Abstract
The use of adult stem cells for therapeutic purposes has met with great success in recent years. Among several types of adult stem cells, mesenchymal stem cells (MSCs) derived from bone marrow (BM) and other sources have gained popularity for basic research and clinical applications because of their therapeutic potential in treating a variety of diseases. Because of their tissue regeneration potential and immune modulation effect, MSCs were recently used as cell-based therapy to promote revascularization, increase pancreatic β-cell proliferation and avoid allograft rejection in islet transplantation. Taking advantage of the recent progress in gene therapy, genetically modified MSCs can further enhance and expand the therapeutic benefit of primary MSCs while retaining their stem-cell like properties. This review aims to gain a thorough understanding of the current obstacles to successful islet transplantation and discusses the potential role of primary MSCs before or after genetic modification in islet transplantation
Keywords: mesenchymal stem cells, islet transplantation, gene therapy, immune tolerance
Introduction
Stem cells exist in all multicellular organisms and share two characteristic properties. They have prolonged or unlimited self-renewal capacity and the potential to differentiate into a variety of specialized cell types. The earliest stem cells in human life are embryonic stem (ES) cells, which are pluripotent stem cells derived from the inner cell mass of the blastocyst and capable of differentiatng into all derivatives of the three primary germ layers: ectoderm, endoderm, and mesoderm. Except the ES cells which can only be isolated from early embryo, there are other types of stem cells in the mature tissues of all aged mammals. These adult stem cells have unlimited self-renewal capacity and more restricted differentiation potential. They multiply by cell division to replenish dying cells and regenerate damaged tissues. The most famous adult stem cells are hematopoietic stem cells (HSCs) which give rise to all the blood cell types and lymphoid lineages. Bone marrow (BM) also contains a population of adult stem cells named mesenchymal stem cells (MSCs).
MSCs can be isolated from multiple tissues such as BM, adipose tissue, umbilical cord blood, adult muscle and the dental pulp of deciduous baby teeth.1–3 After gradient centrifugation in Ficoll-Paque solution and sequential purification by adherence to the flask, MSCs can be cultured, expanded and induced in a standard lab incubator without feeder cells such as fibroblasts.4 Although BM is considered as the primary source of MSCs, they can be isolated from other tissues, including adipose tissue,5 trabecular bone,6 synovium,7 skeletal muscle,8 deciduous teeth,9 and human umbilical cord blood,3 suggesting the diverse distribution of MSCs in a body. However, MSCs derived from diverse origins other than BM exhibit limited differentiation potential.10, 11
MSCs are morphologically defined as plastic, adherent, pluripotent fibroblast-like cells (Fig. 1). MSCs are stem cells because of their stem cell-like properties such as unlimited self-renewal capacity and potential for multilineage differentiation. Primary MSCs can be expanded for 34~50 population doublings (PD) without losing their native characteristics. MSCs can differentiate into a variety of cell types including osteoblasts, chondrocytes, and adipocytes under in vitro and in vivo conditions.4
FIG. 1.
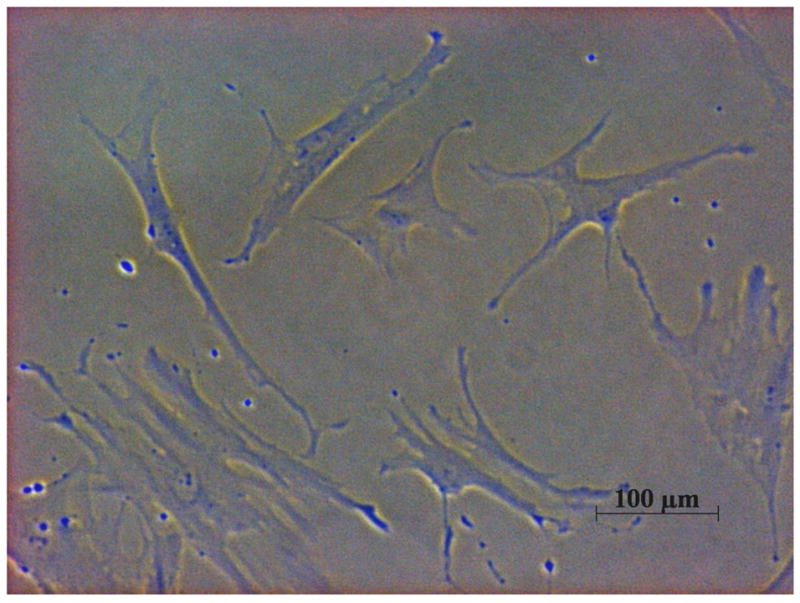
Human bone marrow (BM) derived mesenchymal stem cells (MSCs) are plastic adherent, pluripotent fibroblast-like cells under 100X light microscope.
Among all types of stem cells, MSCs have attracted special attention because of their wide application as regenerative medicine. ES cells were first studied as regenerative medicine because of their self-renewal capacity and differentiation potential. However, direct injection of highly pluripotent ES cells into ectopic organ often give rise to teratoma, a benign tumor containing derivatives of all three germ layers.12 MSCs are less potent to induce teratoma or other malignant transformation as they only have restricted differentiation potential.13 Compared with other adult stem cells such as HSCs, mammary stem cells (MaSCs) or neural stem cells (NSCs), MSCs have a well-characterized trophic effect and immunomodulatory property, making them good candidates in treating degenerative diseases. For example, intravenous transplantation of MSCs was reported to be successful in treating systemic diseases such as graft versus host disease (GVHD) and osteogenesis imperfecta in human.14, 15 Wakitani et al. also reported several successful clinical cases treating cartilage defects with MSCs.16 Nevertheless, primary MSCs or genetically modified MSCs have also been employed in regenerating hematocytes, tendon, BM, muscle, and other connective tissues.17–21
Current Status of Islet Transplantation
Type 1 diabetes is an autoimmune disease resulting from the destruction of insulin-producing pancreatic β-cells, which necessitates a lifelong daily glucose monitoring and injection of insulin. However, the poor control of blood glucose fluctuations with insulin injection leads to many severe complications including neuropathy, nephropathy, retinopathy, heart disease, and atherosclerosis.22 Islet transplantation, which is still an experimental treatment for diabetes, could be a permanent cure for type I diabetes if transplanted islets could actively maintain normal blood glucose under all conditions and escape graft rejection due to inflammatory and immune reactions.
Islets are isolated from deceased organ donors, purified, processed, and transferred into the hepatic portal vein of the diabetic patients, where the islets are deposited in highly perfused liver sinusoids.23 The infused islets produce insulin soon after transplantation to restore “insulin independence,” a status defined as being able to stop insulin injection for at least 14 days following transplantation in diabetic patients.23 However, other reports showed that insulin independence is difficult to maintain over time.24
While significant progress has been made in islet transplantation, many obstacles preclude its widespread application. Two of the most important limitations are the currently limited supply of islets for transplantation and the inadequate means for preventing islet graft rejection.25 Immunosuppressive regimens are capable of preventing islet failure from months to years, but the agents used in these treatments may induce significant side effects, resulting in progressive decline in graft function. Some of the most commonly used immunosuppressive agents such as tacrolimus (also known as FK-506 or Fujimycin), mycophenolic acid, and sirolimus (also known as rapamycin) are also deleterious to islet function and insulin secretion.26, 27 Moreover, because of the extensive posttransplantation challenges, a patient needs at least 10,000 islet equivalents per kilogram of body weight (extracted from two or more donor pancreases) for an optimal transplantation outcome, making the current shortage in islet supply even worse.23, 28
To seek alternative sources of islets, both the use of islets from alternative species and in vitro generation of islets and insulin-producing cells from stem cells have been explored. Porcine islets are widely reported as a competent alternative for xenogeneic islet transplantation with the assistance of biological and biomaterial approaches to prevent enhanced immune destruction of the xenografts.29, 30 For in vitro transdifferentiation strategy, several groups have reported successful generation of insulin-producing β-cells and islet-like structures from ES cells. Lumelsky, et al. demonstrated that ES cells could differentiate into insulin-producing cells which self-assemble into islet-like clusters.31 Blyszczuk et al. reported the differentiation of ES cells into insulin-producing cells through transduction of plasmid vectors encoding paired box gene 4 (Pax-4) and pancreatic duodenal homeobox 1 (Pdx-1).32 However, caution should be exercised as the differentiation from pluripotent ES cells cannot be 100% and the remaining undifferentiated ES cells may still hold tumorigenicity. Several groups used MSCs as a relatively safer source and succeed in generation islet-like cluster or insulin-producing cells.33–35 However, most of them relied on the genetic manipulation of MSCs and the ability of producing large numbers of functional tissues by this means was not proven.
In addition, islets are a cluster of heterogeneous cell types with extensive intra-islet vasculature formed of fenestrated capillary endothelial lining, which gets disrupted during islet isolation, leading to collapse of vasculature, accumulation of endothelial fragments and compromised perfusion in the core of the islets.36 Therefore, unlike whole pancreas and other solid organ transplantations, islet transplantation is ectopic and requires extensive and functional revascularization to promote the posttransplantation survival of islet grafts.37, 38 Less or abnormal revascularization usually lead to hypoxia, apoptotic islet cell death and thus compromised transplantation outcome.
Islet cell destruction following transplantation can be greatly reduced by gene therapy.39 Since islet is a compact cluster of about 1000 non-dividing cells, it is difficult to transfect intact islets by the available non-viral approaches, such as cationic liposomes and polymer-based systems, which are also toxic at high doses.40 In contrast, replication deficient (E1-, E3- deleted) adenoviral (Adv) vectors are known to efficiently transduce islets. In addition, adenovirus vectors can be produced in high titers and there is no risk of insertional mutagenesis as they do not integrate into host genome. We and others have demonstrated adenoviral vector-based gene therapy to be effective in promoting the revascularization and engraftment of human islets posttransplantation.41, 42 For example, growth factor gene expression can significantly improve the islet revascularization after transplantation,41, 43, 44 while anti-apoptotic protein expression can increase the resistance of islet grafts to multiple posttransplantation challenges in human islets.42, 45, 46 The recent success in constructing bipartite viral vectors which can simultaneously promote revascularization and increase the resistance of transplanted islets may highlight the promising future of gene therapy (Fig. 2).43, 44 However, because of the cluster-like property of islets, multiplicity of infection (MOI) higher than 500 is usually required to achieve optimal transduction efficiency as suggested from many reports.43–45, 47 Therefore, despite its effectiveness, the clinical application of gene therapy is still hindered by the high risk of immunogenicity of viral vectors.
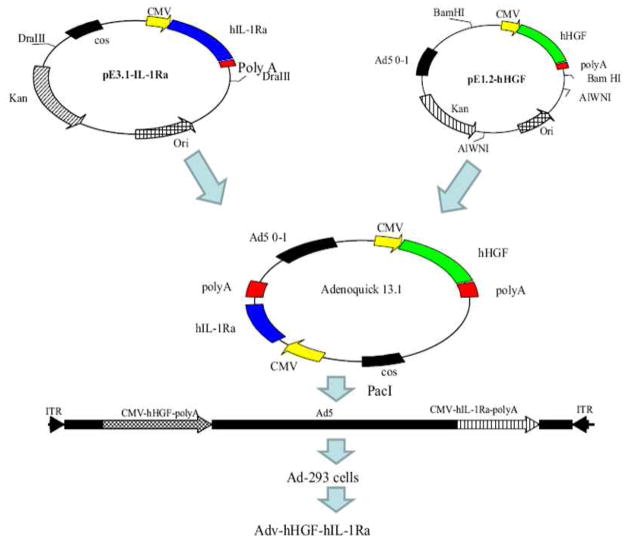
FIG. 2.
Construction of bipartite adenoviral vector encoding human hepatocyte growth factor (HGF) and interleukin 1 receptor antagonist (IL-1Ra) (Adv-hHGF-hIL-1Ra) using AdenoQuick cloning system. Briefly, cDNA of HGF and IL-1Ra was isolated and cloned into the shuttle plasmids pE 3.1 and pE 1.2 under CMV promoter. Then the shuttle plasmids were combined by homologous recombination to generate a cosmid containing the entire sequence of recombinant adenovirus. At last, the cosmid was linearized transfected into 293 cells to produce the recombinant adenovirus Adv-hHGF–hIL-1Ra. Reproduced from Panakanti and Mahato (2009) Pharm Res 26: 587–596.44
Recently, MSCs have demonstrated great potential to address these critical issues encountered in islet transplantation. On one hand, MSCs serve as “helper” cells to support islet function, repair islet injuries and help islet revascularization after transplantation.48–50 On the other hand, MSCs serve as a “border patrol” for transplanted islets to avoid allograft rejection to transplanted islets.51–53
MSCs Support Islet Function
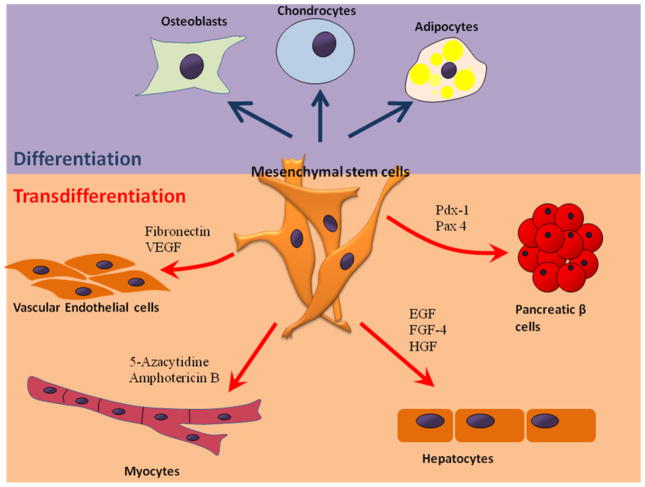
MSCs not only regenerate mesenchymal tissues such as chondrocytes, osteoblasts, and adipocytes, but they also undergo transdifferentiation into cells from other lineages upon proper induction (Fig. 3), indicating plasticity of these adult stem cells and their utility in diverse organ transplantation and cell therapy applications. Transdifferentiation and migration of MSCs may give rise to their organ regeneration potential. Chen et al. first reported in vitro transdifferentiation of rat MSCs into functional insulin-producing islet-like cells that actively controlled blood glucose level in diabetic rats.33 Karnieli et al. and Li et al. independently reported the generation of insulin-producing cells from human MSCs which were genetically manipulated to overexpress Pdx-1 with retroviral and adenoviral vectors, respectively.54, 55
FIG. 3.
Differentiation and transdifferentiation of mesenchymal stem cells (MSCs). MSCs have three established differentiation directions: osteoblasts, chondrocytes and adipocytes. Stimulation with chemical or biological signals can induce transdifferentiation of MSCs into vascular endothelial cell,65, 139 myocytes,1, 140 hepatocytes,141 and pancreatic β cells.32, 54
In vivo studies using MSCs to treat diabetes support the hypothesis that MSCs might differentiate into insulin-producing β cells or they might induce endogenous progenitor proliferation/differentiation. Ezquer et al. demonstrated that systemic administration of MSCs increased β-cell mass and reverted hyperglycemia in streptozotocin induced type 1 diabetic mice.56 However, whether MSCs can directly replenish the loss of β cells is still under debate. The native β cells are derived from neural crest cells during the process of neurulation, while MSCs are derived from mesoderm. Although the number of islets does not increase throughout human life, insulin-producing β cells do proliferate according to several reports.56–58 Hess et al. first raised doubts that MSCs did not directly rescue the pancreatic injuries by in vivo differentiation and migration but instead induced endogenous pancreatic tissue repair in an unknown manner.59 Lately, both Choi et al. 60 and Dor et al. 61 confirmed that new pancreatic β-cells are derived from the expansion of pre-existing β cells rather than exogenous stem cells through the convincing lineage tracing studies. Since then, emerging evidence suggests that MSCs support islet function in an indirect manner such as promoting the proliferation of pre-existing β-cells and angiogenesis, or in other words serve as a “trophic mediator.” For example, in a study using human MSCs to treat streptozotocin induced diabetic mice, Lee et al. demonstrated that the major effect of human MSCs treatment was to increase the number of mouse islets and mouse insulin-producing cells, which were most likely to arise from the proliferation, migration, and neural differentiation of the nearby endogenous mouse neural stem cells.57
Several studies have suggested that MSCs actively participate in angiogenesis. MSCs constitutively express vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), both are potent angiogenic factors.62 HGF is also a potent mitogen to many cells including pancreatic β-cells.63 Izumida et al. reported that proliferative activity and differentiative response in the pancreatic ductal cells were significantly raised once rats were treated with HGF positive MSCs.64 MSCs can also differentiate into endothelial cells and directly assist the neo-vessel formation.65 Silva et al. reported the transdifferentiation of MSCs into an endothelial phenotype in a canine ischemia model, which is supported by the report from Ito et al. that MSCs directly differentiate into a van Willebrand factor-positive vascular endothelial cell type to improve the islet graft morphology and function.66 These features of MSCs are of significant importance since revascularization is crucial for graft survival after islet transplantation. Chao et al. reported that coculturing with human MSCs protected islet-like cell clusters and extended islet cell survival and function in vitro.67 Sordi et al. found that MSCs of BM origin facilitated the restoration of normoglycemia and the neovascularization of the islet graft,68 which was confirmed by a recent study by Rackham et al. that islets co-transplanted with MSCs maintained a morphology that was more closely resembled to that of islets in the endogenous pancreas both in terms of size, endocrine and endothelial cell distribution.69 However, it is still too early to conclude that newly formed vessels arise exclusively from native MSCs or infused MSCs.
MSCs Prevent Graft Rejection
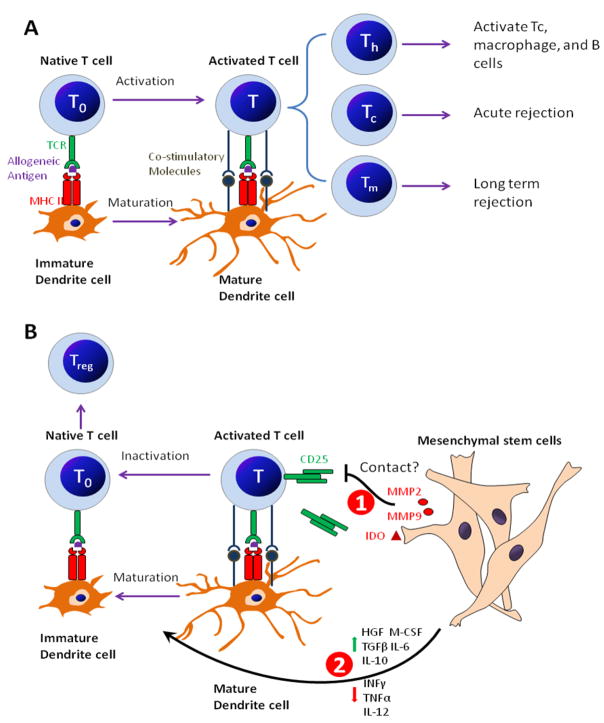
The allograft rejection from the host innate immune system is another major issue for islet transplantation. The most potent antigen presenting cells (APCs), dendritic cells (DCs) play a key role in allograft recognition and rejection. Briefly, the precursor monocytes migrated through the capillaries into tissue and differentiate into immature DCs. Once encountering foreign cells, DCs process and present the allogeneic antigen through major histocompatibility complex class II (MHC II) to the T cell receptor (TCR) of native T cells (T0) and promote T cell activation into cytotoxic T cells (Tc), helper T cells (Th) and memory T cells (Tm), while immature DCs themselves undergo maturation simultaneously. Tc cells then mediate the acute immune attack to the allograft. Th cells recruit and activate more immunocytes including Tc, macrophages and B cells and lead to an enlarged immune response. Tm cells circulate in the host body and mediate the long-term rejection.
MSCs are hypoimmunogenic cells, expressing MHC1 but not MHC II. They also lack co-stimulatory molecules including CD14, CD86, CD40L and CD95L (FasL).70 Therefore, MSCs are capable of evading the alloreactive T cells and natural killer (NK) cells inducing similar immune tolerance like cancer cells.71, 72 Most researchers believe that MSCs avoid allograft rejection by inhibiting T cell activation and proliferation (Fig. 4). Although MSCs can inhibit T cell activation without the involvement of other antigen presenting cells (the direct inhibition model), more emerged evidences support that the soluble factors released by MSCs alone or mixed lymphocyte reaction (MLR)/MSCs co-culture control and reverse the maturation of antigen-presenting DCs and consequently lead to T cell inactivation and tolerance (the indirect inhibition model).
FIG. 4.
Mesenchymal stem cells (MSCs) avoid allograft rejection by T-cell inhibitory effect. (a) The allograft rejection mediated by T cells. Briefly, antigen-presenting DCs present allogeneic antigens through MHC II to the TCR of T0 cells. With assistance from co-stimulatory molecules, DCs become activated into mature DCs and simultaneously promote T cell activation into Tc, Th, and Tm. Then the activated T cells lead to a cascade of immune responses to mediate acute graft rejection and long-term graft rejection. (b) MSCs inhibit T cell activation through two mechanisms: (1) Direct T inhibition which is achieved by a so-far unknown cell-cell contract mechanism or IDO induced T cell inhibition or MMP2/MMP9-mediated CD25 cleavage on the surface of activated T cells. (2) Indirect T cell inhibition, which is achieved by releasing soluble factors to inhibit and reverse DC maturation. The antigen presenting process through immature DCs which in the absence of costimulatory molecules, leads to the inactivation of activated T cells, proliferation of immunosuppressive Treg cells, and allogeneic tolerance.
The direct inhibition model proposed that the rate of T cell inhibition was increased when cell contact between BM-derived MSCs and T cells was allowed.73 Krampera et al. showed that the inhibitory activity of MSCs was abrogated when MSCs were co-cultured with T cells in a transwell system or when MSCs were replaced by MSC culture supernatant.74 Studies by Ding et al. further supported the direct inhibition model that MSCs actively produced matrix metalloproteinase (MMP)-2 and MMP-9 to cleave the high-affinity growth factor receptor CD25 (α-chain of IL-2 receptor) from the surface of infiltrating T cells to suppress T cell responses,52 in great similarity with the immunosuppressive manner of tumor cells.75 However, studies also demonstrated that donor MSCs inhibited the alloreactivity of T cells in recipients and prolonged the allograft survival by actively secreting indoleamine-pyrrole 2, 3-dioxygenase (IDO), which is an immunosuppressive factor.76, 77 This indirect inhibition model is also supported by the studies from Di Nicola et al. that in vitro expanded BM-derived MSCs equally inhibited both CD4+ Th cells and CD8+ Tc cells in MLR by releasing HGF and/or TGF-β.73 Rasmusson et al. demonstrated that blocking the synthesis of prostaglandin E2 (PGE2) could restore part of the proliferation of some T cells,78 which is further supported by the fact that inhibitors of PGE2 production mitigated MSC-mediated immune modulation.79 Beyth et al. reported that MSCs conditioned DCs showed particular expression pattern of surface markers and eventually served as a T-cell inhibitor, possibly mediated by interleukin 10 (IL-10).80 IL-10 had been shown to provoke immunosuppressive regulatory T cells (Treg).79 In addition, IL-6 and macrophage colony-stimulating factor (M-CSF) were also crucial factors in the MSC mediated shift from mature CD1α+ DCs to the immature CD14+ DCs.80, 81 Taken together, all these studies suggest that MSCs actively create an immunosuppressive environment by releasing multiple soluble factors to assist the conversion of mature DCs to an immature status. The following allogeneic antigen-presenting process through immature DCs leads to T cell inactivation and allograft tolerance because of the lack of co-stimulatory molecules.82 This mechanism was also observed when immature DCs take up self apoptotic cells.83
It is worth noting that multiple discrepancies exist among these soluble factors secreted by MSCs. For example, evidences from Beyth et al. showed that adding neutralizing antibody to human TGF-β had no effect on the inhibitory activity of MSCs80, in contrast to the report by Di Nicola et al..73 Ryan et al. reported constitutive expression of IL-10 by MSCs while Beyth et al. only detected IL-10 in MLR/MSCs co-culture.70, 80 Moreover, studies by Tse et al. showed that none of IL-10, TGF-β or PGE2 produced by MSCs was responsible for the T-cell inhibitory effect.84 Although such discrepancies could be explained by the multiple sources and lineages of MSCs and variation in culture conditions among the research groups, the underlying signal transduction pathway between soluble factors released by MSCs and the antigen-presenting DC require further exploration.
Nonetheless, the immune modulation potential of MSCs makes them especially helpful in organ transplantation. Ding et al. reported that MSCs co-transplanted under the kidney capsule of immune competent mice protected islet grafts by inhibiting the alloreactivity of infiltrating T cells.52 Longoni et al. reported that MSCs induced a reduction of pro-inflammatory cytokines and improved the viability of islets infused into the portal vein of diabetic rats.85 Li et al. reported reduced Th1/Th2 ratio, Tc cells and Tm cell number and suppressed DCs maturation once MSCs were co-transplanted with allograft islets under the kidney capsules of diabetic C57LB/6 mouse.86 Kim et al. reported the combined use of autologous MSCs and low-dose cyclosporine A to further prolong graft survival after allogeneic rat islet transplantation.87 MSCs were also used to prevent the allograft rejection in the transplantation studies of other organs including kidney,76, 88 skin89 and heart.77, 90, 91 (Table. 1)
Table 1.
Mesenchymal Stem Cells Improve Organ Transplantation
| Transplanted Organ | Experimental Design | Therapeutic Effects of MSCs |
|---|---|---|
| Kidney | Human of 14 subjects. In vitro MLR study of donor MSCs against recipients lymphocytes. | MSCs inhibit the proliferation of Th and Tc from the recipients by cell-cell contact and IL-10 and IDO. No effect on B or NK cells.76 |
| Human of 2 subjects. Autologous MSCs were injected IV into recipients at day 7 posttransplantation. | MSCs inhibit the proliferation of Tm and Tc. Increase Treg percentage.88 | |
| Skin | Baboons study. Donor MSCs from MHC mismatched donor were injected IV into recipients on the day of transplantation. | MSCs inhibit lymphocyte reactivity and prolong the graft survival and suppress the proliferation. IL-2 partially reverse the effects of MSCs.89 |
| Rat. In vitro expanded MSCs were injected IV 1 week before and on the day of transplantation. | MSCs reduce the alloreactivity of recipient’s T cells and shift the Th1/Th2 balance to immunosuppressive Th2.90 | |
| Heart | Mice. In vitro expanded MSCs were injected IV before transplantation. | MSCs induce donor-specific Treg proliferation and impaired Th1 alloreactivity. Donor-specific Treg do not lead to immune tolerance of allograft from third-party.91 |
| Rat. MSCs were injected IV with a short course of low-dose mycophenolate. | MSCs induce tolerance by secretion IDO and interaction with DCs.77 | |
| Rat. In vitro expanded MSCs coinfused into liver. | Reduce islet number needed for reversal of diabetes promote revascularization.66 | |
| Rat. In vitro expanded MSCs were co-transplanted into omental pouch. | Inhibit Th1 cell activation promote IL-10 producing CD4+ T cells.137 | |
| Islet | Mice. In vitro expanded MSCs were co-transplanted beneath the kidney capsule | MSCs secreted MMP2 and MMP9 to cleave CD25 from IL-1R and thus lead to interleukin-2 hyporesponsiveness in T-cells.52 |
| Monkey. MSCs from donor and third party were coinfused into portal vein and injected IV thrice at day 4, 5 11 after transplantation. | MSCs prolong graft viability and function, probably by increasing Treg proliferation in peripheral blood.138 |
MSCs as Gene Delivery Vehicles
Because of their hypo-immunogenicity and therapeutic potential in tissue regeneration and immune modulation, MSCs are safe and promising therapy to treat degenerative, autoimmune diseases and organ transplantation. However, a better understanding of the characteristics of MSCs would allow us to develop more effective therapeutic approaches, especially through genetic manipulation.
MSCs are transducible by plasmid, retrovirus, lentivirus, adenovirus and adeno-associated virus (AAV) and stably express transgene after genetic modification.92 Despite several successful reports, cationic liposomes and polymers are generally not recommended to deliver plasmid DNA into primary MSCs since it leads to low transfection efficiency and high cell mortality.93, 94 Among different viral vectors, retrovirus is quite effective in gene transduction into MSCs. MSCs do not express the hematopoietic or endothelial surface markers CD11b, CD14, CD31, CD34, or CD45 but do express CD29, CD44, CD73, CD105, CD106 and CD166.95 MSCs also express a low level of coxsackie adenovirus receptor (CAR), high-integrin phenotype.96 All these surface features make MSCs in favor of retrovirus, with a gene transfer efficacy between 50% and 85%.97 Transduced MSCs maintain transgene expression during expansion and differentiation,98 which is an important feature in treating time-costly degenerative diseases and cancer.19, 99 MSCs can also be efficiently transduced with lentivirus which is a subclass of retroviruses.100, 101 Adenoviral (Adv) vectors also efficiently transduce primary MSCs, but with a lower efficiency compared with retrovirus, probably because of low CAR expression on the surface of MSCs. Therefore, MOI higher than 200 is usually necessary to guarantee an optimal Adv transduction efficiency as suggested in many reports.102, 103 To increase the cellular uptake and reduce the MOI, adenovirus modified with Arg-Gly-Asp (RGD) motif is now commonly used to assist the transduction process.96, 104 The transduction efficacy of AAV on primary MSCs is even lower, but several improvements have already been made to overcome this problem. and efforts have been reported to address this issue. Ito et al. developed an UV light activated transduction system to improve the delivery of AAV vectors into human MSCs.105 Stender et al. described optimized conditions for AAV serotype 2 mediated gene transfer into human MSCs.106
Besides the surface features of vectors, the choice of promoters to construct vectors also has great impact on transgene expression in MSCs. Despite some discrepancies,107 overwhelming evidences suggest that cytomegalovirus (CMV) promoter, which is widely used for transgene expression in a variety of mammalian cells, is surprisingly silenced in both ES cells and MSCs.108–111 Qin et al. reported that human elongation factor 1α promoter (EF-1α), chicken β-actin promoter coupled with CMV early enhancer (CAGG), simian virus 40 early promoter (SV40), and tetracycline-responsive element promoter (TRE) promoters are more efficient than CMV promoter to drive the lentivirus mediated transgene expression in rat MSCs,110 which is further supported by the report from McGinley et al. who showed that human EF-1α and PGK promoters have a clear advantage against CMV promoter in transducing rat MSC transduction with lentivirus.111 Further exploration in this area may improve the efficacy of MSC based therapies.
Because viral gene therapy usually leads to intense immune response which greatly hinders the clinical application, MSCs seem to be perfect vehicles for viral vectors because of their hypoimmunogenicity and immune modulation effects. In addition, upon genetic manipulation, the production of soluble factors of MSCs can be maximized for therapeutic purposes. Duan et al. reported that the angiogenesis effect of MSCs could be enhanced by Adv-mediated HGF overexpression in treating cardiac ischemia injury.112 Peng et al. reported increasing retrovirus mediated VEGF expression by MSCs to promote angiogenesis and osteogenesis.113
Moreover, the differentiation of MSCs can be precisely controlled to meet the requirement of tissue repairing. Moutsatsos et al. transduced MSCs with bone morphogenetic protein 2 (BMP-2) gene, showing a definite differentiation along the osteogenic pathway. 114 On the other hand, MSCs are currently popular gene delivery vehicles for cancer research because of their tendency to migrate towards tumor tissues in vivo. Studeny et al. reported that MSCs preferentially survive and proliferate in the presence of malignant cells and can effectively inhibit the growth of malignant cells after being genetically modified with Adv vectors encoding IFN-β.115 Kim et al. and Loebinger et al. both reported successful treatment of cancer by using TNF-related apoptosis-inducing ligand (TRAIL) expressing MSCs from umbilical cord blood and BM, respectively.116, 117 These findings suggest that genetic manipulation afford MSCs with new potentials in treating diseases.
Genetically Modified MSCs Improve Islet Transplantation
Successful islet transplantation requires rapid and functional revascularization to relieve the hypoxic condition and meet the nutrition requirement of islet grafts. Otherwise transplanted islets lose their morphological integrity and viability in days.118 However, the angiogenic factors produced by primary MSCs are usually insufficient to support a rapid and functional revascularization of islet grafts. Several groups could not detect HGF expression from cultured MSCs.119 Co-transplantation studies did not provide solid evidence for the functional revascularization.66, 69 Genetically modified MSCs can totally reverse the incompetence of primary MSCs. For example, insulin gene enhancer protein (ISL-1) plays an important role in the angiogenesis of pancreatic islets.120 Barzelay et al. reported that MSCs transduced with retrovirus encoding ISL-1 gene showed significantly increased angiogenesis ability.121 We previously reported enhanced revascularization and prolonged graft function by Adv-mediated HGF and VEGF gene delivery to islets.44, 122 Other reports also showed increased angiogenesis in vivo by genetically modified MSCs to over-express HGF and VEGF.112, 123 Taken together, these evidences highlight the future use of genetically modified MSCs to promote the graft revascularization after islet transplantation (Fig. 5).
FIG. 5.
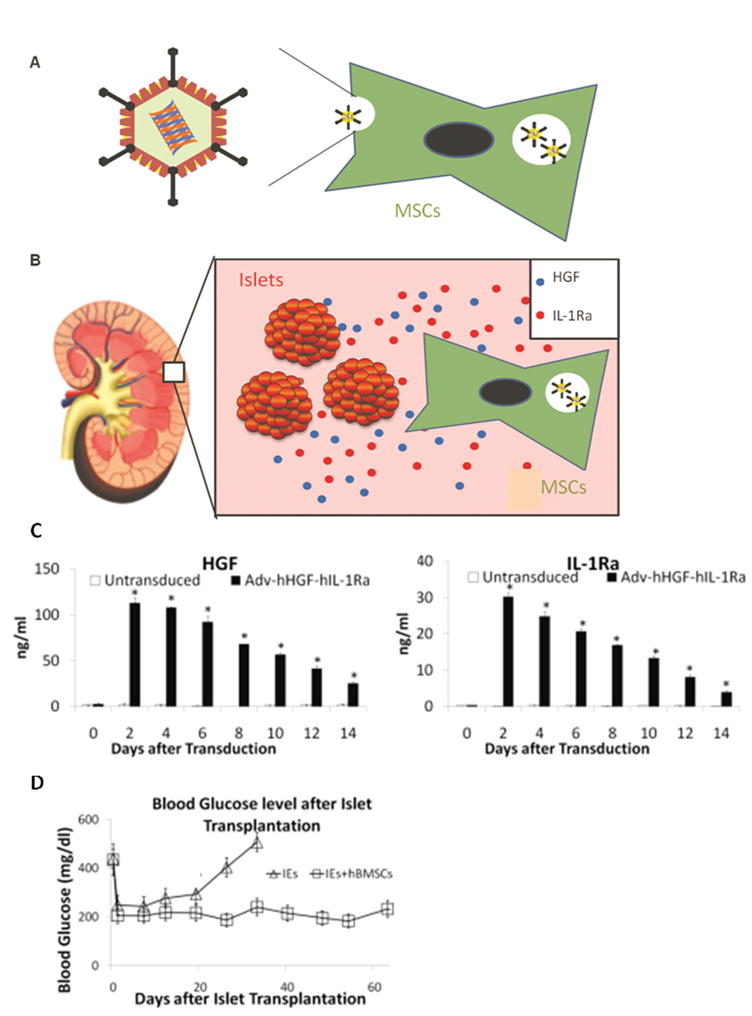
Use of genetically modified MSCs to improve the outcome of human islet transplantation. (a) MSCs were transduced with Adv-hHGF-hIL-1Ra prior to islet transplantation. (b) After co-transplantation with human islets under the kidney capsule of diabetic NOD-SCID mice, MSCs expressed HGF and IL-1Ra into the surrounding microenvironment to support islet viability and function. (c) Genetically modified human BM derived MSCs express elevated level of HGF and IL-1Ra after adv-transduction in transient manner. (d) Genetically modified MSCs prolonged the duration of normoglycemia after co-transplantion with human islets into diabetic NOD-SCID mice. Reproduced from Wu et al. (2011) Pharm Res. 2011 Apr 16. [Epub ahead of print]
Primary MSCs promote the proliferation of pancreatic β-cells by secreting multiple soluble factors.112, 124 How could genetic modification enhance such effect is not well characterized so far. Yu et al. reported that HGF over-expressed by genetically modified MSCs strongly promoted proliferation of hepatocytes and suppressed their apoptosis after liver transplantation.125 Genetically modified MSCs might promote the proliferation of pancreatic β-cells by similar mechanism.
The long-term survival of islet grafts also requires immune suppression. Immunosuppressive drugs cause systemic immune suppression and significant side effects. Genetically modified MSCs can express therapeutic genes and inhibit T-cell-mediated immune responses at the same time, both of which are crucial for the rapid graft settlement and long-term survival of transplanted islets. Unlike primary MSCs which can be expanded only 34~50 times, MSCs genetically modified with human telomerase reverse transcriptase (hTERT) can be expanded more than 260 times in vitro without tumorigenicity,126, 127 making them a safe cell-based therapy to protect the long-term survival of transplanted islets. Cao et al. reported that islets derived from porcine hTERT-transduced MSCs reversed hyperglycemia in streptozotocin-induced diabetic mice and secreted insulin and C-peptide in vitro, suggesting an alternative application of immortalized MSCs for islet replacement therapy.128 However, caution should still be exercised as these hTERT-transduced MSCs usually acquired lots of gene aberrations after several passages, which may lead to MSC transformation and tumorigenicity.129 Current studies using hTERT-transduced MSCs cultured in monolayer could be misleading, since these do not represent all aspects of tumorigenicity, for example, host-tissue interactions that nourish and support expansion of the tumor population and growth in three-dimensional clusters.130 Moreover, hTERT-transduced MSCs may express an extremely high level of osteogenic factors (e.g. osteocalcin) and other factors that may lead to abnormal engraftment after islet transplantation.131, 132
Additionally, genetically modified MSCs might also represent a major source of β-cells as well as islets for transplantation. Karnieli et al. and Li et al. both reported the reversal of hyperglycemia in streptozotocin-induced diabetic mice after transplantation of insulin-producing cells originated from genetically modified Pdx-1 expressing MSCs.54, 55 Li et al. reported the in vitro formation of islet-like structure using genetically modified MSCs.133 These in vitro generated islets from genetically modified MSCs may eventually help to relieve the shortage of donor islet supplies.
To summarize, based on the fast-evolving gene delivery techniques, the well characterized capacity of MSCs to secret angiogenic factors (e.g. VEGF and HGF) and immunomodulatory factors (e.g. IL-10, IDO, PGE2 and TGF-β) thus masking the immunogenicity as well as modulating immune response. Therefore, the use of genetically modified MSCs holds a great promise in improving the outcome of islet transplantation as well as treating many other diseases.134, 135
Conclusion
MSCs are relatively easy to isolate and expand, have immunosuppressive properties, can be transduced efficiently with viral vectors to express therapeutic proteins while retaining their stem-cell like properties even after genetic manipulation. Viral gene therapy is effective and highly selective treatment for multiple diseases but with significant immunogenicity. The progress in MSCs and gene therapy may one day unify and synergistically improve the outcome of islet transplantation by promoting rapid and functional revascularization, increasing pancreatic β-cell proliferation and avoiding allograft rejection. Careful genetic manipulation of MSCs without interfering with their self-renewal and differentiation processes is the prerequisite to their clinical applications. Moreover, the risks accompanying with genetically modified MSCs should not be ignored, such as the risk of tumorigenicity from genetically modified MSCs, the risk of generation of replication competent viral vectors in transduction process and the risk of insertional mutagenesis caused by integration of retrovirus. The risk factors associated with MSCs and genetically modified MSCs may highlight the need of selective elimination of these cells from the mixture of islet and stem cells after successful islet transplantation and engraftment. Unfortunately, no such work has been reported so far on MSCs. Schuldiner et al reported that human ESCs genetically engineered to express a “suicide” gene could be eliminated in vivo by administration of the US Food and Drug Administration (FDA) approved drug ganciclovir.136 This work may provide useful information for the study of selective elimination of MSCs in future. Meanwhile, a more comprehensive understanding of the characterization and the differentiation of genetically modified MSCs may allow us to develop better strategies for their use in cell-based therapeutics.
Acknowledgments
We thank the National Institutes of Health (NIH) for financial support (RO1 DK69968). Funds of the National Natural Science Foundation of China (31000424) and Shanghai Pujiang Program (10PJ1402200) to Z. Ye were also acknowledged. We also thank Dr. David Armbruster of the University of Tennessee Health Science Center for editing this manuscript.
References
- 1.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72(4):570–85. [PubMed] [Google Scholar]
- 2.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 3.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–75. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 6.Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20(5):1060–9. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 7.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Bosch P, Musgrave DS, Lee JY, Cummins J, Shuler T, Ghivizzani TC, Evans T, Robbins TD, Huard Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18(6):933–44. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- 9.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT. Differentiation potential of mesenchymal stem cells of different origin. Bull Exp Biol Med. 2006;141(1):147–51. doi: 10.1007/s10517-006-0115-2. [DOI] [PubMed] [Google Scholar]
- 11.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21(7):1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 13.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65(8):3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 16.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1(1):74–9. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 17.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16(4):406–13. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 18.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 19.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 20.De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160(6):909–18. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 24.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 25.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58(2):194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 26.Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68(3):396–402. doi: 10.1097/00007890-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 27.Polastri L, Galbiati F, Bertuzzi F, Fiorina P, Nano R, Gregori S, Aldrighetti L, Pozza G, Secchi A, Adorini L, Davalli AM. Secretory defects induced by immunosuppressive agents on human pancreatic beta-cells. Acta Diabetol. 2002;39(4):229–33. doi: 10.1007/s005920200039. [DOI] [PubMed] [Google Scholar]
- 28.Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Boyle PJ, Falqui L, Marchetti P, Ricordi C, Gingerich RL, et al. Results of our first nine intraportal islet allografts in type 1, insulin-dependent diabetic patients. Transplantation. 1991;51(1):76–85. doi: 10.1097/00007890-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257(5071):789–92. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98(6):1417–22. doi: 10.1172/JCI118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292(5520):1389–94. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 32.Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100(3):998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10(20):3016–20. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, Favrot M, Benhamou PY. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23(4):594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 35.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3(1):e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng K, Fraga D, Zhang C, Kotb M, Gaber AO, Guntaka RV, Mahato RI. Adenovirus-based vascular endothelial growth factor gene delivery to human pancreatic islets. Gene Ther. 2004;11(14):1105–16. doi: 10.1038/sj.gt.3302267. [DOI] [PubMed] [Google Scholar]
- 37.Menger MD, Beger C, Vajkoczy P. Restitution of intra-islet portal system in pancreatic islet isografts. Transplant Proc. 1994;26(2):688. [PubMed] [Google Scholar]
- 38.Lukinius A, Jansson L, Korsgren O. Ultrastructural evidence for blood microvessels devoid of an endothelial cell lining in transplanted pancreatic islets. Am J Pathol. 1995;146(2):429–35. [PMC free article] [PubMed] [Google Scholar]
- 39.Mahato RI. Gene expression and silencing for improved islet transplantation. J Control Release. 2009;140(3):262–7. doi: 10.1016/j.jconrel.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahato RI, Henry J, Narang AS, Sabek O, Fraga D, Kotb M, Gaber AO. Cationic lipid and polymer-based gene delivery to human pancreatic islets. Mol Ther. 2003;7(1):89–100. doi: 10.1016/s1525-0016(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 41.Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D, Kotb M, Gaber AO, Mahato RI. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21(1):15–25. doi: 10.1023/b:pham.0000012147.52900.b8. [DOI] [PubMed] [Google Scholar]
- 42.Wu H, Panakanti R, Li F, Mahato RI. XIAP Gene Expression Protects beta-Cells and Human Islets from Apoptotic Cell Death. Mol Pharm. 2010;7(5):1655–1666. doi: 10.1021/mp100070j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panakanti R, Mahato RI. Bipartite vector encoding hVEGF and hIL-1Ra for ex vivo transduction into human islets. Mol Pharm. 2009;6(1):274–84. doi: 10.1021/mp800183b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panakanti R, Mahato RI. Bipartite adenoviral vector encoding hHGF and hIL-1Ra for improved human islet transplantation. Pharm Res. 2009;26(3):587–96. doi: 10.1007/s11095-008-9777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng G, Zhu L, Mahato RI. Caspase-3 gene silencing for inhibiting apoptosis in insulinoma cells and human islets. Mol Pharm. 2008;5(6):1093–102. doi: 10.1021/mp800093f. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Mahato RI. iNOS gene silencing prevents inflammatory cytokine-induced beta-cell apoptosis. Mol Pharm. 2008;5(3):407–17. doi: 10.1021/mp700145f. [DOI] [PubMed] [Google Scholar]
- 47.Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JR, Shapiro AM, Elliott JF. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54(9):2541–8. doi: 10.2337/diabetes.54.9.2541. [DOI] [PubMed] [Google Scholar]
- 48.Luo JZ, Xiong F, Al-Homsi AS, Roy T, Luo LG. Human BM stem cells initiate angiogenesis in human islets in vitro. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y, Jin X, Chen Y, Li S, Yuan Y, Mai G, Tian B, Long D, Zhang J, Zeng L, Li Y, Cheng J. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem Funct. 2010;28(8):637–43. doi: 10.1002/cbf.1701. [DOI] [PubMed] [Google Scholar]
- 50.Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation. 2010;89(6):686–93. doi: 10.1097/TP.0b013e3181cb3e8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itakura S, Asari S, Rawson J, Ito T, Todorov I, Liu CP, Sasaki N, Kandeel F, Mullen Y. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7(2):336–46. doi: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 52.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58(8):1797–806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brusko TM. Mesenchymal stem cells: a potential border patrol for transplanted islets? Diabetes. 2009;58(8):1728–9. doi: 10.2337/db09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25(11):2837–44. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H, Liu Q, Liu D, Chen L, Pei X. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol. 2007;211(1):36–44. doi: 10.1002/jcp.20897. [DOI] [PubMed] [Google Scholar]
- 56.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14(6):631–40. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103(46):17438–43. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urban VS, Kiss J, Kovacs J, Gocza E, Vas V, Monostori E, Uher F. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26(1):244–53. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 59.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21(7):763–70. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 60.Choi JB, Uchino H, Azuma K, Iwashita N, Tanaka Y, Mochizuki H, Migita M, Shimada T, Kawamori R, Watada H. Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic beta cells. Diabetologia. 2003;46(10):1366–74. doi: 10.1007/s00125-003-1182-9. [DOI] [PubMed] [Google Scholar]
- 61.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 62.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117(1):3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS. Growth factor/matrix-induced proliferation of human adult beta-cells. Diabetes. 1995;44(12):1458–60. doi: 10.2337/diab.44.12.1458. [DOI] [PubMed] [Google Scholar]
- 64.Izumida Y, Aoki T, Yasuda D, Koizumi T, Suganuma C, Saito K, Murai N, Shimizu Y, Hayashi K, Odaira M, Kusano T, Kushima M, Kusano M. Hepatocyte growth factor is constitutively produced by donor-derived bone marrow cells and promotes regeneration of pancreatic beta-cells. Biochem Biophys Res Commun. 2005;333(1):273–282. doi: 10.1016/j.bbrc.2005.05.100. [DOI] [PubMed] [Google Scholar]
- 65.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 66.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89(12):1438–45. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 67.Chao KC, Chao KF, Chen CF, Liu SH. A novel human stem cell coculture system that maintains the survival and function of culture islet-like cell clusters. Cell Transplant. 2008;17(6):657–64. doi: 10.3727/096368908786092801. [DOI] [PubMed] [Google Scholar]
- 68.Sordi V, Melzi R, Mercalli A, Formicola R, Doglioni C, Tiboni F, Ferrari G, Nano R, Chwalek K, Lammert E, Bonifacio E, Borg D, Piemonti L. Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells. 2010;28(1):140–51. doi: 10.1002/stem.259. [DOI] [PubMed] [Google Scholar]
- 69.Rackham CL, Chagastelles PC, Nardi NB, Hauge-Evans AC, Jones PM, King AJ. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia. 2011 doi: 10.1007/s00125-011-2053-4. [DOI] [PubMed] [Google Scholar]
- 70.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 72.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–74. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 73.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 74.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 75.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61(1):237–42. [PubMed] [Google Scholar]
- 76.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87(6):896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- 77.Popp FC, Eggenhofer E, Renner P, Slowik P, Lang SA, Kaspar H, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20(1–2):55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305(1):33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 79.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 80.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 81.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 82.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193(2):233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191(3):411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 85.Longoni B, Szilagyi E, Quaranta P, Paoli GT, Tripodi S, Urbani S, Mazzanti B, Rossi B, Fanci R, Demontis GC, Marzola P, Saccardi R, Cintorino M, Mosca F. Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol Ther. 2010;12(6):435–46. doi: 10.1089/dia.2009.0154. [DOI] [PubMed] [Google Scholar]
- 86.Li FR, Wang XG, Deng CY, Qi H, Ren LL, Zhou HX. Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clin Exp Immunol. 2010;161(2):357–63. doi: 10.1111/j.1365-2249.2010.04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim YH, Wee YM, Choi MY, Lim DG, Kim SC, Han DJ. IL-10 induced by CD11b+cells and IL-10 activated regulatory T cells play a role in immune modulation of mesenchymal stem cells in rat islet allograft. Mol Med. 2011 doi: 10.2119/molmed.2010.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Marasa M, Golay J, Noris M, Remuzzi G. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6(2):412–22. doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 90.Zhou HP, Yi DH, Yu SQ, Sun GC, Cui Q, Zhu HL, Liu JC, Zhang JZ, Wu TJ. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc. 2006;38(9):3046–51. doi: 10.1016/j.transproceed.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, Perico N, Remuzzi G, Noris M. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181(6):3933–46. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 92.Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5(12):1571–84. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peister A, Mellad JA, Wang M, Tucker HA, Prockop DJ. Stable transfection of MSCs by electroporation. Gene Ther. 2004;11(2):224–8. doi: 10.1038/sj.gt.3302163. [DOI] [PubMed] [Google Scholar]
- 94.Song L, Chau L, Sakamoto Y, Nakashima J, Koide M, Tuan RS. Electric field-induced molecular vibration for noninvasive, high-efficiency DNA transfection. Mol Ther. 2004;9(4):607–16. doi: 10.1016/j.ymthe.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 95.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 96.Tsuda H, Wada T, Ito Y, Uchida H, Dehari H, Nakamura K, Sasaki K, Kobune M, Yamashita T, Hamada H. Efficient BMP2 gene transfer and bone formation of mesenchymal stem cells by a fiber-mutant adenoviral vector. Mol Ther. 2003;7(3):354–65. doi: 10.1016/s1525-0016(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 97.Jorgensen C, Djouad F, Apparailly F, Noel D. Engineering mesenchymal stem cells for immunotherapy. Gene Ther. 2003;10(10):928–31. doi: 10.1038/sj.gt.3302019. [DOI] [PubMed] [Google Scholar]
- 98.Lee K, Majumdar MK, Buyaner D, Hendricks JK, Pittenger MF, Mosca JD. Human mesenchymal stem cells maintain transgene expression during expansion and differentiation. Mol Ther. 2001;3(6):857–66. doi: 10.1006/mthe.2001.0327. [DOI] [PubMed] [Google Scholar]
- 99.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30(21):2379–87. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 100.Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214(4):472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 101.Kallifatidis G, Beckermann BM, Groth A, Schubert M, Apel A, Khamidjanov A, Ryschich E, Wenger T, Wagner W, Diehlmann A, Saffrich R, Krause U, Eckstein V, Mattern J, Chai M, Schutz G, Ho AD, Gebhard MM, Buchler MW, Friess H, Buchler P, Herr I. Improved lentiviral transduction of human mesenchymal stem cells for therapeutic intervention in pancreatic cancer. Cancer Gene Ther. 2008;15(4):231–40. doi: 10.1038/sj.cgt.7701097. [DOI] [PubMed] [Google Scholar]
- 102.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 103.Meinel L, Hofmann S, Betz O, Fajardo R, Merkle HP, Langer R, Evans CH, Vunjak-Novakovic G, Kaplan DL. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials. 2006;27(28):4993–5002. doi: 10.1016/j.biomaterials.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 104.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9(2):189–97. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 105.Ito H, Goater JJ, Tiyapatanaputi P, Rubery PT, O’Keefe RJ, Schwarz EM. Light-activated gene transduction of recombinant adeno-associated virus in human mesenchymal stem cells. Gene Ther. 2004;11(1):34–41. doi: 10.1038/sj.gt.3302102. [DOI] [PubMed] [Google Scholar]
- 106.Stender S, Murphy M, O’Brien T, Stengaard C, Ulrich-Vinther M, Soballe K, Barry F. Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater. 2007;13:93–9. doi: 10.22203/ecm.v013a10. discussion 99. [DOI] [PubMed] [Google Scholar]
- 107.Ward CM, Stern PL. The human cytomegalovirus immediate-early promoter is transcriptionally active in undifferentiated mouse embryonic stem cells. Stem Cells. 2002;20(5):472–5. doi: 10.1634/stemcells.20-5-472. [DOI] [PubMed] [Google Scholar]
- 108.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 109.Liew CG, Draper JS, Walsh J, Moore H, Andrews PW. Transient and stable transgene expression in human embryonic stem cells. Stem Cells. 2007;25(6):1521–8. doi: 10.1634/stemcells.2006-0634. [DOI] [PubMed] [Google Scholar]
- 110.Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One. 2010;5(5):e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O’Toole D, O’Brien T. Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res Ther. 2011;2(2):12. doi: 10.1186/scrt53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8(3):467–74. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 113.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, Kelley P, Stumm N, Mi S, Muller R, Zilberman Y, Gazit D. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3(4):449–61. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 115.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–8. [PubMed] [Google Scholar]
- 116.Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park SH, Sung YC, Jeun SS. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68(23):9614–23. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 117.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69(10):4134–42. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boker A, Rothenberg L, Hernandez C, Kenyon NS, Ricordi C, Alejandro R. Human islet transplantation: update. World J Surg. 2001;25(4):481–6. doi: 10.1007/s002680020341. [DOI] [PubMed] [Google Scholar]
- 119.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 120.Eberhardt M, Salmon P, von Mach MA, Hengstler JG, Brulport M, Linscheid P, Seboek D, Oberholzer J, Barbero A, Martin I, Muller B, Trono D, Zulewski H. Multipotential nestin and Isl-1 positive mesenchymal stem cells isolated from human pancreatic islets. Biochem Biophys Res Commun. 2006;345(3):1167–76. doi: 10.1016/j.bbrc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 121.Barzelay A, Ben-Shoshan J, Entin-Meer M, Maysel-Auslender S, Afek A, Barshack I, Keren G, George J. A potential role for islet-1 in post-natal angiogenesis and vasculogenesis. Thromb Haemost. 2010;103(1):188–97. doi: 10.1160/TH09-07-0433. [DOI] [PubMed] [Google Scholar]
- 122.Narang AS, Sabek O, Gaber AO, Mahato RI. Co-expression of vascular endothelial growth factor and interleukin-1 receptor antagonist improves human islet survival and function. Pharm Res. 2006;23(9):1970–82. doi: 10.1007/s11095-006-9065-7. [DOI] [PubMed] [Google Scholar]
- 123.Shi ZB, Wang KZ. Effects of recombinant adeno-associated viral vectors on angiopoiesis and osteogenesis in cultured rabbit bone marrow stem cells via co-expressing hVEGF and hBMP genes: a preliminary study in vitro. Tissue Cell. 2010;42(5):314–21. doi: 10.1016/j.tice.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 124.Dinarvand P, Hashemi SM, Soleimani M. Effect of transplantation of mesenchymal stem cells induced into early hepatic cells in streptozotocin-induced diabetic mice. Biol Pharm Bull. 2010;33(7):1212–7. doi: 10.1248/bpb.33.1212. [DOI] [PubMed] [Google Scholar]
- 125.Yu Y, Yao AH, Chen N, Pu LY, Fan Y, Lv L, Sun BC, Li GQ, Wang XH. Mesenchymal stem cells over-expressing hepatocyte growth factor improve small-for-size liver grafts regeneration. Mol Ther. 2007;15(7):1382–9. doi: 10.1038/sj.mt.6300202. [DOI] [PubMed] [Google Scholar]
- 126.Abdallah BM, Haack-Sorensen M, Burns JS, Elsnab B, Jakob F, Hokland P, Kassem M. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005;326(3):527–38. doi: 10.1016/j.bbrc.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 127.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao H, Chu Y, Zhu H, Sun J, Pu Y, Gao Z, Yang C, Peng S, Dou Z, Hua J. Characterization of immortalized mesenchymal stem cells derived from foetal porcine pancreas. Cell Prolif. 2011;44(1):19–32. doi: 10.1111/j.1365-2184.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23(29):5095–8. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- 130.Burns JS, Abdallah BM, Guldberg P, Rygaard J, Schroder HD, Kassem M. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005;65(8):3126–35. doi: 10.1158/0008-5472.CAN-04-2218. [DOI] [PubMed] [Google Scholar]
- 131.Gronthos S, Chen S, Wang CY, Robey PG, Shi S. Telomerase accelerates osteogenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J Bone Miner Res. 2003;18(4):716–22. doi: 10.1359/jbmr.2003.18.4.716. [DOI] [PubMed] [Google Scholar]
- 132.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–27. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li L, Li F, Qi H, Feng G, Yuan K, Deng H, Zhou H. Coexpression of Pdx1 and betacellulin in mesenchymal stem cells could promote the differentiation of nestin-positive epithelium-like progenitors and pancreatic islet-like spheroids. Stem Cells Dev. 2008;17(4):815–23. doi: 10.1089/scd.2008.0060. [DOI] [PubMed] [Google Scholar]
- 134.Calne RY, Gan SU, Lee KO. Stem cell and gene therapies for diabetes mellitus. Nat Rev Endocrinol. 2010;6(3):173–7. doi: 10.1038/nrendo.2009.276. [DOI] [PubMed] [Google Scholar]
- 135.Chen NK, Tan SY, Udolph G, Kon OL. Insulin expressed from endogenously active glucose-responsive EGR1 promoter in bone marrow mesenchymal stromal cells as diabetes therapy. Gene Ther. 2010;17(5):592–605. doi: 10.1038/gt.2010.12. [DOI] [PubMed] [Google Scholar]
- 136.Schuldiner M, Itskovitz-Eldor J, Benvenisty N. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells. 2003;21(3):257–65. doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- 137.Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun. 2009;32(2):116–24. doi: 10.1016/j.jaut.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O’Connor DH, Bartholomew AM, Kenyon NS. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59(10):2558–68. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 140.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18(12):1417–26. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 141.Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40(6):1275–84. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]