Abstract
Background
Blood alcohol levels (BAL) cycle up and down over a 7–8 day period when ethanol is fed continuously for one month in the intragastric tube feeding rat model (ITFRM) of alcoholic liver disease. The cycling phenomenon is due to an alternating increase and decrease in the metabolic rate. Recently, we found that S-adenosyl-methionine (SAMe) fed with alcohol prevented the BAL cycle.
Method
Using the ITFRM we fed rats betaine (2 g/kg/day) with ethanol for 1 month and recorded the daily 24 h urine ethanol level (UAL) to measure the BAL cycle. UAL is equivalent to BAL because of the constant ethanol infusion. Liver histology, steatosis and BAL were measured terminally after 1 month of treatment. Microarray analysis was done on the mRNA extracted from the liver to determine the effects of betaine and alcohol on changes in gene expression.
Results
Betaine fed with ethanol completely prevented the BAL cycle similar to SAMe. Betaine also significantly reduced the BAL compared to ethanol fed rats without betaine. This was also observed when SAMe was fed with ethanol. The mechanism involved in both cases is that SAMe is required for the conversion of epinephrine from norepinephrine by phenylethanolamine methyltransferase (PNMT). Epinephrine is 5 to 10 fold more potent than norepinephrine in increasing the metabolic rate. The increase in the metabolic rate generates NAD, permitting ADH to increase the oxidation of alcohol. NAD is the rate limiting factor in oxidation of alcohol by alcohol dehydrogenase (ADH). This explains how SAMe and betaine prevented the cycle. Microarray analysis showed that betaine feeding prevented the up regulation of a large number of genes including TLR2/4, Il-1b, Jax3, Sirt3, Fas, Ifngr1, Tgfgr2, Tnfrsf21, Lbp and Stat 3 which could explain how betaine prevented fatty liver.
Conclusion
Betaine feeding lowers the BAL and prevents the BAL cycle by increasing the metabolic rate. This increases the rate of ethanol elimination by generating NAD.
Keywords: S-adenosylmethionine (SAMe), Betaine, Blood Alcohol Cycle (BAL), Microarray Analysis, NAD
INTRODUCTION
Ethanol fed intragastrically continuously with a liquid diet for 1 month induces a cyclic increase (peak) blood alcohol level, (BAL) followed by a decrease (trough) in BAL over a 6–7 day period (Tsukamoto et al., 1985). The cycle is important in the pathogenesis of alcoholic liver disease (ALD) because hypoxic liver injury occurs at the peaks of BAL where ATP levels are reduced. At the peak BAL, there is an increase in the NADH/NAD ratio in the liver, an increase in the expression of vascular endothelial growth factor, an increase in nitrates, an increase in hypoxia and an increase in the pathology score (Bardag-Gorce et al., 2002; French et al., 1984; Li et al., 2004a,).
Gene expression changes are greatly increased at the peaks of the BAL cycle (Bardag-Gorce et al., 2006b; Li et al., 2010a, 2010b). For the cycle to occur there are three essential factors. First, the thyroid must be normal because cutting the pituitary stalk, giving propylthiouracil or excess T4 prevents the cycle (Li et al., 2000, 2001, 2007). Second, there must be a generation of NAD to increase the oxidation of alcohol by alcohol dehydrogenase (ADH). When complex 1 (NADH dehydrogenase) of the mitochondrial electron transport chain is inhibited by rotenone to reduce NAD generation, the rate of ethanol oxidation is reduced at the peaks (Li et al., 2004b). But when oxidative phosphorylation is uncoupled by dinitrophenol to increase the generation of NAD at the troughs, the oxidation of ethanol by ADH is increased and the cycle is prevented (Li et al., 2005). Third, an increase of catecholamines at the peak of the cycle (Li et al., 2003) increases the metabolic rate and O2 consumption, which increases NAD levels to drive down BALs by increasing the rate of alcohol elimination by ADH. The metabolic rate is increased at the peaks, which is indicated by the body temperature. The body temperature is cyclically increased at the peaks (Li et al., 2000) and liver O2 levels are decreased at the peaks of the cycle (Li et al., 2004a). Epinephrine increases body temperature and the metabolic rate (Staten et al., 1989). Adrenergic drugs and catecholamines have been shown to accelerate the metabolic rate and thus accelerate the ethanol elimination rate (Li et al., 2003; Schola and Schwabe, 1980). Both propranolol and phenoxybenzamine prevent the cycle (Li et al., 2004c). Feeding ephedrine and caffeine with ethanol prevents the cycle from occurring, as with feeding T4, by increasing the metabolic rate. This increases the elimination rate of ethanol (Li et al., 2001, 2003).
Feeding S-adenosylmethionine (SAMe) with alcohol also prevented the BAL cycle (Bardag-Gorce et al., 2010a). SAMe fed with ethanol (6 g/kg body weight bolus) significantly lowered the BAL and urinary alcohol levels (UAL) 3 h after the ethanol bolus, indicating that SAMe feeding increased the metabolic rate and the rate of ethanol elimination (Li et al., 2010b). The levels of alcohol were kept at the Trough levels by feeding SAMe. Likewise, betaine, fed with a bolus of ethanol, also reduced the BAL and UAL 3 h after the ethanol bolus (Li et al., 2010c). Dietary betaine promotes the generation of liver SAMe levels (Barak et al., 1993). Betaine doubled the levels of SAMe in controls and increased SAMe 4 fold in alcohol fed rats. The mechanism involved is that both SAMe feeding directly and betaine feeding indirectly, increased the levels of SAMe. SAMe is required for the conversion of norepinephrine to epinephrine by phenylethanolamine N-methyltransferase (PNMT) (Wong et al., 1992). Epinephrine induction of an increased metabolic rate (thermogenesis) is 5 to 10 fold greater than norepinephrine (Landsberge et al., 1984). In this way SAMe prevents the cycle from occurring by increasing the levels of epinephrine, which accelerates the rate of ethanol elimination by increasing the metabolic rate. In the present study we show that betaine also prevented the BAL cycle by increasing the metabolic rate. Microarrays were done to mine the genes regulated by ethanol and compared with rats fed betaine with ethanol for 1 month. Betaine and its metabolites choline and dimethylglycine were measured in the liver, blood and urine to determine the effects of alcohol and betaine feeding on the metabolism of betaine.
MATERIAL AND METHODS
Animals
Twelve male Wistar rats (Harlico, Hollister, CA) weighing 300 g were divided into 4 groups of 3 rats each and were fed intragastrically at a constant rate (Li et al., 2000). The liquid diet was high in protein content and contained 500 mg. choline/kg. The diet was fed with ethanol (13 g/kg body weight/day) or without ethanol, and with or without betaine (Sigma, St. Louis, MO) 2 g/kg body weight/day. Group 1 was fed ethanol; group 2 was pair fed dextrose isocaloric to replace ethanol calories; group 3 was fed betaine with ethanol; group 4 was fed betaine and isocaloric dextrose. The body weight of the rats and their serum alanine aminotransferase (ALT) levels were determined before and at the end of feeding ethanol. Serum ALT was measured using an automatic clinical analyzer. Urine was collected over 24 h under toluene for all ethanol fed rats over the last 14 days of the one month long experiment where 24 h urinary alcohol levels were measured daily using a kit (QED saliva alcohol test A 150, STC Technologies, Bethlehem, PA). Choline, betaine and dimethylglycine were quantitated in the urine, serum and liver collected from the rats. The rats were maintained according to the guidelines of Animal Care as described by the National Academy of Sciences published by NIH (1996).
RNA Extraction and Microarray Analysis
A portion of the liver was first frozen in liquid nitrogen and then stored at −80° until analysis. Total liver RNAs were extracted by Ultraspec™ RNA Isolation Systemic (Biotechs Laboratories, Houston, TX) and were cleaned up with Rneasy Columns (Qiagen, Valencia, CA). Microarray targets were prepared using MessageAmp Premier RNA Amplification Kit (Ambion, Inc), and then hybridized to the Affymetrix Rat Genome 230 2.0 Array. The acquisition of array image was undertaken by using Affymetrix GeneChip Command Console. Data analyses were performed using Partek® Genomics Suite Version 6.5. Thresholds for selecting significant genes were set at >= 1.5-fold and p<0.05. Genes met both criteria simultaneously were considered as significant changes. Cluster analysis was performed using default settings.
Measurement of Betaine, Dimethylglycine and Choline: Liquid chromatography-mass spectrometry was used to measure betaine, free choline and dimethylglycine according to the method of Holm et al. (2003).
Liver Histology
Portions of the livers were fixed in 10% zinc formalin for 4 h and then kept in 80% ethanol until they were embedded in paraffin. Sections 4 μm thick were cut and stained with hematoxylin and eosin. The sections were then used to score the pathology and measure the amount of fat morphometrically using Nikon morphometric software (fat pixels/total pixels).
Statistical Analysis
Results among the 4 groups of rats were determined by ANOVA and Student-Newman-Keuls for multiple group comparisons using Sigma-Stat software (San Francisco, CA).
RESULTS
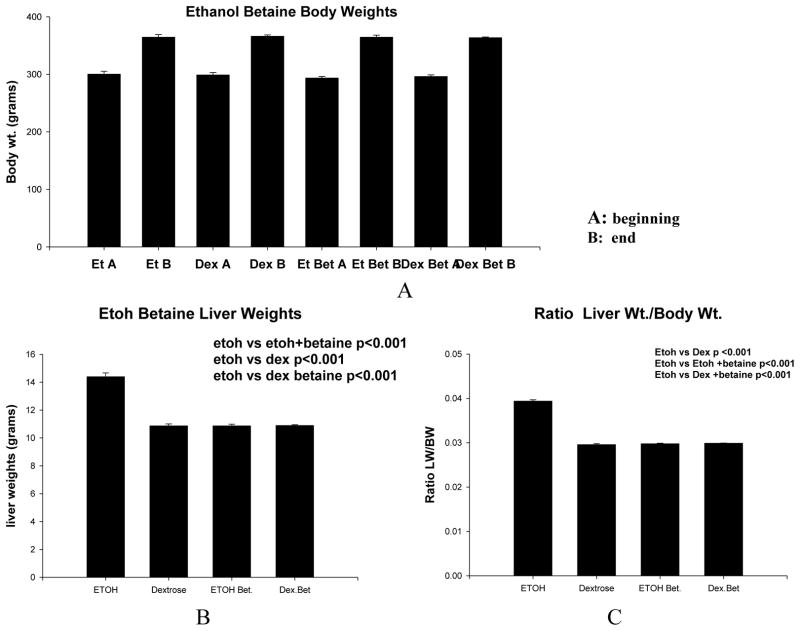
The body weights of the rats in the 4 groups are shown in Figure 1A. There was no difference between the groups at the start or at the end of the 1 month experiment. There was a significant increase in liver weight in group 1 ethanol fed rats when compared to the other 3 groups (p<0.001) (Fig 1B). The liver weight/body weight ratio was increased only in the group 1 ethanol fed rats when compared to the other 3 groups (p<0.001) (Fig 1C).
Fig 1.
A. There was no significant difference in body weights among the 4 groups of rats at either the beginning or the end of the experiment (ET=ethanol, Bet=betaine, Dex=dextrose). B. All 4 groups gained the same amount of weight. There was a significant increase in the liver weights in the group 1 ethanol fed rats compared to the other 3 groups (Mean ±S.E.M., n=3). C. The liver/body weight ratios were also increased when ethanol alone was fed (Mean ±S.E.M., n=3).
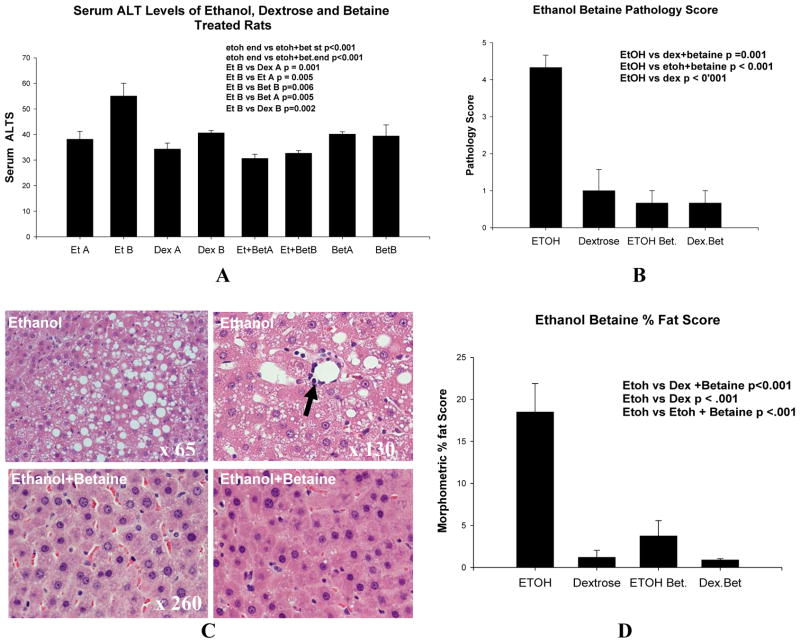
The serum ALT levels measured in the rats before starting feeding and after 1 month feeding was significantly increased only in the group 1 rats fed ethanol for 1 month (p<=0.001 to 0.006) (Fig 1A). The pathology score of the group 1 ethanol fed rats was significantly increased when compared with the 3 other groups (p<0.001) (Fig 2B). The histology of the liver is shown in figure 2C. Note that only the livers from the group 1 ethanol fed rats showed an increase in macrovesicular fat (Fig 2C). The amount of fat formed in the group 1 ethanol fed rats was significantly increased compared to the other 3 groups of rats (p<0.001, Mean ± SEM, n=3 (Fig 2D).
Fig 2.
A. There was a significant increase in ALT only in the group 1 ethanol fed rats when compared with the other 3 groups at the end of the experiment (Mean ±S.E.M., n=3). B. The total pathology score was markedly increased in the group 1 ethanol-fed rats compared to the other 3 groups of rats (Mean ±S.E.M., n=3). C. The representative liver histology from the 3 groups of rats fed ethanol or betaine are shown. The control fed dextrose showed the same histology as the control fed betaine (results not shown). Note that the group 1 ethanol fed rat livers showed an increase in macrovesicular fat and inflammation (arrow). H&E. D. Morphometric measurement of fat in histologic sections of the liver of the 4 groups of rats showed a significant increase of fat in the group 1 ethanol-fed rats (Mean ±S.E.M., n=3).
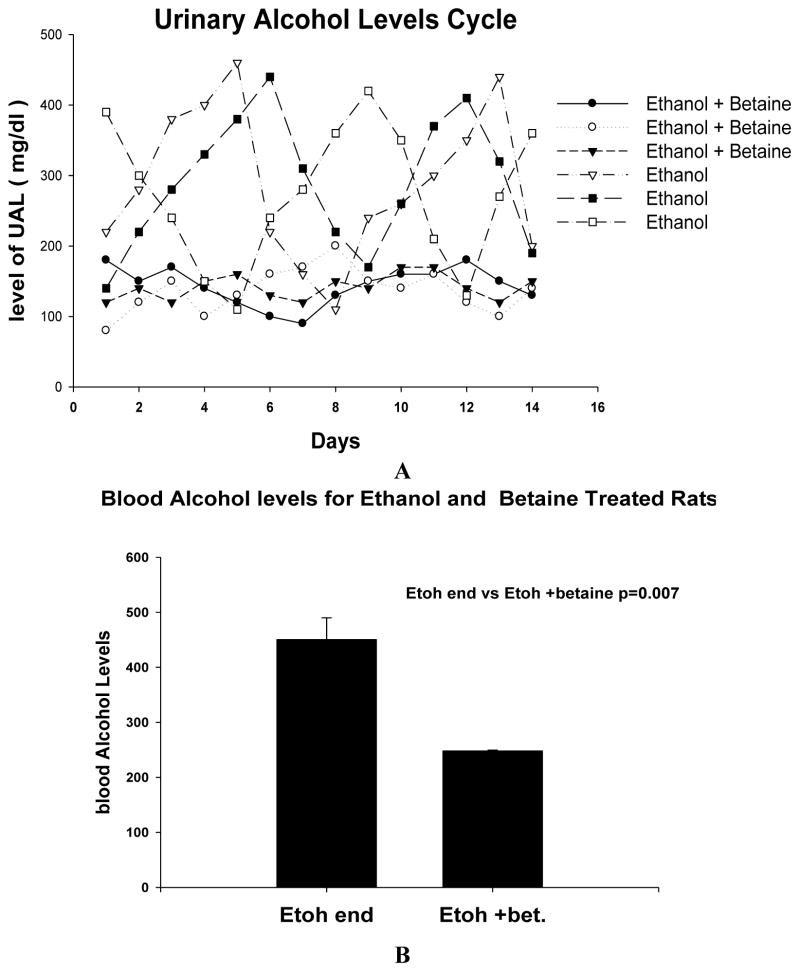
The UAL cycle showed that it was manifested when the 24 h group urines from rats fed ethanol were assayed daily for the last 14 days of the experiment (Fig 3A). Group 3 rats fed ethanol with betaine did not cycle, but rather the UAL hovered between 100 and 200 mg%. Thus, when betaine was fed with ethanol the UAL cycle did not develop. The terminal blood alcohol levels in group 1 rats were 450.5 mg% (Mean ± S.E.M., n=3) compared with 248 ± 1.2 mg% in the group 3 rats fed ethanol and betaine (p<0.007) (Fig 3B) indicating that betaine maintained the ethanol elimination rate at a higher level than when ethanol was fed alone.
Fig 3.
A. The UAL cycle was present when group 1 ethanol fed rats were monitored. However, the group 3 rats fed ethanol with betaine did not develop the UAL cycle. Instead the UAL hovered between 100 and 200 mg%. B. The terminal BAL of group 1 ethanol fed rats was significantly higher when compared with the rats fed betaine with ethanol (Mean ± SEM, n = 3).
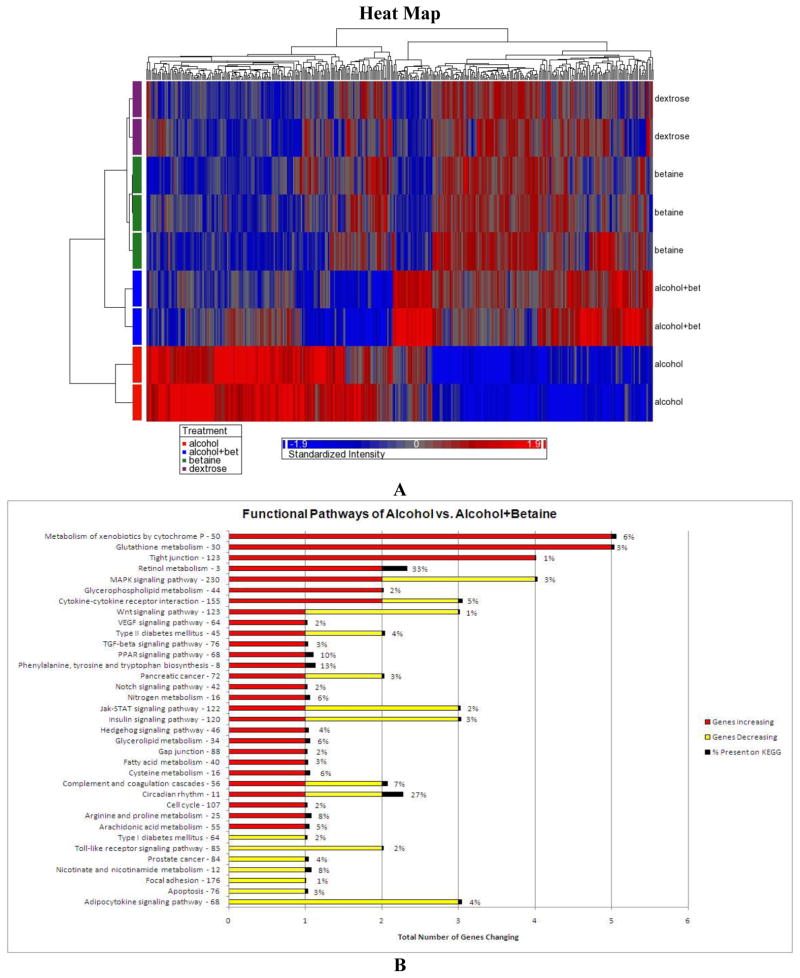
Microarray heat maps were done to compare the global changes in gene expression. They showed that the pattern of gene clusters found in the rats fed ethanol alone (group 1) differed markedly from the rats fed ethanol plus betaine (group 3) and from as the group 2 and 4 rats (Fig 4A). There were 397 genes which differed significantly in their expression when group 1 rats fed ethanol was compared with group 3 rats fed ethanol plus betaine. Overall the difference was highly significant (15-fold, p<0.005). Likewise, betaine (group 3) prevented most of the functional pathway gene expressions induced by ethanol feeding for 1 month (group 1) (Fig 4b). The expressions of many of the genes up regulated by ethanol and prevented by betaine are listed in Table 1. Of the affected genes, the most important changes, which could cause liver injury induced by ethanol feeding include Lbp, Dapk1, Gadd 45b, Wnt2, Lepr, Tlr2 and 4, Tfngr1, Tgfgb2, Tnfrsf1b, Stat 3, Sirt 3, Jak 3, Nos 3 and Fas. The prevention of the up regulation of these genes by betaine feeding could account for the prevention of steatosis and inflammation. Steatosis and inflammation were observed when ethanol was fed without betaine added to the diet (Fig 2C).
Fig 4.
A. The heat map of gene expression changes showed that rats fed alcohol alone differed from the other 3 groups of rats (15 fold, p<0.005). (Red is up regulated, blue is down regulated). B. The KEGG functional pathways changed when group 1 rats fed ethanol were compared with group 3 rats fed ethanol plus betaine. Ethanol up regulated (red bars) most functional pathways compared to the group 3 rats fed ethanol plus betaine (red= up regulated, yellow down regulated).
Table 1.
The expression of genes up regulated by ethanol feeding and prevented by betaine feeding with ethanol.
| ETOH vs. DEX | ETOH vs. ETOH + BETAINE | ||||
|---|---|---|---|---|---|
| Gene Title | Gene Symbol | Fold Change | P Value | Fold Change | P Value |
| Glucose-6-phosphatase, catalytic | G6pc | 8.06 | 0.022 | NC | |
| lipopolysaccharide binding protein | Lbp | 5.91 | 0.0004 | NC | |
| death associated protein kinase 1 | Dapk1 | 4.98 | 0.0016 | NC | |
| growth arrest and DNA-damage-inducible 45 beta | Gadd45b | 3.86 | 0.0118 | 2.72 | 0.0352 |
| alpha-2-macroglobulin | A2m | 3.59 | 0.0270 | −3.23 | 0.0362 |
| wingless-related MMTV integration site 2 | Wnt2 | 3.10 | 0.0031 | 2.07 | 0.0199 |
| leptin receptor | Lepr | 3.09 | 0.0035 | NC | |
| toll-like receptor 2 | Tlr2 | 3.06 | 0.0017 | NC | |
| tumor necrosis factor receptor superfamily, member 21 | Tnfrsf21 | 2.47 | 0.0067 | NC | |
| CD68 antigen | CD68 | 2.46 | 0.0045 | NC | |
| interleukin 1 beta | Il1b | 2.43 | 0.0176 | NC | |
| Transforming growth factor, beta 2 | Tgfb2 | 2.32 | 0.0120 | NC | |
| tumor necrosis factor receptor superfamily, member 1b | Tnfrsf1b | 2.28 | 0.0007 | NC | |
| toll-like receptor 4 | Tlr4 | 2.27 | 0.0089 | NC | |
| signal transducer and activator of transcription 3 | Stat3 | 2.25 | 0.0014 | NC | |
| LPS-induced TN factor | Litaf | 2.00 | 0.0183 | 1.84 | 0.0287 |
| sirtuin 3 | Sirt3 | 1.95 | 0.0053 | NC | |
| death-associated kinase 2 | Dapk2 | 1.92 | 0.0073 | NC | |
| Janus kinase 3 | Jak3 | 1.89 | 0.0088 | −1.63 | 0.0241 |
| autophagy-related 10 | Atg10 | 1.74 | 0.0392 | NC | |
| interferon gamma receptor 1 | Ifngr1 | 1.74 | 0.0086 | NC | |
| cystathionase | Cth | 1.67 | 0.0384 | 1.86 | 0.0199 |
| nitric oxide synthase 3, endothelial cell | Nos3 | 1.61 | 0.0499 | NC | |
| aryl hydrocarbon receptor | Ahr | 1.58 | 0.0263 | NC | |
| Fas (TNF receptor superfamily, member 6) | Fas | 1.54 | 0.0080 | NC | |
| histone deacetylase 8 | Hdac8 | 1.54 | 0.0131 | NC | |
| programmed cell death 4 | Pdcd4 | 1.54 | 0.0399 | NC | |
| uncoupling protein 2 (mitochondrial, proton carrier) | Ucp2 | 1.51 | 0.0464 | NC | |
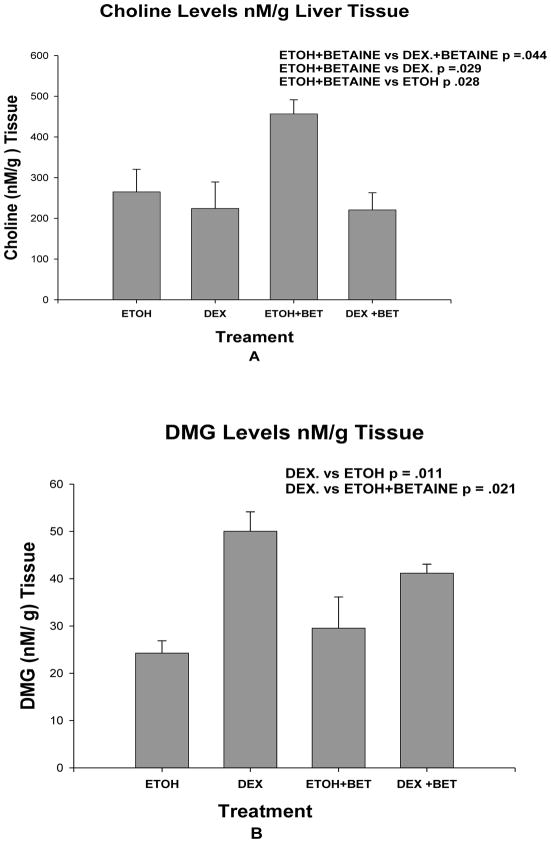
The liver tissue measurements of choline, and dimethylglycine (DMG) showed differences between groups. Choline levels were significantly increased in group 3 fed ethanol plus betaine compared with groups 1, 2 and 4 (Fig 5A). DMG levels were decreased in group 1 rats fed ethanol and rats fed ethanol and betaine compared with group 2 rats fed isocaloric dextrose (Fig 5B). Betaine levels were not significantly different among the different groups (results not shown).
Fig 5.
A. Liver tissue levels of choline were significantly increased in group 3 rats fed ethanol and betaine (Mean ±SEM, n=3). B. DMG levels in liver tissue were decreased in group 1 rats fed ethanol and group 3 rats fed ethanol plus betaine compared to group 2 rats fed isocaloric dextrose. (Mean ±SEM, n=3).
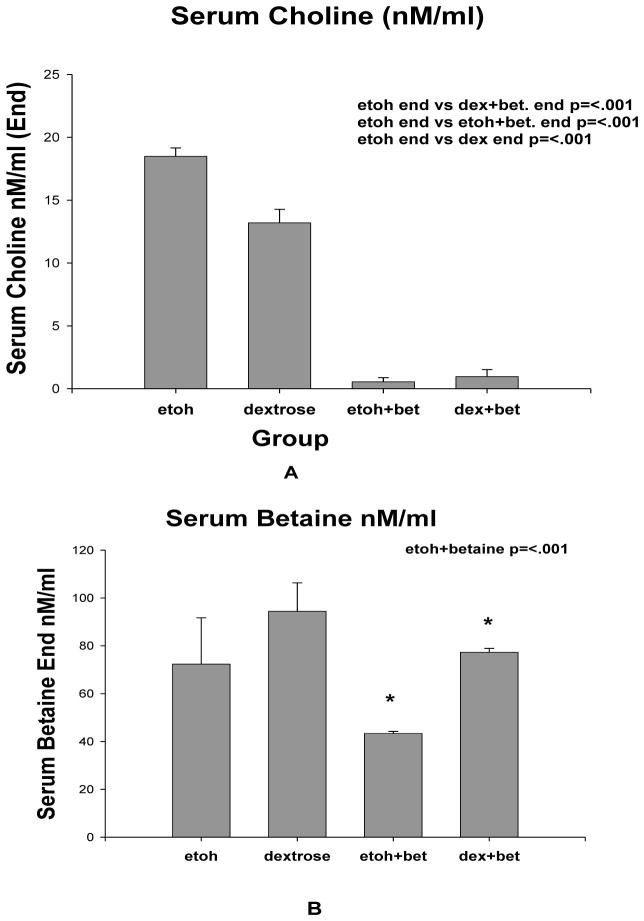
The level of choline, DMG and betaine were measured in the serum. Serum choline levels in group 1 rats fed ethanol were significantly higher than groups 2, 3 and 4. (Fig 6A). The serum levels of choline in groups 3 and 4 fed betaine with or without ethanol were markedly reduced by betaine. Ethanol plus betaine fed rats (group 3) had decreased betaine levels when compared to group 4 rats fed betaine with isocaloric dextrose (Fig 6B). Serum DMG levels were not different among the 4 rat groups (1–4 groups) (results not shown).
Fig 6.
A. Betaine with or without ethanol markedly reduced serum choline levels (Mean +SEM, n=3). B. The serum betaine levels were decreased by feeding ethanol and betaine (group 3) compared to isocaloric dextrose with betaine (group 4) (Mean ±SEM, n=3).
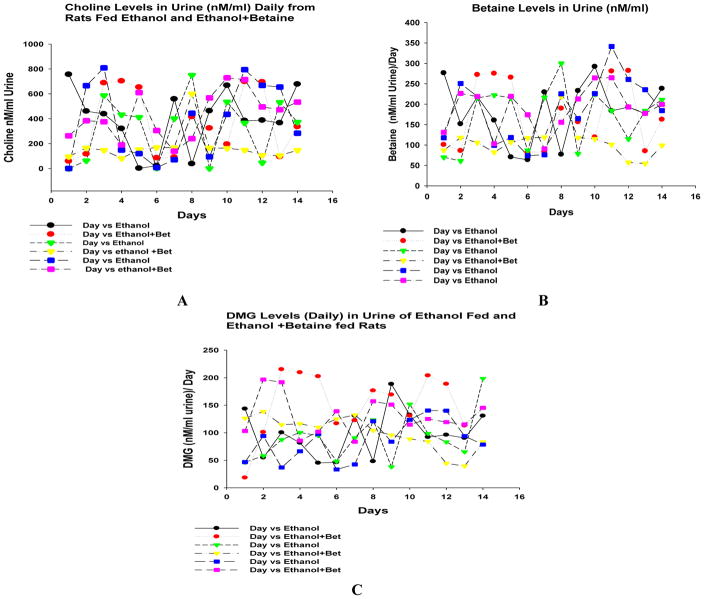
Urine DMG levels were measured over the last 14 days of ethanol feeding in groups 1 and 3 where ethanol was fed with or without betaine (Fig 7). The DMG levels were increased in the urine when ethanol was fed with betaine (group 3) compared with ethanol fed alone (group 1) (Fig 7). There were no significant differences in the levels of choline or betaine in the urine when the 4 groups of rats were compare (data not shown). Urinary choline, betaine and DMG levels varied from day to day during this final 14 day period. Each rat had a different pattern of changing daily levels (Fig 8A, B, C).
Fig 7.
The urine DMG levels were increased when group 3 alcohol plus betaine fed rats were compared to group 1 rats fed ethanol alone. (Mean ±SEM, n=3).
Fig 8.
A. Urine, choline (A), betaine (B) and DMG (C) levels varied daily up and down over the 14 day feeding period, for ethanol group 1, and ethanol plus betaine, group 3.
DISCUSSION
As predicted, betaine fed with ethanol prevented the BAL/UAL cycle caused by feeding alcohol alone. The mechanism of the prevention of the cycle by betaine may be the same as when SAMe was fed. SAMe is essential for the conversion of norepinephrine to epinephrine by PNMT (Fig 9). Epinephrine is 5 to 10 times more effective in increasing the metabolic rate than is norepinephrine. This increase in epinephrine prevents the rise of the BAL/UAL levels over 200 mg% because the increase in the metabolic rate generates NAD. Low levels of NAD would limit the rate of oxidation of ethanol by ADH. In the absence of SAMe or betaine feeding the rate of ethanol elimination is limited by the availability of NAD so that the UAL/BAL levels climb above 200 mg% to 400–500 mg% when ethanol is fed at a constant rate of 13 g/kg body wt/day. The levels of BAL peak at 500 mg% because catecholamine levels rise to a very high level at the peak (Li et al., 2003, 2004c). They then drive down the BAL to trough levels where the cycle finishes.
Fig 9.
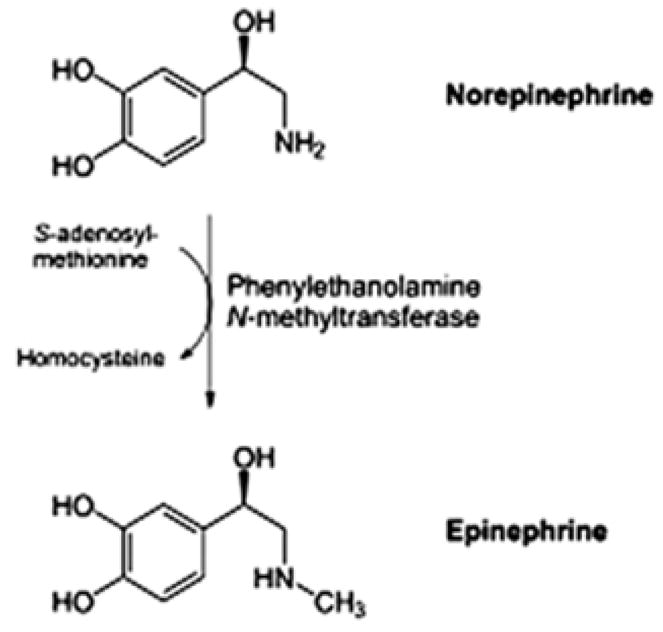
SAMe is required for the enzymatic conversion from norepinephrine to epinephrine.
When alpha or beta blockers (propranolol and phenoxybenzamine) are given, the BAL at the peaks continues upwards reaching fatal levels (Li et al., 2003). Mice fed ethanol and a high fat diet developed the UAL cycle (Powell et al., 2010). In mice the amplitude of the cycle was reduced and the ethanol elimination rate increased when a diet rich in methyl donors including betaine was fed with ethanol (Powell et al., 2010). Both betaine and SAMe increased the rate of the elimination of BAL and the BAL levels 3 h after an alcohol bolus (Bardag-Gorce et al., 2010a; Li et al., 2010b and Li et al., 2010c).
Betaine like SAMe, prevents liver injury when fed with ethanol. The reduction in steatosis was greater when betaine was used compared to SAMe (Bardag-Gorce et al., 2010b). Betaine, like SAMe prevented many of the changes in gene expression induced by ethanol feeding for 1 month (Bardag-Gorce et al., 2010b). The expressions of many genes were up regulated by ethanol feeding and the up regulation was prevented by betaine fed with ethanol as found in the present study. A few genes listed in Table 1 are noted here: Gadd 45b, Tir 2, Tir 4, Tnfrst 16, Jak 3 and Pdcd4. Perhaps the most important genes that were up regulated, which would induce the inflammation observed in the ethanol fed rats (group 1) and were not up regulated when betaine was fed, were the expressions of Tlr2 and 4. Shi reported that betaine inhibited Tlr4 expression induced by ethanol feeding (Shi et al., 2010). In mice fed ethanol and methyl donors, PPARα was up regulated, which could explain the prevention of steatosis observed (Powell et al., 2010). We did not observe an increase in PPARα. Betaine feeding corrected defective VLDL secretion induced by ethanol (Kharbanda et al., 2009) which helped preventing steatosis from developing by removing lipid from liver cells. The prevention by betaine feeding of both steatosis and inflammation observed in the ethanol fed rats could be the result of the prevention of oxidative stress by Betaine. This was shown in ethanol treated HepG2 cells where Betaine completely prevented oxidative stress in vitro (Oliva et al., 2011).
Little is known regarding the metabolism of betaine, choline and DMG intermediates when ethanol and betaine are fed (Holm et al., 2003). We found that liver choline levels were increased by ethanol plus betaine (group 3), suggesting that the oxidation of choline to betaine, an NAD consuming metabolic reaction, was curtailed in a high betaine, high alcohol environment. Although liver betaine was increased by ethanol plus betaine (group 3), concentrations of hepatic dimethylglycine, the metabolite produced when betaine is used as a methyl donor, were decreased by feeding ethanol plus betaine (group 3) and by ethanol alone (group 1). This could be due to an increased conversion of DMG to glycine in rats fed ethanol (DMG→sarcosine→glycine). Glycine decreases the blood alcohol levels in rats fed ethanol intragastrically by accelerating the first pass metabolism in the stomach (Limuro et al, 1996) and diminishes liver steatosis and circulating ALT levels (Limuro et al, 1996). This observation may not be valid because isolated liver perfusion was used to assess the ethanol elimination rate. The perfused liver would not be subject to changes in blood catecholamines, which causes the change in the metabolism of ethanol in vivo.
Alcohol and/or betaine administration also modified the concentration of circulating free choline and betaine.
Research Highlights.
Betaine fed with ethanol:
Prevented the BAL Cycle by converting norepinephrine to epinephrine
Reduced the BAL by increasing the metabolic rate
Prevented the up regulation of TLR4/2 by ethanol, by donating methyl groups to histones which silence genes
Acknowledgments
The authors thank Adriana Flores for typing the manuscript. This study was supported by the grant NIH/NIAAA 8116 and the morphology core of P50-11999 Alcohol Center Grant on the liver and pancreas.
Abbreviations
- ETOH, Et, etoh
Ethanol
- BET, bet
Betaine
- Dex, dex
Dextrose
- BAL
Blood alcohol level
- UAL
Urinary alcohol level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barak AJ, Beckenhauer HC, Junnia M, Tuma DJ. Dietary betaine promotes generation of hepatitic adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcoholism Clin Exp Res. 1993;17:552–555. doi: 10.1111/j.1530-0277.1993.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, French BA, Dedes J, Li J, French SW. Gene expression patterns of the liver in response to alcohol: in vivo and in vitro models compared. Exp Molec Pathol. 2006;80:241–251. doi: 10.1016/j.yexmp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, French BA, Li J, Riley NE, Yuan QX, Reitz R, Cai Y, Wan YJY, French SW. The importance of cycling of blood alcohol levels in the pathogenesis of experimental alcoholic liver disease in rats fed ethanol intragastrically. Gastroenterology. 2002;123:325–335. doi: 10.1053/gast.2002.34177. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Oliva J, Wong W, Fong S, Li J, French BA, French SW. S-adenosylmethionine decreases the peak blood alcohol levels 3 h after an acute bolus of ethanol by inducing alcohol metabolizing enzymes in the liver. Exp Mol Pathol. 2010a;89:217–21. doi: 10.1016/j.yexmp.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Li J, Oliva J, Lu SC, French BA, French SW. The cyclic pattern of blood alcohol levels during continuous ethanol feeding in rats. The effect of feeding S-adenosylmethionine. Exp Mol Pathol. 2010b;88:380–387. doi: 10.1016/j.yexmp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW, Benson NC, Sun PS. Centrilobular liver necrosis induced by hypoxia in chronic ethanol-fed rats. Hepatology. 1984;4:912–917. doi: 10.1002/hep.1840040521. [DOI] [PubMed] [Google Scholar]
- Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem Feb. 2003;49(2):286–94. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, Ward BW, Cannella JJ, III, Tuma DJ. Betaine administration corrects ethanol induced defective VLDL secretion. Mol Cell Biochem. 2009;327:75–78. doi: 10.1007/s11010-009-0044-2. [DOI] [PubMed] [Google Scholar]
- Landsberg L, Saville ME, Young JB. Sympathoadrenal system and regulation of thermogenesis. A J Physiol 247 (Endocrine) Metab. 1984;10:E181–E189. doi: 10.1152/ajpendo.1984.247.2.E181. [DOI] [PubMed] [Google Scholar]
- Li J, Bardag-Gorce F, French BA, Dedes J, French SW. The effect of propranolol on gene expression during blood alcohol cycle of rats fed ethanol intragastrically. Exp Mol Pathol. 2010a;88:32–37. doi: 10.1016/j.yexmp.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bardag-Gorce F, Oliva J, Dedes J, French BA, French SW. Gene expression modifications in the liver caused by binge drinking and S-adenosylmethionine feeding. The role of epigenetic changes. Genes Nutr. 2010b;5:169–179. doi: 10.1007/s12263-009-0158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bardag-Gorce F, Oliva J, French BA, Dedes J, French SW. The effect of betaine treatment on rats fed an acute bolus of ethanol at 3 h and 12 h post bolus: a microarray analysis. Genes Nutr. 2010c;5:321–324. doi: 10.1007/s12263-010-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, French BA, Fu P, Bardag-Gorce F, French SW. Mechanism of alcohol cyclic pattern. Role of catecholamines. Am J Physiol Gastrointestinal Liver Physiol. 2003;285:G442–G448. doi: 10.1152/ajpgi.00093.2003. [DOI] [PubMed] [Google Scholar]
- Li J, French BA, Fu P, Bardag-Gorce F, French SW. Catecholamines are involved in the mechanism of the urinary alcohol level cycle in rats fed ethanol intragastrically at a constant rate. Life Sci. 2004c;75:3043–3051. doi: 10.1016/j.lfs.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Li J, French BA, Fu P, French SW. Liver necrosis induced by thyroid hormone administration in rats fed ethanol. Exp Molec Pathol. 2001;71:79–88. doi: 10.1006/exmp.2001.2381. [DOI] [PubMed] [Google Scholar]
- Li J, French BA, Nan L, Fu P, French SW. Uncoupling of oxidative phosphorylation prevents the urinary alcohol level cycling caused by feeding ethanol continuously at a constant rate. Exp Mol Pathol. 2005;78:228–232. doi: 10.1016/j.yexmp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Li J, French B, Wu Y, Vankatesh R, Montgomery R, Bardag-Gorce F, Kitto J, French SW. Liver hypoxia and lack of recovery after reperfusion at high blood alcohol levels in the intragastric feeding model of alcohol liver disease. Exp Mol Pathol. 2004a;77:184–192. doi: 10.1016/j.yexmp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Li J, Fu P, French BA, French SW. The effect of rotenone on the urinary ethanol cycle in rats fed ethanol intragastrically. Exp Mol Path. 2004b;77:210–213. doi: 10.1016/j.yexmp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Li J, Nguyen V, French BA, Parlow AF, Su GL, Fu P, Yuan QX, French SW. Mechanism of the cyclic pattern of urinary ethanol levels in rats fed ethanol. The role of the hypothalamic-pituitary-thyroid axis. Am J Physiol. 2000;279:G118–125. doi: 10.1152/ajpgi.2000.279.1.G118. [DOI] [PubMed] [Google Scholar]
- Limuro Y, Bradford BU, Forman DT, Thurman RG. Glycine prevents alcohol-induced liver injury by decreasing alcohol in the rat stomach. Gastroenterology. 1996;110:1536–42. doi: 10.1053/gast.1996.v110.pm8613061. [DOI] [PubMed] [Google Scholar]
- Powell CL, Bradford BU, Craig CP, Tsuchiya M, Uehara T, O’Connell TM, Pogribny CP, Melny KS, Koop DR, Bleyle L, Threadgill DW, Rusyn I. Mechanism for prevention of alcohol-induced liver injury by dietary methyl donors. Toxicology Sci. 2010;115:131–139. doi: 10.1093/toxsci/kfq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, Bardag-Gorce F, Tillman B, French SW. Protective effects of quercetin, EGCG, catechin and betaine against oxidative stress induced by ethanol in vitro. Exp Molec Pathol. 2011;90:295–299. doi: 10.1016/j.yexmp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schola R, Schwabe U. In: Stimulation of ethanol metabolism by catecholamine in alcohol and aldehyde metabolising systems. Thurman RG, editor. New York: Plenum; 1980. pp. 601–618. [Google Scholar]
- Shi QZ, Wang LW, Zhang W, Gong ZJ. Betaine inhibits Toll-like receptor 4 expression in rats with ethanol-induced liver injury. World J Gastroenterol. 2010;16:897–903. doi: 10.3748/wjg.v16.i7.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staten MA, Mathews DE, Cryer PE, Bier DM. Epinephrine’s effect on metabolic rate is independent of changes in plasma insulin or glucagon. Am J Physiol. 1989;257 doi: 10.1152/ajpendo.1989.257.2.E185. [DOI] [PubMed] [Google Scholar]; Edcrine Metab. 20:E185–E192. [Google Scholar]
- Tsukamoto H, French SW, Reidelberger RD, Largman C. Cyclic pattern of blood alcohol levels during continuous intragastric ethanol infusion in rats. Alcohol Clin Exp Res. 1985;9:31–37. doi: 10.1111/j.1530-0277.1985.tb05046.x. [DOI] [PubMed] [Google Scholar]
- Wong DL, Lesage A, Siddall B, Funder JW. Glucocorticoid regulation of phenylethanolamine N-methyltransferase in vivo. FASEB J. 1992;6:3310–3315. doi: 10.1096/fasebj.6.14.1426768. [DOI] [PubMed] [Google Scholar]