Abstract
Interleukin-24 (mda-7/IL-24) is a cytokine in the IL-10 family that has received a great deal of attention for its properties as a tumor suppressor and as a potential treatment for cancer. In this study, we have identified and characterized five alternatively spliced isoforms of this gene. Several, but not all of these isoforms induce apoptosis in the osteosarcoma cell line U2OS, while none affect the survival of the non-cancerous NOK cell line. One of these isoforms, lacking three exons and encoding the N-terminal end of the mda-7/IL-24 protein sequence, caused levels of apoptosis that were higher than those caused by the full-length mda-7/IL-24 variant. Additionally, we found that the ratio of isoform expression can be modified by the splice factor SRp55. This regulation suggests that alternative splicing of mda-7/IL-24 is under tight control in the cell, and can be modified under various cellular conditions, such as DNA damage. In addition to providing new insights into the function of an important tumor suppressor gene, these findings may also point toward new avenues for cancer treatment.
Keywords: mda-7, IL-24, mda-7/IL-24, alternative splicing, SRp55, apoptosis
1. Introduction
Alternative mRNA splicing is emerging as a critical player in the regulation of gene expression and as a method for expanding the proteome [1]. As might be predicted for such a system, many diseases, including cancer, are increasingly associated with or caused by aberrations in alternative splicing [2–5]. A complex of proteins and ribonucleoproteins known as the spliceosome carries out the splicing procedure at sites that are defined by somewhat variable sequences [6]. If alternative splicing is to occur, the selection of the alternative splice site is governed by at least two families of proteins, hnRNPs and the SR family [7,8]. Alternative splicing enables much more sophisticated control over gene expression and cell function than is possible through modification of promoter activity alone. Alternative splicing can produce mRNAs with different stability, alter the function of the protein product by splicing out regions coding for a particular domain, change enzymatic activity or kinetics, alter intracellular localization, or introduce stop codons leading to a truncated protein product [9]. Additionally, there are many cases now described where protein isoforms produced by alternatively spliced transcripts modify the activity of the primary isoform by heterodimerizing, blocking receptor sites, or by other mechanisms [10]. In this study, we investigated the splicing of the tumor suppressor Interleukin-24.
Interleukin-24 (mda-7/IL-24) is a cytokine within the IL-10 family [11] that was originally described as melanoma differentiation-associated gene 7 (mda-7) in studies designed to identify transcripts that were differentially expressed in melanoma cells induced to terminally differentiate [12]. This gene was found to be constitutively expressed in normal melanocytes, with the expression level decreasing progressively in benign nevi, in situ melanoma, invasive melanoma and metastatic melanoma [13].
Many of the initial studies into the function of mda-7/IL-24 took place prior to its classification as an interleukin, and utilized an adenoviral vector delivery system (Ad-mda7) that expressed the protein intracellularly. In this context, mda-7/IL-24 was found to activate multiple apoptotic signaling pathways, both intrinsic and extrinsic, some of which appeared to be cell-type specific. Apoptosis induced by mda-7/IL-24 involved the caspase cascade [14], occurred independently of p53, Ras, Rb, and p21 [15–19], and was observed in a wide variety of tumor cell types, including those representing melanoma, breast cancer, pancreatic cancer, prostate cancer, lung cancer, malignant gliomas, hepatomas, renal carcinoma, ovarian cancer, and other tumors, while having no apparent effect on normal cells and cell lines [14,15,19–24]. Additionally, injection of the construct into solid tumors caused a reduction in the size of tumors in animal models, enhanced the lethal effects of radiation on tumor cells, and displayed potent antitumor bystander activity [25,26]. These observations provided evidence of the ability of mda-7/IL-24 to act as a tumor suppressor from inside the cell, and served as the basis for the development of clinical trials. Phase I/II clinical trials are currently underway in humans [27,28].
In contrast, a separate set of events occurs when mda-7/IL-24 acts as a cytokine. These functions of mda-7/IL-24 are mediated through the IL-20R1/IL20R2 and IL-22-R1/IL-20R2 receptors, and it has been speculated that secretion and receptor-mediated activity may be responsible for the previously-observed anti-tumor “bystander” activity observed in cells not directly infected with the mda-7/IL-24 expressing virus Ad-mda-7 [25,26,29]. The receptors for mda-7/IL-24 are associated with the JAK/STAT pathway, which affects transcriptional regulation, particularly of genes involved in cell differentiation, proliferation and apoptosis [30,31], providing a mechanism by which mda-7/IL-24 can also act as a tumor suppressor from outside the cell.
Mda-7/IL-24 is indeed glycosylated and secreted [17,18], and is secreted even from cells infected with Ad-mda-7 [29,31]. However, while the exogenous protein is cytotoxic to tumor cells, these mechanisms are not yet well-defined. Following binding to its receptors, mda-7/IL-24 activates STAT3, and to a lesser extent STAT1 [32], but this activation is not essential for tumor cell killing [33,34]. Further, the purified protein exerts its cytotoxic activity regardless of the receptor status of the tumor cells [16]. It should also be noted that some groups have contested the ability of mda-7/IL-24 to induce apoptosis in cancer cells as recently as 2007 [35].
In addition to triggering apoptotic pathways, mda-7/IL-24 may also work through the immune system. Unlike IL-10, which exhibits primarily immunosuppressive Th2-type activity, mda-7/IL-24 is immunostimulatory. When applied to PBMCs in culture, mda-7/IL-24 induced activation and production of TNF-α, IL-1, IFN-γ, IL-6 and GM-CSF [11]. This immunostimulatory function was confirmed in a clinical trial, which observed an increase in these cytokines in the serum of patients who had received an intratumoral injection of Ad-Mda-7 [28].
Interleukin-24 consists of 7 exons and 6 introns [36] (Figure 1A), with three different splice variants reported in the literature. Allen et al describe a splice variant lacking exons 3 and 5, termed mda-7s [37]. They predicted that mda-7s would heterodimerize with full-length mda-7/IL-24, but found no effect of mda-7s on the kinetics of cell death induced by full-length mda-7/IL-24. Expression analysis found mda-7s in normal melanocytes, but reduced or absent expression in melanomas, leading the researchers to speculate that loss of this splice variant is associated with tumor progression. A second report by the same group identified splice variants of mda-7/IL-24 lacking either exon 3 or exon 5, but no functional analysis of these variants was conducted, and these variants have not yet been validated by other studies [38]. A recent study of the mouse analog for mda-7/IL-24 (FISP) found a novel splice variant that lacks 29 nucleotides from the 5′ end of exon 4 [39]. The results from this study indicated that the variant (FISP-sp) dimerizes with the full-length version, blocks its secretion, and inhibits the induction of apoptosis. Alternative splicing is commonly reported for other human interleukin genes as well, including IL-2, IL-4, IL-6, IL-7, IL-10 and IL-21 [40–45]. In many of these cases, the alternative protein isoform functions to modulate the activity of the primary interleukin.
Figure 1. Identification of mda-7/IL-24 splice variants.
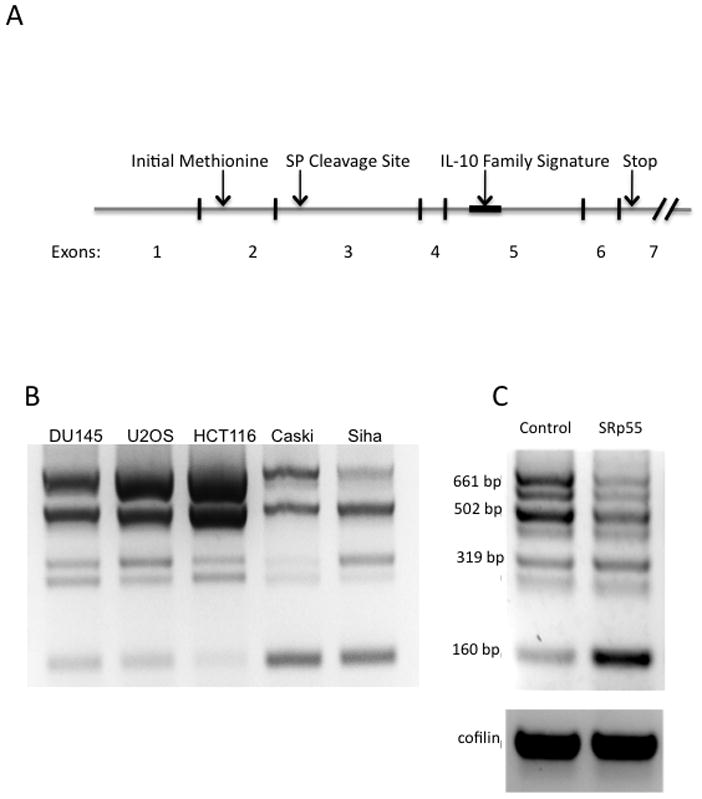
A) The exon structure and key features of full length mda-7/IL-24. The processed mRNA of full-length mda-7/IL24 includes all 7 exons. The open reading frame of mda-7/IL-24 spans exons 2 through 7. Exon 3 contains the signal peptide cleavage site and exon 5 contains the IL-10 family signature sequence, which contributed to the classification of mda-7/IL-24 as a cytokine. B) RT-PCR products amplified by primers corresponding to regions on exon 1 (forward) and exon 7 (reverse) of mda-7/IL-24 reveals multiple isoforms in five cancer cell lines: DU145, U2OS, HCT116, Caski, and Siha. C) U2OS cells were transiently transfected with a vector control (V) or a plasmid expressing the SFRS6 gene. RNA was extracted and RT-PCR was performed using primers designed to amplify mda-7/IL-24. Expression of splice factor SFRS6 led to a dramatic shift in the ratio of splice isoforms, most notably a decrease in expression of the full length isoform (661 bp) and an increase in the expression of the shortest isoform, a 160 bp splice variant.
We previously observed that mda-7/IL-24 is transcribed into at least 4 distinguishable isoforms when expressed in the U2OS cell line [46]. This observation suggested the possibility that differences between these isoforms could account for some of the previous discrepant observations, and prompted us to investigate further. In this study, we set out to characterize and compare the pro-apoptotic properties of these four isoforms, and found that these mda-7/IL-24 isoforms displayed varying effects on the viability of osteosarcoma-derived U2OS cells. Interestingly, one of the shortest isoforms, which lacks exons 2, 3 and 5, and shares similarity to the last 63 aa of full-length mda-7/IL-24, proved to be the most potent apoptotic inducer of all the isoforms tested, including the full-length variant. These results suggest the potential for increased efficacy of mda-7/IL-24 related protein products in the treatment of human cancers.
The current interest in clinical applications of mda-7/IL-24 is largely due to its unique ability to induce apoptosis in a wide variety of cancer cells while having no apparent effect on normal cells. Further development of the clinical potential of mda-7/IL-24 will require a more complete understanding of how this molecule functions, including an appreciation and optimization of the biological activities exerted by the different isoforms.
2. Materials and Methods
2.1 Cell lines and cell culture
U2OS cells, derived from a human osteosarcoma, were obtained from the ATCC. HCT116 cells were a kind gift from Dr. Bert Vogelstein (Johns Hopkins University) [47]. These two cell lines were cultured in McCoy’s 5A medium (Invitrogen) supplemented with 10% fetal bovine Serum (PAA Laboratories), penicillin (100 U/ml) and streptomycin (100 μg/ml) (Sigma-Aldrich). DU145 cells were obtained from the ATCC and cultured in MEM (Invitrogen) supplemented with 10% fetal bovine Serum (PAA Laboratories), penicillin (100 U/ml) and streptomycin (100 μg/ml) (Sigma-Aldrich). NOK cells, a normal oral keratinocyte cell line immortalized by hTERT, were a kind gift from Dr. Karl Munger [48]. NOK cells were cultured in KSFM medium (Invitrogen).
2.2 Cloning and sequence analysis of mda-7/IL-24 splice variants
Total RNA was isolated from U2OS, HCT116, and DU145 cells using TRIzol Reagent (Invitrogen) and used as a template to produce cDNA using Superscript III Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. Primers were designed complementary to exons 1 and 7 of IL24 to encompass the entire ORF of any splice isoform containing the first and last exons: GAAGATCTGAATTTTCAACAGAGGCTGCAA and GCTCTAGAGAGCTTGTAGAATTTCTGCA (includes an added XbaI restriction site). RT-PCR was performed at 94°C for 30 sec, at 57°C for 30 sec, and 72°C for 1 minute for 35 cycles using Taq DNA Polymerase (NEB, Beverly MA). Total PCR product was cloned into the pCR Blunt-TOPO vector (Invitrogen) according to the manufacturer’s instructions. Plasmids from the TOPO cloning reaction were transformed into competent E. coli, plated on selective medium and grown into colonies. Individual colonies were screened by RT-PCR using the primers described above. Colonies containing different sized fragments were amplified in selective medium, DNA was isolated using the Zyppy Plasmid Miniprep Kit (Zymo Research) and sequenced.
2.3 Subcloning
Constructs were subcloned from the pTOPO vector into the pFLAG-Myc-CMV-22 vector (Sigma-Aldrich). Sequences correctly oriented in TOPO were cloned by cutting at the SacI and XbaI sites and cloned into the same cut sites on pFLAG-Myc-CMV-22 (removing the FLAG tag but maintaining the Myc tag). Sequences that were reversed in TOPO were cloned by cutting at the XbaI and EcoRV (blunt) sites and cloned into the XbaI and Ecl136II (blunt) sites on pFLAG-Myc-CMV-22. For subcloning into the retroviral pLNCX vector (Clontech), the gene sequence together with the Myc tag was amplified by RT-PCR (primer sequences: ATGGGCGGTAGGCGTGTAC and GCTCTAGAGAGCTTGTAGAATTTCTGCA) and blunt cloned into the pLNCX vector, which was digested with HpaI and dephosphorylated with alkaline phosphatase (Roche).
2.4 Mutagenesis of mda-7/IL-24 clones
Single nucleotide mutations were introduced into the plasmid carrying the IL-24δ2,3,5 isoform in order to change the start Met codon ATG into the Ile codon ATC. To do this, we used the QuickChange II Site-Directed Mutagenesis Kit (Agilent Technologies) according to the manufacturer’s protocol. The Reverse primer was a complement to the Forward primer: 5′-CTC GCA AGA AAA TGA GAT CTT TTC CAT CAG AGA CAG TGC ACA CAG GCG-3′. The bolded base C mutagenized the G in the ATG codon, and also introduced the BglII site AGATCT. Mutagenized clones were selected by direct sequencing, and the clone containing the mutation was named mda-7/IL-24δ2,3,5 Mutant A. To produce a frame shift in mutant A, the construct was digested with BglII, ends were polished with Klenow fragment in the presence of 4dNTP, and the construct was treated with ligase. The sequence of Mutant B, containing the shifted open reading frame, was confirmed by DNA sequencing.
2.5 Transfections
Transfections were carried out using the TransIT-LT1 Reagent (Mirus Bio) according to the manufacturer’s instructions.
2.6 Immunoblotting
Cells (4 × 105 to 1 × 106) were lysed in 50 to 100 μl Laemmli buffer for 10 minutes on ice. Lysates were subjected to 8% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon FL membranes (Millipore). Antic-myc Clone 4AB monoclonal antibodies (Sigma) were applied at a 1:1000 dilution. HRP-conjugated secondary anti-mouse antibodies (Pierce) or fluorescently tagged secondary anti-mouse antibodies (Li-Cor Biosciences) (1:5000 and 1:15000 dilution, respectively) were used for detection. HRP conjugated secondary antibodies were detected using the chemiluminescent SuperSignal West Pico or Femto Maximum Sensitivity substrate (Pierce). Fluorescent antibodies were detected using the Odyssey Infrared Imaging system (Li-Cor Biosciences).
2.7 Cell viability assays
MTT Assay
Cells were plated in 96 well tissue culture plates (5 × 104 cells per well), transfected as described above, and at the indicated incubation times, cell viability was measured. 20 μl of MTT was added (5 mg/ml stock) to the medium in each well and cells were incubated at 37°C for 2 hours. The medium was removed and 150 μl of Me2SO was added to each well, mixed by pipetting, and the absorbance of each well was measured at 490 nm. Cell Titer-Glo Assay (Promega): This assay was used instead of MTT for NOK cells; the two assays were first compared for the same transfection, and the data correlated. Cells were grown in 96 well tissue culture plates. At the noted time points after transfection, the Cell Titer-Glo reagent was added to the cell culture medium and mixed on a rotating shaker. After 10 minutes incubating at room temperature, light emission was read using a luminometer.
2.8 Colony forming assays
Cells were plated in 6 well tissue culture plates, and transiently transfected as described above with one of the various IL24 isoforms. Parallel plates were co-transfected with GFP to normalize for transfection efficiency. After 48 hours, cells were trypsinized. GFP co-transfected cells were lysed and GFP intensity was measured using a fluorescent plate reader. The remaining cells were resuspended in 1 ml of medium, re-seeded to different densities (500 μl, 100 μl and 20 μl per well) and then passaged in selective medium containing G418 (500 μg/ml). Following 2 weeks of selection, colonies were fixed with formalin, stained with crystal violet, and the colonies were counted.
2.9 Caspase 3/7 activity assay
Cells were plated in 24 well tissue culture plates and transiently transfected with the vector control, or the mda-7/IL-24 constructs. After 24 hours, cells were lysed in 100 μl Passive Lysis Buffer (Promega). 50 μl was used to normalize for cell number using the Cytotox-Glo assay (Promega) according to the manufacturer’s instructions. The rest of the lysate was subjected to a fluorescence based Caspase 3/7-Glo Assay (Promega) according to the manufacturer’s instructions.
3. Results
3.1 Expression of mda-7/IL-24 splice isoforms in mammalian cells
To analyze the variety and abundance of mda-7/IL-24 isoforms, we performed RT-PCR using primers specific to the first and last (7th) exons of mda-7/IL-24 (Figure 1A). The PCR products were separated by electrophoresis in an ethidium bromide-containing agarose gel and visualized under UV light. The mRNA expression of these splice variants in several cell lines is shown in Figure 1B. Within this set of cell lines, we detected at least 7 distinguishable mRNA isoforms of mda-7/IL-24, several of which are found in similar abundance as the full-length isoform. The distinctive mda-7/IL-24 splicing profiles observed in these different cancer cell lines demonstrates the high sensitivity of mda-7/IL-24 splicing to regulation of the splicing machinery. Since our initial analysis of mda-7/IL-24 isoforms [46] determined that one of the splice variants seen in U2OS cells was up-regulated by the silencing of splice factor SRp55, we further investigated the regulation of mda-7/IL-24 splicing by over-expressing this splice factor. U2OS cells were transiently transfected with a plasmid expressing the SFRS6 gene which codes for splice factor SRp55, or with a vector control. After 48 hours, total RNA was isolated from the cells, and RT-PCR was performed. We found that over-expression of SFRS6 caused a dramatic shift in mda-7/IL-24 splicing away from the larger isoforms, and in particular, toward an increase in the smallest isoform, represented in this PCR by the 160 bp band (Figure 1C). In contrast, repression of SRp55 activity was previously shown to increase levels of the 319 bp isoform [46]. This data suggests that the distribution of mda-7/IL-24 isoforms is responsive to the activity of the SRp55 splicing factor, and that the pattern of splicing isoforms of this gene can be modified by changes in splicing machinery.
3.2 Characterization of alternatively spliced isoforms of mda-7/IL-24
We set about to characterize these isoforms by cloning the RT-PCR products (Figure 1B) into the pCR-Blunt-TOPO vector and sequencing the resulting plasmids. Our analysis revealed 6 differentially spliced transcripts from the mda-7/IL-24 gene in addition to the full-length transcript. The full-length transcript contains all 7 exons. Isoform 2, as previously described (mda-7S, or mda-7/IL-24δ3,5) lacks exons 3 and 5 [37]. In addition, we characterized several transcripts lacking exons 2, 3, and 5 in various combinations (Figure 2A). In all cases, exons were spliced in or out in their entirety. Transcripts were sequenced that lack exon 5 (mda-7/IL-24δ5), exons 2 and 3 (mda-7/IL-24δ2,3), exons 2 and 5 (mda-7/IL-24δ2,5), exons 2, 3 and 5 (mda-7/IL-24δ2,3,5) and exon 2 (mda-7/IL-24δ2) (Figure 2A).
Figure 2. Characterization of mda-7/IL-24 splice variants.
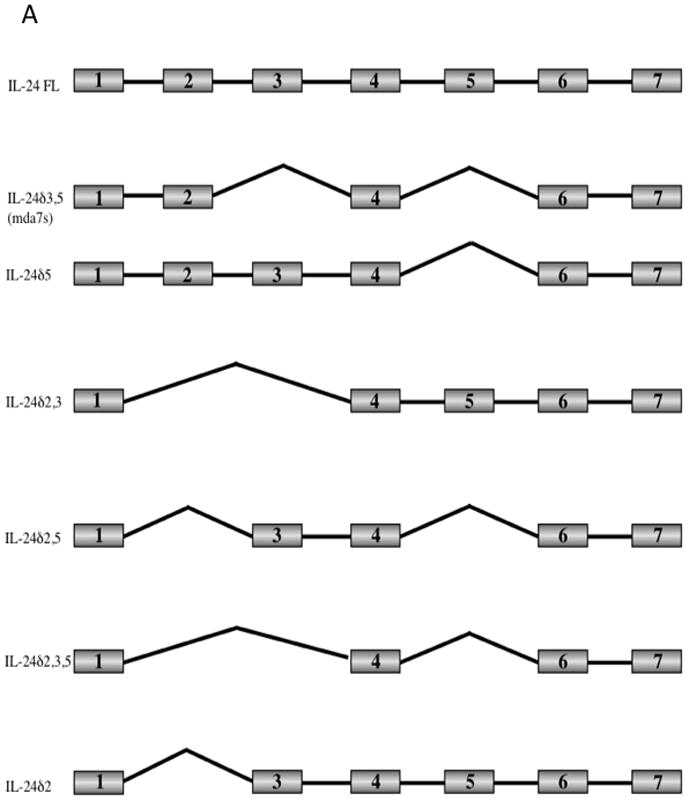
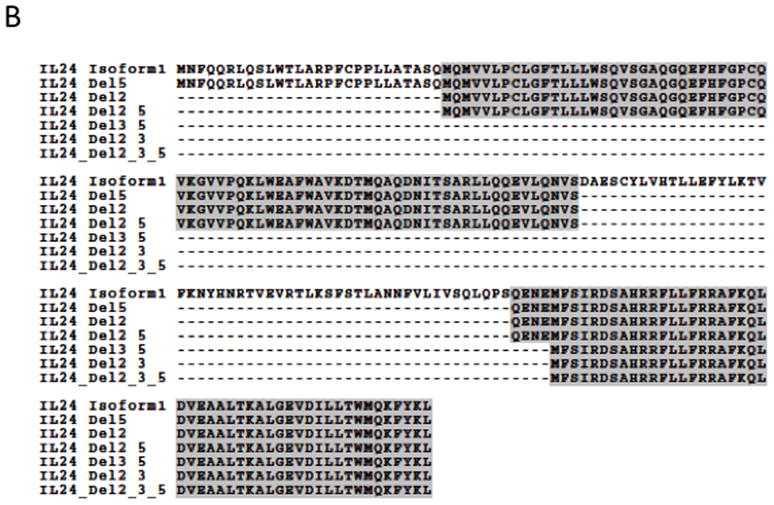
A) The PCR products representing the mda-7/IL-24 splice variants were cloned into vectors and sequenced. A schematic representation of the exon structure of the mda-7/IL-24 splice variants is shown. The exons that are spliced out in the various isoforms include exon 2 which contains the initial methionine, exon 3 which contains the signal peptide cleavage site, and exon 5 which contains the IL-10 family signature sequence. B) Clustal alignment of amino acid sequences of the mda-7/IL-24 splice variants, both observed and predicted.
Predicted open reading frames are shown in a ClustalW alignment in Figure 2B. The open reading frame (ORF) of the full-length mda-7/IL-24 generates a protein with 206 amino acids (aa), and the longest ORF of mda-7s generates a 36 aa protein. The predicted ORF for mda-7/IL-24δ5 spans exons 2 through 7 (153 aa), and the ORF for mda-7/IL-24δ2,3 is predicted to span exon 6 and terminate on exon 7 (48 aa). The ORF for mda-7/IL-24δ2,5 encompasses sequences from exons 3, 4, 6, and 7 (126 aa), and the ORF for mda-7/IL-24δ2 includes sequences from exons 3 through 7 (179 aa). The ORF for mda-7/IL-24δ2,3,5 is predicted to include sequences from exons 6 and 7 (48 aa), as do the predicted ORFs for mda-7/IL-24δ3,5 and mda-7/IL-24δ2,3. For further experimentation, we chose to focus on the novel isoforms, and in particular, on one of the three with redundant ORFs (mda-7/IL-24δ2,3,5).
3.3 mRNA and protein expression
The full-length mda-7/IL-24 isoform and the five novel isoforms described were subcloned from the pCR-Blunt-TOPO vector into the pFLAG-Myc-CMV-22 vector for expression of Myc-tagged proteins in mammalian cells. Plasmids were transfected into U2OS cells, and after 48 hours, mRNA was isolated and used to synthesize cDNA. Mda-7/IL-24-specific fragments were amplified by RT-PCR: mda-7/IL-24δ5 at 502 bp, mda-7/IL-24δ2,3,5 at 160 bp, mda-7/IL-24δ2,3 at 219 bp, mda-7/IL-24δ2,5 at 324 bp, and mda-7/IL-24δ2 at 518 bp. Expression of all 5 novel isoforms was verified at the mRNA level (Figure 3A). To monitor protein expression of cloned isoforms, plasmids were transfected into U2OS cells, which were lysed 48 hours later. The lysates were separated by SDS-PAGE, transferred to a PVDF membrane and probed with antibodies to the c-myc terminal tag. With immunoblotting, the full-length mda-7/IL-24 protein, as well as mda-7/IL-24δ2,5 and mda-7/IL-24δ2 were observable (Figure 3B). We were unable to detect mda-7/IL-24δ3,5, mda-7/IL-24δ2,3,5, or mda-7/IL-24δ2,3 by immunoblot. Immunoprecipitation using polyclonal anti-c-myc anbibodies and Protein G-Agarose beads for IP and monoclonal c-myc antibodies for detection was also unsuccessful in detecting these three isoforms at the protein level. It is possible that the protein products of these three mda-7/IL-24 isoforms, mda-7/IL-24δ2,3, mda-7/IL-24δ3,5, and mda-7/IL-24δ2,3,5 are too short (approximately 6 kDa) to be detected by conventional immunoblot methods, that their structure prevents the c-myc tag from being accessible to antibodies, or that they are relatively unstable proteins.
Figure 3. Expression of mda-7/IL-24 splice variants.
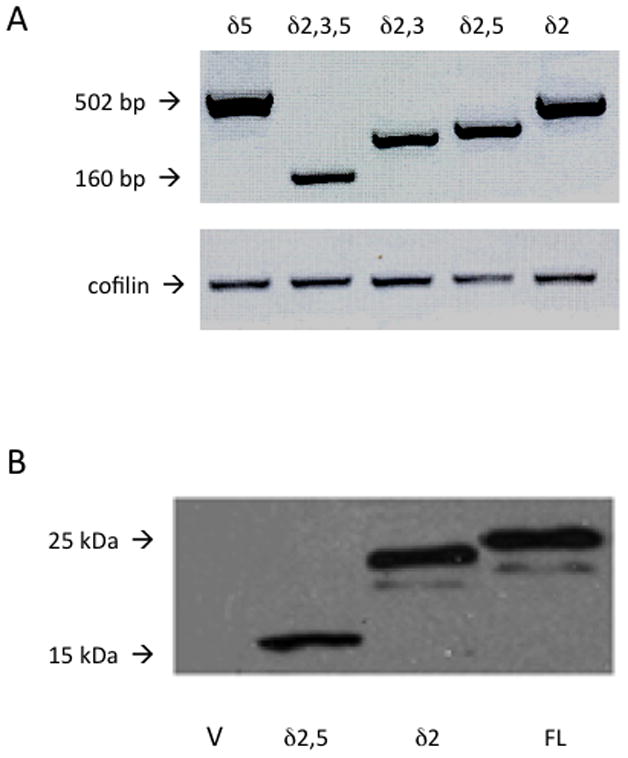
A) Expression of mda-7/IL-24 constructs after transient transfection into U2OS cells. Total mRNA was isolated, and cDNA was reverse-transcribed. Upper panel: mda-7/IL-24 primers. Lower panel: primers for the housekeeping gene cofilin. B) Expression of mda-7/IL-24 constructs after transient transfection into U2OS cells. Total cell lysates were collected using Laemli lysis buffer, sonicated, separated by SDS-PAGE, transferred to PVDF membrane and probed using anti-c-myc antibodies. V represents the transfection with a vector control. FL indicates full length mda-7/IL-24. δ2,5 and δ2 show the expression pattern of clones mda-7/IL-24δ2,5 and mda-7/IL-24δ2.
3.4 Full length mda-7/IL-24, mda-7/IL-24δ5, mda-7/IL-24δ2,3,5, mda-7/IL-24δ2,5 and mda-7/IL-24δ2 reduce cell viability in U2OS cells
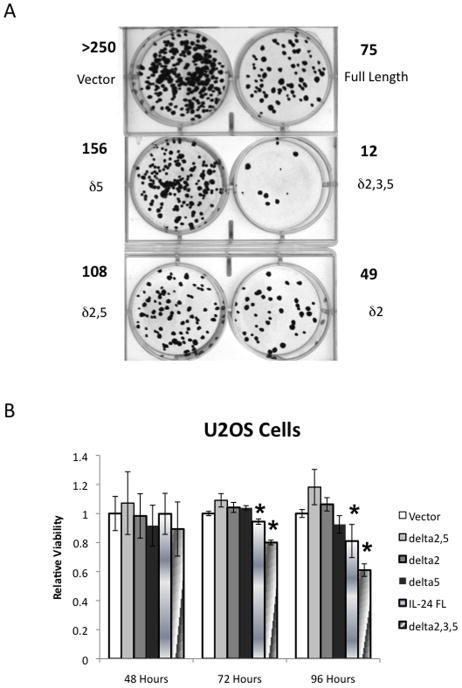
mda-7/IL-24 has attracted research attention for its ability to selectively induce apoptosis in cancer cells while having relatively little effect on normal cells. We therefore wanted to determine if the individual splice isoforms of mda-7/IL-24 were capable of inducing apoptotic events in U2OS cancer cells. To evaluate this, a colony-forming assay was performed. The individual isoforms were transiently transfected into U2OS cells, and parallel plates of cells were co-transfected with GFP to normalize for transfection efficiency. After 48 hours, GFP was measured; the rest of the cells were resuspended and split to multiple densities. Once they had attached, they were placed under neomycin selection for 2 weeks. The cells were fixed with formalin, stained with crystal violet and the colonies were counted. Representative plates, along with the number of colonies in these plates are shown in Figure 4A. The results showed that all of the tested mda-7/IL-24 isoforms significantly reduced the number of surviving cells. Interestingly, the isoform that yields the shortest protein product (48 aa), mda-7/IL-24δ2,3,5, exerted the strongest negative effect on colony formation. To determine how quickly mda-7/IL-24 splice variants can produce significant changes in cell viability, we tested their effect on transiently transfected U2OS cells, measuring cell viability using the MTT assay at 48, 72, and 96 hours post-transfection (Figure 4B). These results show significant differences in cell survival (as compared to the vector control) with only two isoforms: the full-length variant and the mda-7/IL-24δ2,3,5 splice variant. Similar to our results with the colony formation assay, this method also showed that the splice variant mda-7/IL-24δ2,3,5 exerted a more profound effect on cancer cell survival than did the full-length protein.
Figure 4. mda-7/IL-24 splice variants reduce cell viability in U2OS but not NOK cells.
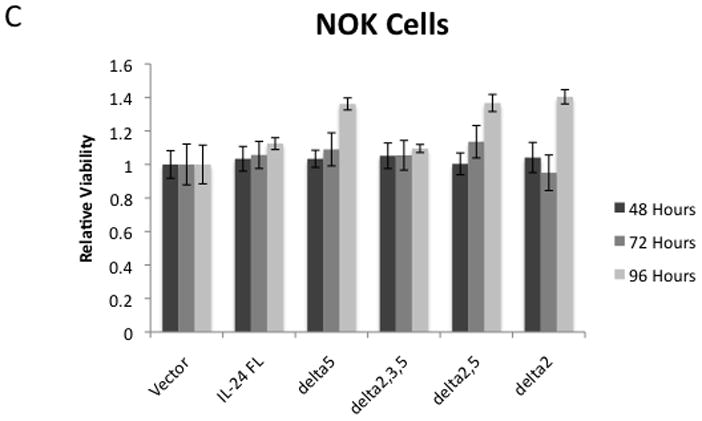
A colony forming assay was performed by transiently transfecting U2OS cells with the mda-7/IL-24 constructs and growing in selective media until colonies were visible. A) Representative plates showing the crystal violet stained colonies. The number of colonies are noted in bold type. B) To evaluate how quickly this change in viability occurred, U2OS cells were transiently transfected with the mda-7/IL-24 splice variants, and a viability assay using MTT was performed in triplicate. C) NOK cells, a non-cancer immortalized cell line, were transiently transfected with the mda-7/IL-24 splice variants, and a luminescence-based viability assay was performed in triplicate. An * indicates a statistically significant decrease in viability compared to the vector control for the same time point (p < 0.05).
3.5 The mda-7/IL-24 isoforms have no effect on viability of NOK cells
One of the major features of mda-7/IL-24 that makes it particularly attractive for cancer therapy is its specificity in inducing apoptosis only in tumor cells, while having no appreciable effect most on non-cancerous cells. In order to evaluate the cancer-specific effects of our novel splice isoforms, we utilized NOK cells, a non-cancerous immortalized cell line derived from normal oral keratinocytes [48]. Cells were seeded in 96 well plates and transiently transfected with the splice isoforms. A luminescence-based cell viability assay was performed at 48, 72, and 96 hours post-transfection. None of our mda-7/IL-24 isoforms caused a significant decrease in viability in the NOK cells, indicating that the isoforms share the cancer-specificity of full length mda-7/IL-24 (Figure 4C).
3.6 Full-length mda-7/IL-24 and its isoforms activate caspases, with the exception of mda-7/IL-24δ2
The previously described experiments measured cell viability, but did not determine whether the reduced viability we observed was due to a halting of proliferation, apoptosis, or another form of cell death. Full-length mda-7/IL-24 has a well-demonstrated ability to induce apoptosis in many cancer cell types. To determine if the observed decrease in viability was the result of apoptosis, a caspase 3/7 assay was performed. 24 hours after transfection, cells were lysed and the lysates were divided. Half of the lysate from each transfection was subjected to assessment of caspase 3/7 activity, and the other half of the lysate was subjected to a cell viability assay to normalize for cell number. The results of these assays demonstrate that full-length mda-7/IL-24 and its isoforms, with the exception of mda-7/IL-24δ2, induced activation of caspases 3 and 7 more than 2-fold when compared to the vector control (Figure 5).
Figure 5. Expression of all but one of the mda-7/IL-24 splice variants increased Caspase 3/7 activity.
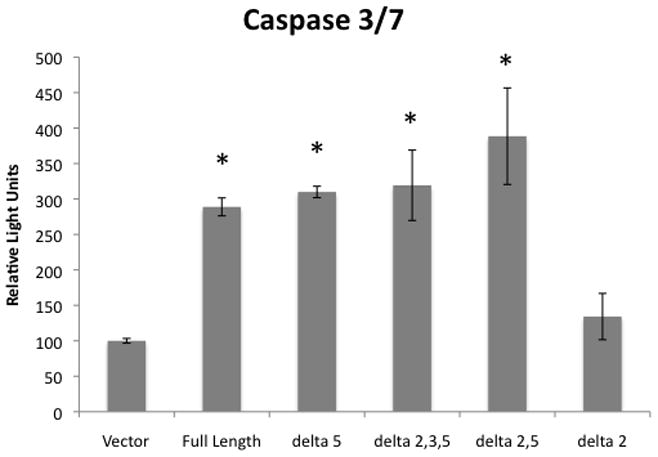
U2OS cells were transiently transfected with the indicated mda-7/IL-24 isoforms or with a vector control, and allowed to grow in culture for 24 hours. Cells were then lysed with a passive lysis buffer and the lysates were subjected to a luciferase-based caspase 3/7 activity assay. Results shown are normalized for cell number. Experiments were performed in triplicate. An * indicates a statistically significant increase in caspase 3/7 activity when compared to the vector control (p < 0.05).
3.7 Pro-apoptotic activity of mda-7/IL-24δ2,3,5 is due to its short protein product
One of the most striking results noted above is the increased pro-apoptotic activity of the clone encoding mda-7/IL-24δ2,3,5. However, this isoform is capable of producing only a short 48 aa protein with a molecular weight of 5.77 kDa. Unfortunately, we were unable to detect the protein product in U2OS cells, likely due to the technical difficulties involved in the detection of such a small protein in mammalian cells. To determine whether the pro-apoptotic activity of this isoform is due to expression of its protein product, we mutagenized the clone in two ways in order to disrupt its expression. The first mutant, mutant A, lacks the initiation Met codon. However, it should be able to express protein from the same ORF due to upstream alternative translation sites (Figure 6A). Another mutant, mutant B, has a mutation in the region of the original Met site that shifts the open reading frame. For this reason, its translated protein product is not similar to that of the mda-7/IL-24 protein (Figure 6A). Analysis of U2OS cell survival after transient transfection (96 hours) with the original mda-7/IL-24δ2,3,5 construct and its mutant variants A and B showed that while mutation of the Met codon only did not eliminate the pro-apoptotic activity of the clone, the shift in translation frame did completely eliminate this activity in mutant B (Figure 6B). These results indicate that production of the protein product of mda-7/IL-24δ2,3,5 is necessary for its pro-apoptotic activity.
Figure 6. The mda-7/IL-24δ2,3,5 isoform reduces U2OS cell viability due to expression of a 48 aa protein that is similar to the C-end of the full-length mda-7/IL-24 isoform.
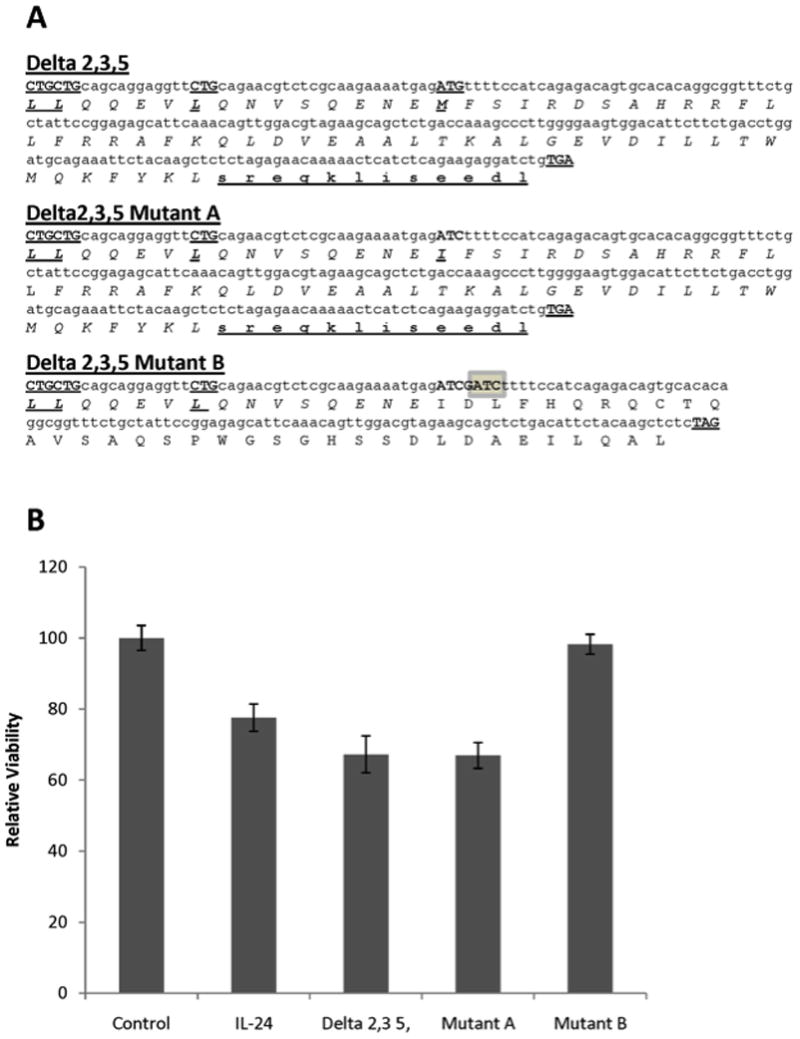
A) DNA and protein sequences of the myc-tagged mda-7/IL-24δ2,3,5 isoform. Potential start sites, including an alternative, and the ending site of translation are bolded and underlined. The protein sequence, which is similar to the last 36 aa of the full-length mda-7/IL-24 sequence, is shown in italic. c-Myc protein sequences derived from the vector in the Delta 2,3,5 and Mutant A constructs are shown in bold underlined lower case. Mutant A has a G→C mutation that changes the ATG initiation codon into ATC. Mutant B has a GATC insertion (highlighted), which induces a frame shift. B) MTT analysis of the viability of U2OS cells transiently transfected with vector (Control), the full-length mda-7/IL-24 isoform (IL-24), mda-7/IL-24δ2,3,5 (Delta2,3,5), or its mutant clones A and B. The viability assay was performed in triplicate after 94 hrs of transfection, and error bars show the standard deviation.
4 Discussion and Conclusions
In this study, we extended our previous observation of multiple distinct transcripts of mda-7/IL-24 [47] by identifying and characterizing five mRNA splice isoforms of mda-7/IL-24, defining their mRNA and predicted protein structures, and determining their effects on cell viability. The profound biological effects observed for this gene makes our observation of multiple distinct transcripts of mda-7/IL-24 particularly interesting. These five splice variants, not previously characterized, were identified in five different tumor cell lines (U2OS, DU145, HCT116, Caski, and Siha). Previous reports in the literature have provided some information regarding the biological effects of both the full length mda-7/IL-24 (Isoform 1), which contains all 7 exons, and a shorter isoform lacking exons 3 and 5, (Isoform 2, or mda7-S) [37]. In this study, we characterized 5 novel isoforms lacking various combinations of exons 2, 3, and 5. Although one report [38] describes isoforms lacking either exon 3 or exon 5, no follow-up to this study provided any confirmation or functional characterization. Interestingly, while we have described isoform mda-7/IL-24δ5, we were unable to isolate the second isoform described by Allen et al, mda-7/IL-24δ3. The different sets of isoforms detected by ourselves and others suggests that the expression and distribution of mda-7/IL-24 isoforms may be cell-type specific.
Initially, we predicted that the potential protein products for the 5 novel isoforms would share sequences with the full-length isoform of the gene, and attempted to confirm their expression in mammalian cells. While we were able to consistently express all 5 novel isoforms at the mRNA level, we were able to visualize only two of them (mda-7/IL-24δ2 and mda-7/IL-24δ2,5) at the protein level. This was not entirely unexpected, as genes often create nonsense splice isoforms, or mRNA splice variants that are not expressed at the protein level. Furthermore, technical and/or stability issues particularly applicable to small proteins could have contributed to our inability to detect these isoforms. In any case, we chose to continue our analysis by examining each of these five splice variants for biological function.
The most intriguing aspect of our analysis into the function of the novel mda-7/IL-24 splice isoforms was the survival data. None of the splice variants had any effect on the non-cancerous NOK cell line, but several, and in particular mda-7/IL-24δ2,3,5, dramatically reduced viability in the osteosarcoma-derived cell line U2OS. mda-7/IL-24δ2,3,5 is one of the splice isoforms for which we were unable to demonstrate expression at the protein level. It is predicted to encode the short 48 aa c-myc-tagged protein, identical to that encoded by two other mda-7/IL-24 isoforms, mda-7/IL-24δ2,5 and mda-7/IL-24δ3,5. However, its effect on cells is clear, as it reproducibly reduces cell viability and activates caspases 3 and 7. There are several possible reasons for this. The first possibility, that the c-terminal myc tag that we used for immunoblotting was out of sequence with the protein product of mda-7/IL-24δ2,3,5, was quickly ruled out. Sequence analysis of the three reading frames of this mRNA product confirmed that our predicted 48 amino acid protein product was the longest potential product that could be generated, and it is in frame with the c-terminal myc tag. The next possible longest potential peptide product is only 25 amino acids in length, and it is highly unlikely that it would be preferentially expressed over a protein more than twice as long. The second possibility is that the protein product is not detectable by immunoblot, either because its small size is allowing it to pass through the pores of the membrane, or because the protein is folding in such a way as to make the myc tag inaccessible to antibodies. We attempted to overcome the potential size issue by immunoprecipitation utilizing anti-c-myc antibodies and protein A/G agarose beads, followed by a polyclonal antic-myc antibody for detection. However, we were unable to detect the protein product even after immunoprecipitation.
Failure to detect the 48 aa protein using these methods prompted us to employ site-specific mutagenesis to modify translation initiation and to introduce a frame shift. These mutations enabled us to demonstrate that the anti-survival effect of this clone was indeed due to expression of its protein sequence, predicted to be similar to the C-end of the full-length isoform. Only by shifting the open reading frame, such that any protein produced would lack any similarity to the mda-7/IL-24 protein sequence, were we able to eliminate the pro-apoptotic activity of the mda-7/IL-24δ2,3,5 isoform (Figure 6B).
An important question arises as to the regulation of these alternative splice isoforms of mda-7/IL-24. While splice site selection is a complicated process that has not been completely elucidated, there is growing understanding of the role of the SR family of proteins. We initially observed additional splice isoforms of mda-7/IL-24 in a study examining the splice factor gene SFRS6, which codes for the SRp55 protein [46]. Silencing of that splice factor was shown to increase the expression of mda-7/IL-24δ2,3. In this study we have demonstrated that over-expression of SFRS6 causes an overall shift in the splicing of mda-7/IL-24 away from the larger isoforms, and particularly toward the smallest isoform, mda-7/IL-24δ2,3,5. While the reason for this shift is still unknown, our data from these studies suggests that mda-7/IL-24 splice site selection is not random, but is governed at least in part by the SR family protein SRp55. The non-random nature of mda-7/IL-24 splice site selection is also supported by the observation that Isoform 2 (mda7-S) expression decreases with tumor progression [37]. Our data supports and extends the literature and suggests a specific role for mda-7/IL-24 splicing in the cell’s response to different conditions, including DNA damage, stress, and potentially cancer.
In summary, we have identified and characterized five mRNA splice isoforms of the tumor suppressor gene mda-7/IL-24. These isoforms have varying effects on cell survival in an osteosarcoma tumor cell model (U2OS). Specifically, several of the isoforms - mda-7/IL-24δ5, mda-7/IL-24δ2,3, and mda-7/IL-24δ2,5—activate caspases and induce apoptosis with similar efficacy as full length mda-7/IL-24. One of the isoforms, mda-7/IL-24δ2,3,5, demonstrated a consistent ability to induce apoptosis even more effectively than the full-length mda-7/IL-24, while having no effect on a non-cancerous cell line. Finally, one of the isoforms, mda-7/IL-24δ2, did not induce caspase activity or affect cell survival. These results indicate that some of these isoforms are more effective than the full-length mda-7/IL-24 in inducing apoptosis, suggesting their use as a potential cancer treatment. Our discovery that the short 36 aa C-end of the mda-7/IL-24 protein fragment has a potent pro-apoptotic ability presents an opportunity to dissect the mechanism(s) that trigger(s) cancer specific apoptosis by these mda-7/IL-24 derived proteins, an investigation of potential clinical significance. Additionally, the presence of multiple isoforms with differing levels of effectiveness could provide some explanation for the multiple pathways reported to be involved in mda-7/IL-24-induced, cancer-specific apoptosis.
Seven splice isoforms of cytokine mda-7/IL-24 were characterized.
The shortest isoform, lacking exons 2,3, and 5, induced more apoptosis in U2OS cancer cells than did the full-length isoform.
However, this short isoform displayed no effect on the viability of non-transformed NOK cells.
Acknowledgments
This work was supported in part by the NCI grant R01 CA095461 (PDH), and grant 5 P20 MD001632 (supporting CFGJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–4. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 2.Brinkman BM. Splice variants as cancer biomarkers. Clin Biochem. 2004;37:584–94. doi: 10.1016/j.clinbiochem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Skotheim RI, Nees M. Alternative splicing in cancer: noise, functional, or systematic? Int J Biochem Cell Biol. 2007;39:1432–49. doi: 10.1016/j.biocel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Venables JP. Aberrant and alternative splicing in cancer. Cancer Res. 2004;64:7647–54. doi: 10.1158/0008-5472.CAN-04-1910. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Lo HS, Yang H, Gere S, Hu Y, Buetow KH, Lee MP. Computational analysis and experimental validation of tumor-associated alternative RNA splicing in human cancer. Cancer Res. 2003;63:655–7. [PubMed] [Google Scholar]
- 6.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 7.Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krecic AM, Swanson MS. hnRNP complexes: composition, structure, and function. Curr Opin Cell Biol. 1999;11:363–71. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 9.Stamm S, Ben-Ari S, Rafalska I, Tang Y, Zhang Z, Toiber D, et al. Function of alternative splicing. Gene. 2005;344:1–20. doi: 10.1016/j.gene.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Stamm S. Signals and their transduction pathways regulating alternative splicing: a new dimension of the human genome. Hum Mol Genet. 2002;11:2409–16. doi: 10.1093/hmg/11.20.2409. [DOI] [PubMed] [Google Scholar]
- 11.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 12.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–86. [PubMed] [Google Scholar]
- 13.Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, Grimm EA. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20:1069–74. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- 14.Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7:2051–7. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- 15.Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–73. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- 16.Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, et al. mda-7/IL-24: exploiting cancer’s Achilles’ heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Lebedeva IV, Su ZZ, Sarkar D, Gopalkrishnan RV, Waxman S, Yacoub A, et al. Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad. mda-7-induced apoptosis. Oncogene. 2005;24:585–96. doi: 10.1038/sj.onc.1208183. [DOI] [PubMed] [Google Scholar]
- 18.Mhashilkar AM, Schrock RD, Hindi M, Liao J, Sieger K, Kourouma F, et al. Melanoma differentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy. Mol Med. 2001;7:271–82. [PMC free article] [PubMed] [Google Scholar]
- 19.Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–80. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 20.Chen WY, Cheng YT, Lei HY, Chang CP, Wang CW, Chang MS. IL-24 inhibits the growth of hepatoma cells in vivo. Genes Immun. 2005;6:493–9. doi: 10.1038/sj.gene.6364233. [DOI] [PubMed] [Google Scholar]
- 21.Leath CA, Kataram M, Bhagavatula P, Gopalkrishnan RV, Dent P, Fisher PB, et al. Infectivity enhanced adenoviral-mediated mda-7/IL-24 gene therapy for ovarian carcinoma. Gynecol Oncol. 2004;94:352–62. doi: 10.1016/j.ygyno.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–18. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 23.Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci U S A. 1998;95:14400–5. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, et al. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol Cancer Ther. 2003;2:623–32. [PubMed] [Google Scholar]
- 25.Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, et al. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review) Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- 26.Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–66. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–59. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–72. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, et al. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–95. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Gopalkrishnan RV, Sauane M, Fisher PB. Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24. Int Immunopharmacol. 2004;4:635–47. doi: 10.1016/j.intimp.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, et al. Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analysed via a bacterial fusion protein. Oncogene. 2004;23:7679–90. doi: 10.1038/sj.onc.1207958. [DOI] [PubMed] [Google Scholar]
- 32.Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–23. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 33.Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, Zheng M. mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther. 2005;11:724–33. doi: 10.1016/j.ymthe.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Zheng M, Bocangel D, Doneske B, Mhashilkar A, Ramesh R, Hunt KK, et al. Human interleukin 24 (MDA-7/IL-24) protein kills breast cancer cells via the IL-20 receptor and is antagonized by IL-10. Cancer Immunol Immunother. 2007;56:205–15. doi: 10.1007/s00262-006-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreis S, Philippidou D, Margue C, Rolvering C, Haan C, Dumoutier L, et al. Recombinant interleukin-24 lacks apoptosis-inducing properties in melanoma cells. PLoS ONE. 2007;2:e1300. doi: 10.1371/journal.pone.0001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–63. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 37.Allen M, Pratscher B, Roka F, Krepler C, Wacheck V, Schöfer C, et al. Loss of novel mda-7 splice variant (mda-7s) expression is associated with metastatic melanoma. J Invest Dermatol. 2004;123:583–8. doi: 10.1111/j.0022-202X.2004.23321.x. [DOI] [PubMed] [Google Scholar]
- 38.Allen M, Pratscher B, Krepler C, Frei K, Schöfer C, Pehamberger H, et al. Alternative splicing of IL-24 in melanocytes by deletion of exons 3 and 5. Int J Immunogenet. 2005;32:375–8. doi: 10.1111/j.1744-313X.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 39.Sahoo A, Jung YM, Kwon HK, Yi HJ, Lee S, Chang S, et al. A novel splicing variant of mouse interleukin (IL)-24 antagonizes IL-24-induced apoptosis. J Biol Chem. 2008;283:28860–72. doi: 10.1074/jbc.M802510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alms WJ, Atamas SP, Yurovsky VV, White B. Generation of a variant of human interleukin-4 by alternative splicing. Mol Immunol. 1996;33:361–70. doi: 10.1016/0161-5890(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 41.Bihl MP, Heinimann K, Rüdiger JJ, Eickelberg O, Perruchoud AP, Tamm M, Roth M. Identification of a novel IL-6 isoform binding to the endogenous IL-6 receptor. Am J Respir Cell Mol Biol. 2002;27:48–56. doi: 10.1165/ajrcmb.27.1.4637. [DOI] [PubMed] [Google Scholar]
- 42.Korte A, Möricke A, Beyermann B, Köchling J, Taube T, Kebelmann-Betzing C, et al. Extensive alternative splicing of interleukin-7 in malignant hematopoietic cells: implication of distinct isoforms in modulating IL-7 activity. J Interferon Cytokine Res. 1999;19:495–503. doi: 10.1089/107999099313947. [DOI] [PubMed] [Google Scholar]
- 43.Rahman M, Nara H, Onoda T, Araki A, Li J, Hoshino T, Asao H. Cloning and characterization of an isoform of interleukin-21. FEBS Lett. 2007;581:4001–9. doi: 10.1016/j.febslet.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Tsytsikov VN, Yurovsky VV, Atamas SP, Alms WJ, White B. Identification and characterization of two alternative splice variants of human interleukin-2. J Biol Chem. 1996;271:23055–60. doi: 10.1074/jbc.271.38.23055. [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Gessner R, Taube T, von Stackelberg A, Henze G, Seeger K. Expression of interleukin-10 splicing variants is a positive prognostic feature in relapsed childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:3038–42. doi: 10.1200/JCO.2005.00.885. [DOI] [PubMed] [Google Scholar]
- 46.Filippov V, Schmidt EL, Filippova M, Duerksen-Hughes PJ. Splicing and splice factor SRp55 participate in the response to DNA damage by changing isoform ratios of target genes. Gene. 2008;420:34–41. doi: 10.1016/j.gene.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 48.Piboonniyom SO, Duensing S, Swilling NW, Hasskarl J, Hinds PW, Münger K. Abrogation of the retinoblastoma tumor suppressor checkpoint during keratinocyte immortalization is not sufficient for induction of centrosome-mediated genomic instability. Cancer Res. 2003;63:476–83. [PubMed] [Google Scholar]