9. Interactions of endothelial selectins and VCAM-1 with their HSPC-expressed counter-receptors, PSGL-1 and low affinity α4β1 (VLA-4), respectively, supports HSPC tethering and rolling, whereas sticking is mediated by the (chemoattractant-induced) high affinity form of α4β1 8. The role of another α4 integrin – α4β7 – is not completely understood. Some studies have implicated α4β7 in HSPC rolling 86 and reported expression of the α4β7 ligand, MAdCAM-1, on BM endothelium 87, but others could not detect a role for this pathway in HSPC homing to BM 8. In addition, HSPCs also express CD44, a molecule involved in rolling of hematopoietic cells 88. A major ligand of CD44 is hyaluronate, an anionic extracellular matrix glycosaminoglycan that is widely expressed in connective, epithelial and neural tissues, including the BM 89,90. In addition, a specialized glycoform of CD44, called HCELL (hematopoietic cell E- and L-selectin ligand), has been identified on early immature HSPCs. In vitro studies indicate that HCELL binds to E- and L-selectin with higher affinity than PSGL-1, the most common selectin ligand on differentiated leukocytes 88,91,92.
Although there are numerous chemokines in the BM 93,94, only CXCL12 has been shown to be functionally relevant for HSPC recruitment 95,96. CXCL12/CXCR4 signaling is not only responsible for HSPC retention and mobilization, but also for activation of integrins, which in turn mediate firm adhesion of HSPCs 96. However, HSPCs are capable of homing to adult BM also via CXCL12-independent mechanisms 50, indicating that additional, yet unknown route(s) are involved in colonization of this organ. Accordingly, although CXCR4 and CXCL12-deficient mice die during fetal development, they do develop a BM compartment 52,53. Nevertheless, in adult BM, primitive HSPCs (Lin−Thy1locKit+Sca1+) express a very restricted repertoire of chemokine receptors and respond only to CXCL12 97. This raises the possibility that HSPCs may respond to other (non-chemokine) chemoattractants. Indeed, HSPCs migrate towards gradients of the sphingolipid sphingosine 1-phosphate (S1P) to access lymph vessels from peripheral tissues (see below) 12. However, the concentration of S1P in the BM is high in blood and low in the extravascular space 98, making it unlikely that this pathway contributes to HSPC homing to the BM.
How HSPC migration to and from adult peripheral tissues is controlled
The exact mechanisms mediating the retention of HSPCs in, as well as the egress from peripheral organs and the recruitment pathway(s) responsible for HSPC trafficking from blood into extramyeloid compartments remain(s) to be elucidated. Here we summarize what is known so far with respect to migration into and exit from peripheral (non-BM) tissues.
Pathways for HSPC migration into non-BM tissues
Under physiological conditions, HSPCs of different maturation stages (i.e. ranging from the most primitive stem cells to lineage-committed progenitors) are found in the liver, lungs, intestines, kidneys 12,19, thymus (reviewed in 99, and the skin (reviewed in 100). Tissue damage facilitates HSPC homing into sites of injury 101–103. The observation that HSPC lodge in multiple organs is not surprising, because CXCL12 is produced constitutively not only by stromal cells in the BM, but also in various other tissues by different cell types, including endothelial cells and tissue DCs 70,104,105. Moreover, CXCL12 up-regulation was noticed in stress conditions 103,106. Apart from CXCL12, E-selectin has been implicated in HSPC homing to both the BM and the skin (reviewed in 100). In contrast stem cell homing to the spleen, a hematopoietic site in mice, does not depend on VCAM-1/α4β1 interaction, which is essential for BM colonization 107. Thus, some but not all peripheral sites use HSPC recruitment signals similar to the ones that operate within the BM.
Pathways for HSPC exit from non-BM tissues
Little is known about the signals, which regulate the exit of HSPCs out of tissues other than the BM. HSPCs arrive in peripheral organs via blood, but leave them predominantly via the draining lymphatics 12,39. It was recently shown that egress of HSPCs from peripheral tissues depends on sphingosine-1 phosphate (S1P) and S1P receptors (S1P receptor 1, or S1P1, in particular), a signaling pathway, which has been implicated previously in lymphocyte exit from secondary lymphoid organs into the lymph 108–110. The concentration of S1P is high in the lymph, but low in tissues due to degradation by S1P lyase, and HSPCs follow this gradient in order to leave peripheral organs (Fig. 3). Drug-induced inhibition of S1P-S1P receptor signaling decreases the number of HSPCs in tissue-draining lymph due to impaired egress from the tissue. Interestingly, stress signals that mimic an infection (induced by administration of a TLR4 agonist) reduce S1P1 expression on HSPCs resulting in prolonged retention in peripheral organs, thus providing necessary time for HSPCs to differentiate into immune cells required to eliminate the danger. Tuning of HSPC retention in peripheral tissues could support innate immune responses by fostering a local and versatile supply of effector cells.
Clinical perspective and concluding remarks
A better understanding of the molecular cues that control the trafficking of HSPCs is critical in several clinical settings, most prominently BM transplantation, and may lead to novel options to treat leukemias and other malignancies.
A successful outcome after BM transplantation (BMT) largely depends on the ability of intravenously injected HSPCs to rapidly find their way to specific hematopoietic niches. Several potential sources of HSPCs are routinely used in clinical protocols, including (i) HSPC directly derived from the BM or (ii) from the cord blood, as well as (iii) HSPCs isolated from the peripheral blood after their mobilization from the BM by administration of drugs or cytokines. While HSPCs from the BM reconstitute the myeloablated recipient BM faster 111, their use in allogeneic BM transplantation is limited by the availability of fully HLA-matched donors 112. In this respect, HSPCs from cord blood proved to provide a sufficient engraftment in adoptive recipients 113,114. However, a potential caveat of this approach lies in the delayed hematopoietic reconstitution afforded by cord blood-derived HSPCs. This in turn leads to increased incidents of early post-transplant complications. Reduced levels of surface-expressed homing-related molecules by cord blood HSPCs 115 is considered, at least in part, to contribute to this problem. As a consequence, various protocols utilizing panels of cytokines are in use to enhance the expression of adhesion molecules, MMPs and CXCR4 on transplanted cells 115,116. Indeed, faster hematopoietic recovery directly correlates with the efficacy of migration of HSPC to the BM 117.
A better understanding of the signals that control HSPCs trafficking could also be clinical relevance beyond their obvious utility in BM transplantation. Similar to many forms of cancer, certain leukemias are considered cell autonomous stem cell disorders. For example, it has been demonstrated in acute myeloid leukemia (AML) that a small subset of AML cells has long-term repopulation potential and is capable of propagating and maintaining the disease 118. These so-called “leukemia stem cells” (LSCs) possess similar self-renewal capacity as normal HSCs. Unlike non-hematopoietic cancer stem cells, LSCs depend on a specific microenvironment and microdomains within the BM (reviewed in119 and 120), and analogous to normal HSPCs, the trafficking of LSCs relies on expression of distinct adhesion and chemotactic molecules. In fact, recent findings suggest that the molecular machinery used by normal HSPCs for homing and migration to supportive niches may be “hijacked” by LSCs and that LSCs occupy and receive critical signals from the microenvironment that usually supports self-renewal of healthy HSCs. Correspondingly, molecular signatures involved in the trafficking of HSPCs, such as CXCL12 and CXCR4 or α4β1 integrin, have also been implicated in promoting LSC migration into the BM 121.
Since LSC migration and lodgment in niches is not only involved in leukemia maintenance and spreading, but also has been implicated in primary resistance to chemotherapy, the dissection and targeting of LSC trafficking could offer new possibilities to treat leukemic diseases in the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uchida N, Weissman I. Searching for hematopoietic stem cells. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shizuru JA, et al. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Sieburg CE, et al. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986;44 (4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- 4.Mempel T. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Current Opinion in Immunology. 2004;16 (4):406–417. doi: 10.1016/j.coi.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Sumen C, et al. Intravital microscopy; visualizing immunity in context. Immunity. 2004;21 (3):315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Kohler A, et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011;117 (16):4349–4357. doi: 10.1182/blood-2010-09-308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCuskey RS, et al. Microscopy of living bone marrow in situ. Blood. 1971;38:87–95. [PubMed] [Google Scholar]
- 8.Mazo IB, et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: Parallel contributions by endothelial selectins and VCAM-1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazo IB, von Andrian UH. Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol. 1999;66(1):25–32. doi: 10.1002/jlb.66.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Hidalgo A, et al. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110(4):559–569. doi: 10.1172/JCI14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazo IB, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22 (2):259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131 (5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medvinsky A, et al. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138 (6):1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 14.Corbel C, et al. Hematopoietic potential of the pre-fusion allantois. Dev Biol. 2007;301 (2):478–488. doi: 10.1016/j.ydbio.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson EJ, et al. The thymus and T-cell commitment: the right niche for Notch? Nat Rev Immunol. 2006;6 (7):551–555. doi: 10.1038/nri1883. [DOI] [PubMed] [Google Scholar]
- 16.Djaldetti M, et al. Hematopoiesis in the embryonic mouse spleen: an electron microscopic study. Blood. 1972;39 (6):826–841. [PubMed] [Google Scholar]
- 17.Christensen JL, et al. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2 (3):E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowie MB, et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116 (10):2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright DE, et al. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294 (5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 20.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4 (1–2):7–25. [PubMed] [Google Scholar]
- 21.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132 (4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9 (1):11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya D, et al. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203 (1):73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czechowicz A, et al. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318 (5854):1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokoyoda K, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20 (6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Taichman RS, et al. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87 (2):518–524. [PubMed] [Google Scholar]
- 27.Adams GB, et al. Haematopoietic stem cells depend on Gαs-mediated signalling to engraft bone marrow. Nature. 2009:1–6. doi: 10.1038/nature07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425 (6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 29.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425 (6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 30.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118 (2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466 (7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord BI, et al. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46 (1):65–72. [PubMed] [Google Scholar]
- 33.Nilsson SK, et al. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97 (8):2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 34.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109 (5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junt T, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317 (5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 36.Grassinger J, et al. Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood. 2010;116 (17):3185–3196. doi: 10.1182/blood-2009-12-260703. [DOI] [PubMed] [Google Scholar]
- 37.Nagai Y, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24 (6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sioud M, et al. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol. 2006;364 (5):945–954. doi: 10.1016/j.jmb.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 39.Laird DJ, et al. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132 (4):612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 40.Lagasse E, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6 (11):1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 41.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464 (7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464 (7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 43.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464 (7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 44.Taoudi S, et al. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132 (18):4179–4191. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- 45.Kim I, et al. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106 (3):903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133 (19):3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 47.Emambokus NR, Frampton J. The glycoprotein IIb molecule is expressed on early murine hematopoietic progenitors and regulates their numbers in sites of hematopoiesis. Immunity. 2003;19 (1):33–45. doi: 10.1016/s1074-7613(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 48.Hirsch E, et al. Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 49.Yoder MC, et al. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7 (3):335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 50.Mazo IB, et al. Total body irradiation causes profound changes in endothelial traffic molecules for hematopoietic progenitor cell recruitment to bone marrow. Blood. 2002;99 (11):4182–4191. doi: 10.1182/blood.v99.11.4182. [DOI] [PubMed] [Google Scholar]
- 51.Ma Q, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 53.Zou YR, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 54.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci USA. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abraham M, et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 2007;25 (9):2158–2166. doi: 10.1634/stemcells.2007-0161. [DOI] [PubMed] [Google Scholar]
- 56.Scott LM, et al. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23 (24):9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Priestley GV, et al. Sustained alterations in biodistribution of stem/progenitor cells in Tie2Cre+ alpha4(f/f) mice are hematopoietic cell autonomous. Blood. 2007;109 (1):109–111. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foudi A, et al. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4−/− chimeric mice. Blood. 2006;107 (6):2243–2251. doi: 10.1182/blood-2005-02-0581. [DOI] [PubMed] [Google Scholar]
- 59.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111 (8):3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velders GA, et al. Enhancement of G-CSF-induced stem cell mobilization by antibodies against the beta 2 integrins LFA-1 and Mac-1. Blood. 2002;100 (1):327–333. doi: 10.1182/blood.v100.1.327. [DOI] [PubMed] [Google Scholar]
- 61.Hidalgo A, et al. The integrin alphaMbeta2 anchors hematopoietic progenitors in the bone marrow during enforced mobilization. Blood. 2004;104 (4):993–1001. doi: 10.1182/blood-2003-10-3702. [DOI] [PubMed] [Google Scholar]
- 62.Papayannopoulou T, et al. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98 (8):2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 63.Kovach NL, et al. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85 (1):159–167. [PubMed] [Google Scholar]
- 64.Lévesque J-P, et al. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83:1033–1038. [PubMed] [Google Scholar]
- 66.Okumura N, et al. Chemotactic and chemokinetic activities of stem cell factor on murine hematopoietic progenitor cells. Blood. 1996;87 (10):4100–4108. [PubMed] [Google Scholar]
- 67.Toksoz D, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992;89 (16):7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adachi S, et al. Necessity of extracellular domain of W (c-kit) receptors for attachment of murine cultured mast cells to fibroblasts. Blood. 1992;79 (3):650–656. [PubMed] [Google Scholar]
- 69.Kiel MJ, et al. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1 (2):204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Ponomaryov T, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106 (11):1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung Y, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38 (4):497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Adams GB, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439 (7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 73.Lam BS, et al. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood. 2011;117 (4):1167–1175. doi: 10.1182/blood-2010-05-286294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12 (6):657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 75.Christopherson KW, 2nd, et al. Endothelial induction of the T-cell chemokine CCL21 in T-cell autoimmune diseases. Blood. 2003;101(3):801–806. doi: 10.1182/blood-2002-05-1586. [DOI] [PubMed] [Google Scholar]
- 76.Mendez-Ferrer S, et al. Circadian rhythms influence hematopoietic stem cells. Current opinion in hematology. 2009;16 (4):235–242. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mendez-Ferrer S, Frenette PS. Hematopoietic stem cell trafficking: regulated adhesion and attraction to bone marrow microenvironment. Annals of the New York Academy of Sciences. 2007;1116:392–413. doi: 10.1196/annals.1402.086. [DOI] [PubMed] [Google Scholar]
- 78.Auffray I, et al. Nerve growth factor is involved in the supportive effect by bone marrow--derived stromal cells of the factor-dependent human cell line UT-7. Blood. 1996;88 (5):1608–1618. [PubMed] [Google Scholar]
- 79.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124 (2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 80.Ross DD, et al. Diurnal variation of circulating human myeloid progenitor cells. Exp Hematol. 1980;8 (7):954–960. [PubMed] [Google Scholar]
- 81.Verma DS, et al. Diurnal changes in circulating myeloid progenitor cells in man. Am J Hematol. 1980;9 (2):185–192. doi: 10.1002/ajh.2830090206. [DOI] [PubMed] [Google Scholar]
- 82.Mendez-Ferrer S, et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452 (7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 83.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76 (2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 84.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7 (9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 85.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286 (5447):2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 86.Katayama Y, et al. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104 (7):2020–2026. doi: 10.1182/blood-2003-12-4157. [DOI] [PubMed] [Google Scholar]
- 87.Feuerer M, et al. Bone marrow microenvironment facilitating dendritic cell: CD4 T cell interactions and maintenance of CD4 memory. Int J Oncol. 2004;25 (4):867–876. [PubMed] [Google Scholar]
- 88.Dimitroff CJ, et al. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153 (6):1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vermeulen M, et al. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 90.Avigdor A, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103 (8):2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 91.Dimitroff CJ, et al. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc Natl Acad Sci U S A. 2000;97 (25):13841–13846. doi: 10.1073/pnas.250484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dimitroff CJ, et al. differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J Biol Chem. 2001;276 (50):47623–47631. doi: 10.1074/jbc.M105997200. [DOI] [PubMed] [Google Scholar]
- 93.Hillyer P, et al. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin Exp Immunol. 2003;134 (3):431–441. doi: 10.1111/j.1365-2249.2003.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shulby SA, et al. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64 (14):4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 95.Nagasawa T, et al. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peled A, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104 (9):1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wright DE, et al. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195(9):1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishii T, et al. The role of sphingosine 1-phosphate in migration of osteoclast precursors; an application of intravital two-photon microscopy. Mol Cells. 2011 doi: 10.1007/s10059-011-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zlotoff DA, Bhandoola A. Hematopoietic progenitor migration to the adult thymus. Annals of the New York Academy of Sciences. 2011;1217:122–138. doi: 10.1111/j.1749-6632.2010.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Invest Dermatol. 2004;122 (5):1061–1069. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 101.Badami CD, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63(3):596–600. doi: 10.1097/TA.0b013e318142d231. discussion 600–592. [DOI] [PubMed] [Google Scholar]
- 102.Dalakas E, et al. Hematopoietic stem cell trafficking in liver injury. Faseb J. 2005;19 (10):1225–1231. doi: 10.1096/fj.04-2604rev. [DOI] [PubMed] [Google Scholar]
- 103.Kollet O, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112 (2):160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pablos JL, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155(5):1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410 (6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 106.Stumm RK, et al. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22 (14):5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Papayannopoulou T, et al. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 109.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5 (7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 110.Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate--modifiers of lymphocyte migration. N Engl J Med. 2006;355 (11):1088–1091. doi: 10.1056/NEJMp068159. [DOI] [PubMed] [Google Scholar]
- 111.Korbling M, et al. Allogeneic blood stem cell transplantation: peripheralization and yield of donor-derived primitive hematopoietic progenitor cells (CD34+ Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood. 1995;86 (7):2842–2848. [PubMed] [Google Scholar]
- 112.Anasetti C, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320 (4):197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 113.Broxmeyer HE, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86 (10):3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Broxmeyer HE, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A. 1992;89 (9):4109–4113. doi: 10.1073/pnas.89.9.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng Y, et al. Ex vivo manipulation of umbilical cord blood-derived hematopoietic stem/progenitor cells with recombinant human stem cell factor can up-regulate levels of homing-essential molecules to increase their transmigratory potential. Exp Hematol. 2003;31 (12):1237–1246. doi: 10.1016/j.exphem.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 116.Ramirez M, et al. Ex vivo expansion of umbilical cord blood (UCB) CD34(+) cells alters the expression and function of alpha 4 beta 1 and alpha 5 beta 1 integrins. Br J Haematol. 2001;115 (1):213–221. doi: 10.1046/j.1365-2141.2001.03084.x. [DOI] [PubMed] [Google Scholar]
- 117.Voermans C, et al. In vitro migratory capacity of CD34+ cells is related to hematopoietic recovery after autologous stem cell transplantation. Blood. 2001;97 (3):799–804. doi: 10.1182/blood.v97.3.799. [DOI] [PubMed] [Google Scholar]
- 118.Glanville SH, et al. Transplantation of embryonic spleen tissue reveals a role for adult non-lymphoid cells in initiating lymphoid tissue organization. Eur J Immunol. 2009;39(1):280–289. doi: 10.1002/eji.200838724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lane SW, et al. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114 (6):1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66 (9):4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 121.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435 (7044):969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fraser ST, et al. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin(+) population. Exp Hematol. 2002;30 (9):1070–1078. doi: 10.1016/s0301-472x(02)00887-1. [DOI] [PubMed] [Google Scholar]
- 123.Ferkowicz MJ, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130 (18):4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 124.Clark RA, et al. CD44 and hyaluronan-dependent rolling interactions of lymphocytes on tonsillar stroma. J Cell Biol. 1996;134:1075–1087. doi: 10.1083/jcb.134.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]