Abstract
Celecoxib is a COX2 inhibitor that reduces the risk of colon cancer. However, the basis for its cancer chemopreventive activity is not fully understood. In this study, we defined a mechanism of celecoxib action based on degradation of c-FLIP, a major regulator of the death receptor pathway of apoptosis. c-FLIP protein levels are regulated by ubiquitination and proteasome-mediated degradation. We found that celecoxib controlled c-FLIP ubiquitination through Akt-independent inhibition of GSK3 kinase, itself a candidate therapeutic target of interest in colon cancer. Celecoxib increased the levels of phosphorylated GSK3 (p-GSK3), including the α and β forms, even in cell lines where p-Akt levels were not increased. PI3K inhibitors abrogated Akt phosphorylation as expected but had no effect on celecoxib-induced GSK3 phosphorylation. In contrast, PKC inhibitors abolished celecoxib-induced GSK3 phosphorylation, implying that celecoxib influenced GSK3 phosphorylation through a mechanism relied upon PKC but not Akt. GSK3 blockade either by siRNA or kinase inhibitors was sufficient to attenuate c-FLIP levels. Combining celecoxib with GSK3 inhibition enhanced attenuation of c-FLIP and increased apoptosis. Proteasome inhibitor MG132 reversed the effects of GSK3 inhibition and increased c-FLIP ubiquitination, confirming that c-FLIP attenuation was mediated by proteasomal turnover as expected. Our findings reveal a novel mechanism through which the regulatory effects of c-FLIP on death receptor signaling are controlled by GSK3, which celecoxib acts at an upstream level to control independently of Akt.
Keywords: Celecoxib, GSK3, c-FLIP, Akt, degradation
Introduction
The cellular FLICE-inhibitory protein (c-FLIP) is the major inhibitor of the extrinsic apoptotic pathway through inhibition of caspase-8 activation (1). c-FLIP has multiple splice variants, and two main forms have been well characterized: c-FLIP short form (c-FLIPS) and long form (c-FLIPL) (1). Generally speaking, elevated c-FLIP expression protects cells from death receptor-mediated apoptosis, whereas downregulation of c-FLIP by chemicals or small interfering RNA (siRNA) augments death receptor-mediated apoptosis (1). Moreover, overexpression of c-FLIP also protects cells from apoptosis induced by certain cancer therapeutic agents such as etoposide and cisplatin (2–4).
c-FLIP is known to be subjected to rapid turnover, regulated by an ubiquitin-proteasome mechanism (5, 6). Certain cancer therapeutic agents stimulate downregulation of c-FLIP expression through this mechanism (5). However, the mechanism underlying drug-induced c-FLIP degradation is unclear. A recent study has demonstrated that c-Jun N-terminal kinase (JNK)-mediated activation of the E3 ubiquitin ligase Itch specifically ubiquitinates c-FLIPL and induces its proteasomal degradation (7).
Glycogen synthase kinase-3 (GSK3) is a ubiquitous serine/threonine kinase that is present in mammals in two isoforms: α and β (8, 9). GSK3 was initially identified as an enzyme involved in the regulation of glycogen metabolism. Increasing evidence during the past decades indicates that GSK3 has a key role in regulating a diverse range of cellular functions including cell survival and death (8, 9). Thus, GSK3 inhibition has been considered an attractive therapeutic strategy for certain diseases such as diabetes, neurodegenerative diseases and mental disorders (10, 11). It has been documented that GSK3 exerts opposing apoptosis-regulating effects: it inhibits the death receptor-mediated extrinsic apoptotic pathway, while promoting cell death caused by the mitochondrial intrinsic apoptotic pathway (8). Inhibition of GSK3 with either small molecule inhibitors or siRNA sensitizes cancer cells to TRAIL- or agonistic death receptor 5 (DR5) antibody-induced apoptosis (12–14). However, it is largely unclear how inhibition of GSK3 enhances death receptor-induced apoptosis (8). Recently, a study demonstrated that GSK3 is involved in forming an antiapoptotic protein complex with DDX3 and cIAP-1, leading to inhibition of apoptotic signaling by preventing formation of the death-inducing signaling complex and caspase-8 activation (14). However, linkage between GSK3 and c-FLIP regulation has not been suggested.
Celecoxib, a marketed anti-inflammatory and anti-pain drug, is being tested in clinical trials for its chemopreventive and therapeutic effects against a broad spectrum of epithelial malignancies either as a single agent or in combination with other agents. The antitumor activity of celecoxib is thought to be associated with its ability to induce apoptosis in a variety of cancer cells (15). The molecular mechanisms underlying celecoxib-mediated apoptosis have not been fully elucidated, although it appears to be associated with inactivation of PDK1/Akt, induction of endoplasmic reticulum stress involving upregulation of CHOP/GADD153 and increase in Ca2+ levels, or downregulation of the anti-apoptotic protein survivin (16). Our previous results have shown that celecoxib induces apoptosis in non-small cell lung cancer (NSCLC) cell lines involving the activation of the extrinsic death receptor pathway through both DR5 induction and c-FLIP downregulation (17, 18).
We have shown that celecoxib downregulates c-FLIP through facilitating ubiquitin/proteasome-dependent protein degradation (18). However, the signaling process leading to celecoxib-induced c-FLIP degradation is unknown. In an effort to demonstrate the mechanism underlying celecoxib-induced c-FLIP degradation, we have revealed a novel mechanism of c-FLIP degradation through GSK3 inhibition. To the best of our knowledge, this is the first study demonstrating a linkage between GSK3 inhibition and c-FLIP downregulation, thus highlighting a new mechanism by which GSK3 modulates the extrinsic apoptotic pathway.
Materials and Methods
Reagents
Celecoxib, dimethy-celecoxib (DMC) and antibodies against caspases and DR5 were the same as described previously (17, 19). Human recombinant TRAIL was purchased from PeproTech, Inc. Rapamycin and LY294002 were purchased from LKT Laboratories, Inc. Wortmannin and Rö-31-8220 were purchased from Biomol. MG132, LiCl, SB216763 and SB415286 were purchased from Sigma Chemicals. Gö6983, Gö6979, GF109203X and rottlerin were purchased from EMD Calbiochem. Rabbit polyclonal antibodies against p-Akt (S473), p-GSK3β (S9), p-GSK3α/β (S21/S9), and p-S6 (S235/S236) were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Rabbit polyclonal antibodies against GSK3α/β and p-FOXO3α (T32) were purchased from Upstate. Mouse monoclonal anti-FLIP antibody (NF6) was purchased from Alexis Biochemicals. Rabbit polyclonal anti-actin antibody was purchased from Sigma Chemicals. Wild-type (WT), constitutively active (CA; R85A) and kinase-dead (KD; B9A) human GSK3β in pCMV-Tag 5A expression vector were generously provided by Dr. B. P. Zhou (University of Kentucky School of Medicine, Lexington, KY).
Cell Lines and Cell Culture
The human NSCLC cell lines used in this study were provided by Dr. R. Lotan (M.D. Anderson Cancer Center, Houston, TX) in 2003 and cultured as previously described (17). H157 and A549 cell lines were recently authenticated by Genetica DNA Laboratories, Inc. (Cincinnati, OH) by analysis of the STR DNA profile. The other cell lines used have not been authenticated. The stable H157-Lac Z-5, H157-FLIPL-21 and H157-FLIPS-1 transfectants were described previously (20). Through the entire study, the concentrations of DMSO (as a solvent control) did not exceed 0.05%.
Western Blot Analysis
Whole-cell protein lysates were prepared and analyzed by Western blotting as described previously (17, 21).
Cell Survival Assay
Cells were seeded in 96-well cell culture plates and treated the next day with the agents indicated. The viable cell number was determined using the sulforhodamine B (SRB) assay, as previously described (22).
Detection of Apoptosis
Apoptosis was evaluated by Annexin V staining using Annexin V-PE apoptosis detection kit purchased from BD Biosciences or by measuring cytoplasmic histone-associated DNA fragments using a Cell Death Detection ELISAPlus kit following the manufacturer’s instructions. We also detected caspase activation by Western blotting as an additional indicator of apoptosis.
Immunoprecipitation for Detection of Ubiqutinated c-FLIP
H157-FLIPL-21 cells, which stably express FLIPL, were transfected with HA-ubiquitin plasmid using the FuGENE 6 transfection reagent (Roche Molecular Biochemicals) following the manufacturer’s instruction. After 24 h, the cells were treated with tested agent or MG132 plus the tested agent for 4 h and then were lysed for immunoprecipitation of Flag-FLIPL using Flag M2 monoclonal antibody (Sigma Chemicals) as previously described (23), followed by the detection of ubiquitinated FLIPL with Western blotting using anti-HA antibody from Abgent.
siRNA-mediated Gene Silencing
GSK-3α, GSK-3β #1 and GSK-3β #2 siRNAs, which target the sequences 5’-AAGTGATTGGCAATGGCTCAT-3’, 5’-AAGAATCGAGAGCTCCAGATC-3’, and 5’-AAGTAATCCACCTCTGGCTAC-3’ (12), respectively, were ordered from Qiagen. Itch #1 and Itch #2 siRNAs, which target the sequences 5’-AAGTGCTTCTCAGAATGATGA-3’ and 5’-AACCACAACACACGAATTACA-3’, respectively, were also ordered from Qiagen. The non-silencing control siRNA duplexes were described previously (17). Transfection of these siRNA duplexes was conducted in 6-well plates using the HiPerFect transfection reagent (Qiagen) following the manufacturer’s manual. Gene-silencing effects were evaluated by Western blot analysis.
RT-PCR for Detection of c-FLIP mRNA
Total cellular RNA was extracted from a given cell line with TRI-Reagent (Sigma Chemicals) and reverse transcribed into cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) following the manufacturer’s instructions. The cDNA was then amplified by PCR using the following primers: FLIP sense 5’-ACAGAGTGAGGCGATTTGAC-3’, FLIP antisense 5’-GAACAGACTGCTTGTACTTCT-3’; actin sense 5’-GGCACCCAGCACAATGAAGATCAA-3’, and actin antisense 5’-ACTCGTCATACTCCTGCTTGCTGA-3’. The 25-µL amplification mixture contained 1 µL of the cDNA, 0.5 µL of deoxynucleotide triphosphate (25 mM each), 1 µL each of the sense and antisense primers (20 µM each), 5 µL of TaqMaster PCR enhancer, 1 µL of Taq DNA polymerase (5 units/µL; Eppendorf), 2.5 µL 10 × reaction buffer, and sterile H2O. PCR was performed for 35 cycles. After an initial step at 95°C for 3 minutes, each cycle consisted of 1 minute of denaturation at 94°C, 1 minute of annealing at 55°C, and 1 minute of extension at 72°C. This was followed by an additional extension step at 72°C for 7 minutes. The housekeeping gene actin was also amplified as an internal reference. PCR products were resolved by electrophoresis on a 1.5% agarose gel, stained, and directly visualized under UV illumination.
Statistical Analysis
The statistical significances among treatment groups were analyzed with one-way analysis of variance (ANOVA) by use of Graphpad InStat 3 software (GraphPad Software; La Jolla, CA). Results were considered to be statistically significant at P < 0.05.
Results
Celecoxib Increases Akt and GSK3 Phosphorylation in Human NSCLC Cells
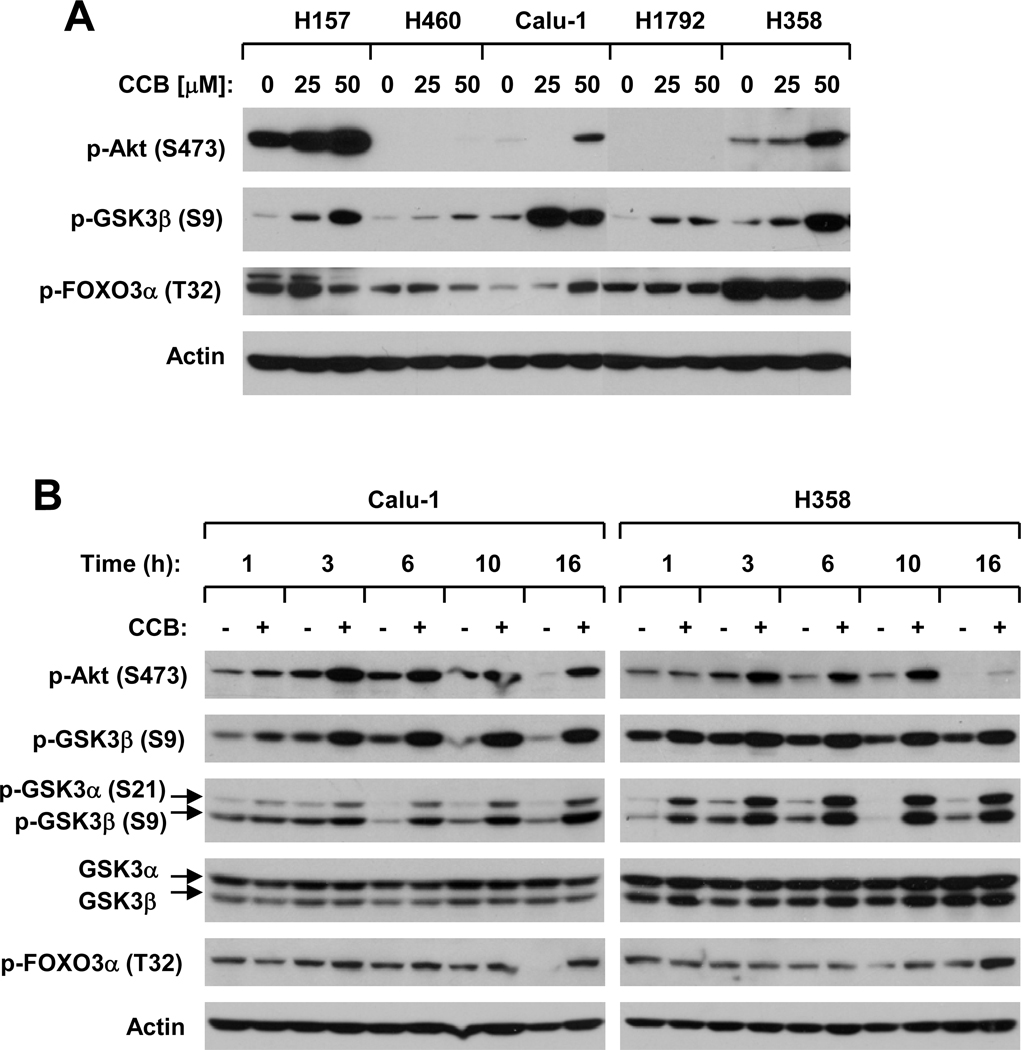
It has been suggested that PI3K/Akt signaling positively regulates c-FLIP expression in tumor cells (24, 25). Given that celecoxib was shown in some studies to inhibit PDK1/Akt signaling in certain types of cancer cells such as prostate cancer cells (26–29), we questioned whether there is a link between celecoxib-induced c-FLIP downregulation and Akt inhibition. To this end, we first determined whether celecoxib affects Akt phosphorylation in a panel of human NSCLC cell lines. In our cell systems, we did not find that celecoxib inhibited Akt phosphorylation in any tested NSCLC cell lines. Instead, we detected increased levels of p-Akt in some cell lines exposed to celecoxib (e.g., H157, Calu1 and H358) (Fig. 1A). In some cell lines such as H1792, we did not detect either basal levels or increased levels of p-Akt when treated with celecoxib (Fig. 1A). Furthermore, we examined the effects of celecoxib on the phosphorylation of two well-known Akt substrates, GSK3β and FOXO3α. As presented in Fig. 1A, celecoxib weakly increased p-FOXO1α levels in only one of 5 cell lines (i.e., Calu-1), whereas it increased p-GSK3β levels in all the tested cell lines. Through detailed time-course analysis, we found that the observed increase in p-Akt levels occurred at 3 h post celecoxib treatment and was sustained to 16 h in both Calu-1 and H358 cell lines. Accordingly, p-FOXO1α levels were weakly increased after 3 h in Calu-1 cells and after 10 h in H358 cells post exposure to celecoxib. In Calu-1 cells, celecoxib increased the levels of p-GSK3β or α/β in a fashion similar to the p-Akt increase; however, in H358 cells, celecoxib increased p-GSK3 levels even at 1 h post treatment (Fig. 1B). Thus, these data clearly indicate that celecoxib exerts more pronounced effects on increasing the phosphorylation of GSK3 (both α and β) than on Akt in human NSCLC cells.
Fig. 1. Celecoxib increases Akt and GSK3 phosphorylation in human NSCLC cells.
The indicated cell lines were treated with the given concentrations of celecoxib (CCB) for 6 h (A) or with 50 µM celecoxib for the given times (B). The cells were then subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis for the indicated proteins.
DMC is a celecoxib derivative lacking COX-2-inhibitory activity (30). It possesses more potent effects than celecoxib on induction of apoptosis, downregulation of c-FLIP and enhancement of TRAIL-induced apoptosis (19). DMC even at 15 µM increased the levels of p-GSK3β in H157, H460 and Calu-1 cells, whereas it increased p-Akt levels only at 30 µM in these cell lines (supplemental Fig. S1A).
Celecoxib Increases GSK3 Phosphorylation Independent of Akt and mTOR/p70S6K Signaling
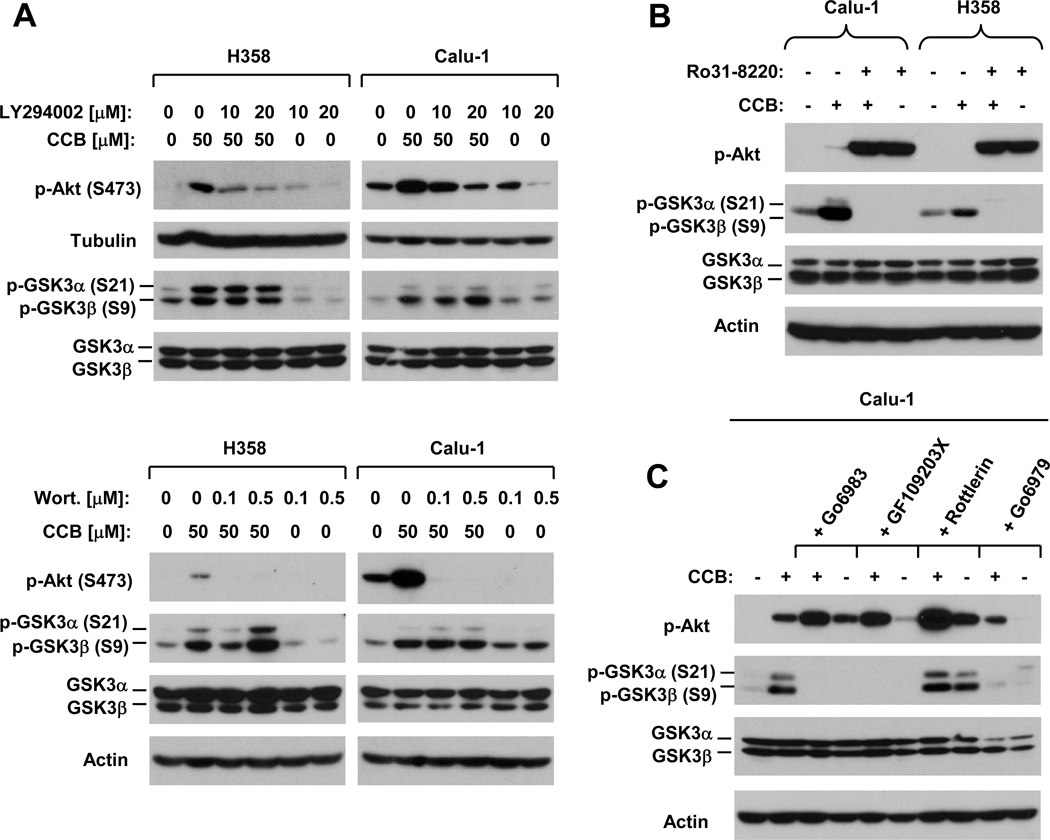
It is well-known that Akt phosphorylates GSK3 resulting in its inactivation (31). To demonstrate whether the celecoxib-induced increase in GSK3 phosphorylation is due to an increase in Akt phosphorylation, we compared the effects of celecoxib on GSK3 phosphorylation in the absence and presence of the PI3K inhibitor LY294002 or wortmannin. Both LY294002 and wortmannin abrogated celecoxib-induced Akt phosphorylation, but failed to prevent the increase in GSK3 phosphorylation (Fig. 2A). Similarly, LY294002 blocked DMC-induced Akt phosphorylation, but failed to affect DMC-induced increase in p-GSK3β (Fig. S1B). These results indicate that celecoxib and DMC increase GSK3 phosphorylation independent of Akt.
Fig. 2. Celecoxib induces PKC-mediated GSK3 phosphorylation (B and C) independent of Akt (A).
A, The indicated cell lines were exposed to the given treatments as indicated for 6 h. LY294002 or wortmannin (Wort.) was pre-incubated with the cells for 30 min before addition of celecoxib (CCB). B, The indicated cell lines were pre-treated with 2 µM Rö31-8220 for 30 min and then co-treated with 50 µM celecoxib for 6 h. C, Calu-1 cells were pre-treated with 20 µM Gö6983, 2 µM GF109203X, 20 µM Rottlerin or 0.5 µM Gö6979 for 30 min and then co-treated with 50 µM celecoxib for 6 h. After the aforementioned treatments, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis for the indicated proteins.
It has been suggested that p70S6K also regulates or phosphorylates GSK3 under certain conditions (32, 33). Thus, we next asked whether this mechanism is involved in mediating celecoxib-induced GSK3 phosphorylation. To this end, we treated two NSCLC cell lines with celecoxib in the absence and presence of the mTOR inhibitor rapamycin, which is known to shut down mTOR/p70S6K signaling (34), and detected p-GSK3 and p-S6 (a readout of p70S6K activity) levels. As shown in supplemental Fig. S2, rapamycin abolished basal levels of p-S6 despite no increase in p-S6 levels by celecoxib, indicating the successful inhibition of p70S6K activity. However, rapamycin did not affect celecoxib-induced GSK3 phosphorylation at all. These results suggest that celecoxib also induces GSK3 phosphorylation independent of mTOR/p70S6K. We noted that rapamycin alone strongly increased p-Akt levels in both cell lines, as we previously reported (35); however, it either did not increase p-GSK3β levels (i.e., Calu-1) or induced a weaker p-GSK3β elevation than celecoxib (i.e., H358) (Fig. S2).
Celecoxib Induces Protein Kinase C (PKC)-dependent GSK3 Phosphorylation
PKC has been documented to phosphorylate GSK3 (36–41). Thus, we next determined whether PKC is involved in mediating GSK3 phosphorylation by celecoxib. As presented in Fig. 2B, the presence of the pan PKC inhibitor Rö31-8220 abolished celecoxib’s ability to increase GSK3 phosphorylation in both Calu-1 and H358 cells. Moreover, we examined the effects of other PKC inhibitors on celecoxib-induced GSK3 phosphorylation and found that another pan PKC inhibitor GF1092303X, the PKC α and β inhibitor Gö9679 and the PKC α–ξ inhibitor Gö6983 were also able to abolish celecoxib-induced GSK3 phosphorylation. In contrast, the PKC δ inhibitor Rottlerin did not inhibit celecoxib-induced GSK3 phosphorylation (Fig. 2C). Collectively, these results clearly suggest that celecoxib induces GSK3 phosphorylation through a PKC-mediated mechanism, likely involving PKC α and β. We also examined p-Akt levels in cells exposed to these treatments and found that the presence of these PKC inhibitors except for Gö6976 actually exerted enhanced effects on Akt phosphorylation (Figs. 2B and 2C). This result further supports that celecoxib-induced GSK3 phosphorylation is separated from the increase in Akt phosphorylation.
Inhibition of GSK3 Enhances the Ability of Celecoxib to Downregulate c-FLIP
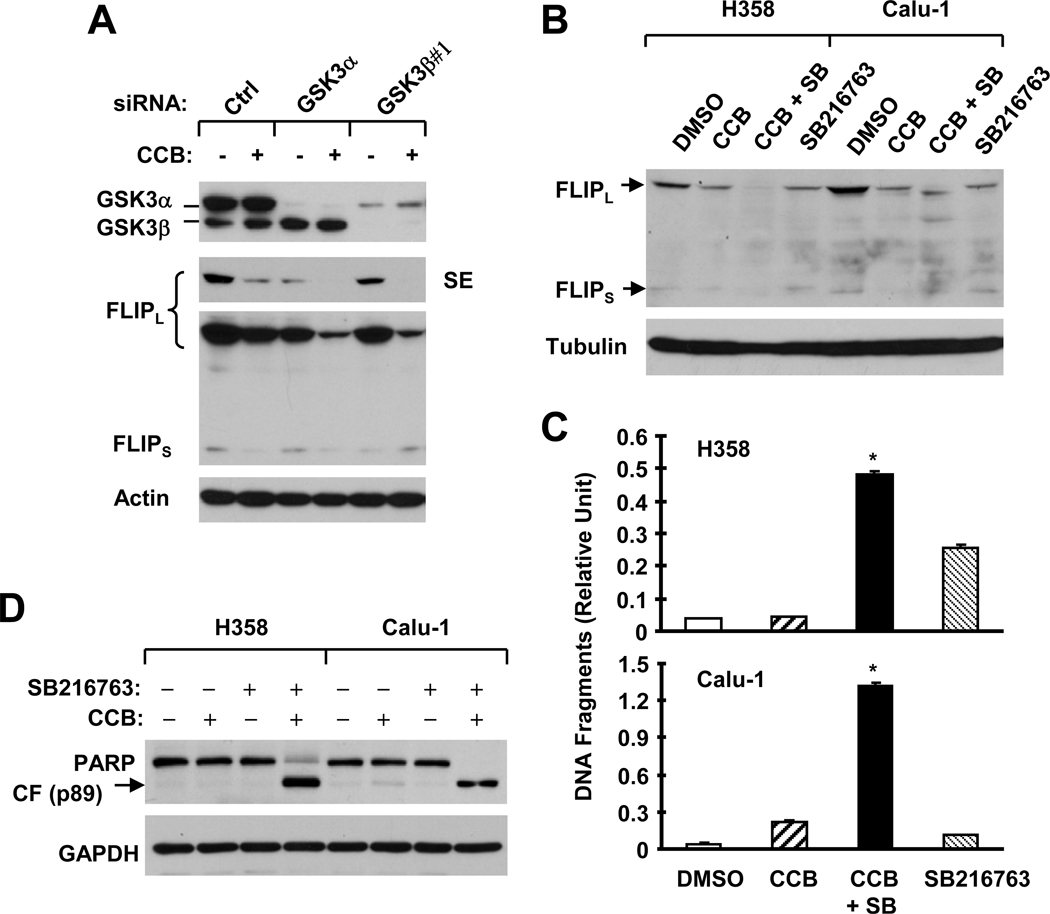
To determine the impact of GSK3 phosphorylation on celecoxib-induced c-FLIP downregulation, we used GSK3 siRNAs to knock down GSK3α and GSK3β, respectively, and then examined their effects on celecoxib-induced c-FLIP reduction. In H358 cells, GSK3α siRNA reduced the levels of GSK3α only, whereas GSK3β siRNA (#1) reduced the levels of not only GSK3β, but also GSK3α (Fig. 3A). Silencing of GSK3 with both GSK3α and GSK3β siRNAs reduced basal levels of FLIPL, suggesting that GSK3 regulates c-FLIP. Treatment of these cells, particularly GSK3α siRNA- or GSK3β #1 siRNA-transfected cells, with celecoxib resulted in further reduction of FLIPL levels, which was lower than in cells treated with celecoxib alone or GSK3 siRNA transfection alone (Fig. 3A). These results indicate that silencing of GSK3 enhances celecoxib’s effect on downregulation of c-FLIP (i.e., FLIPL).
Fig. 3. The combination of celecoxib with a GSK3 siRNA (A) or a small molecule GSK3 inhibitor (B–D) exerts enhanced effects on downregulation of c-FLIP (A and B) and apoptosis (C and D).
A, H358 cells were transfected twice with the given siRNAs in a 48 h interval. Twenty-four hours after the second transfection, the cells were treated with 50 µM celecoxib (CCB) for 8 h (A) and then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. Ctrl, control. B–D, The indicated cell lines were treated with 50 µM celecoxib alone, 20 µM SB216763 (SB) alone or their combination. After 8 h (B) or 24 h (C and D), the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis (B and D) or for analysis of apoptosis using the Cell Death Detection ELISA kit (C). Columns, means of triplicate determinations; Bars, ± SDs. *, P < 0.001 compared with DMSO, celecoxib or SB216763 with ANOVA test.
We further examined the effects of celecoxib combined with a GSK3 inhibitor on c-FLIP downregulation. Both celecoxib and SB216763 alone decreased the levels of c-FLIP; however, the combination of celecoxib and SB216763 was even more potent than either agent alone in decreasing c-FLIP levels (Fig. 3B). Moreover, the combination of celecoxib with SB216763 was also much more effective than either celecoxib or SB216763 alone in increasing DNA fragmentation (Fig. 3C) and in inducing PARP cleavage (Fig. 3D). For example, the mean arbitrary units for DNA fragments induced by celecoxib, SB216763 and their combination were 0.224, 0.115 and 1.320, respectively, in comparison with 0.045 in control cells treated with DMSO. Thus, it is clear that the combination of celecoxib and SB216763 increases DNA fragmentation, to a greater level than the sum of that caused by celecoxib or SB216763 alone, suggesting that celecoxib combined with a GSK3 inhibitor results in more than additive (i.e., synergistic) apoptosis-inducing effects in human NSCLC cells.
Modulation of GSK3 Activity Alters c-FLIP Levels
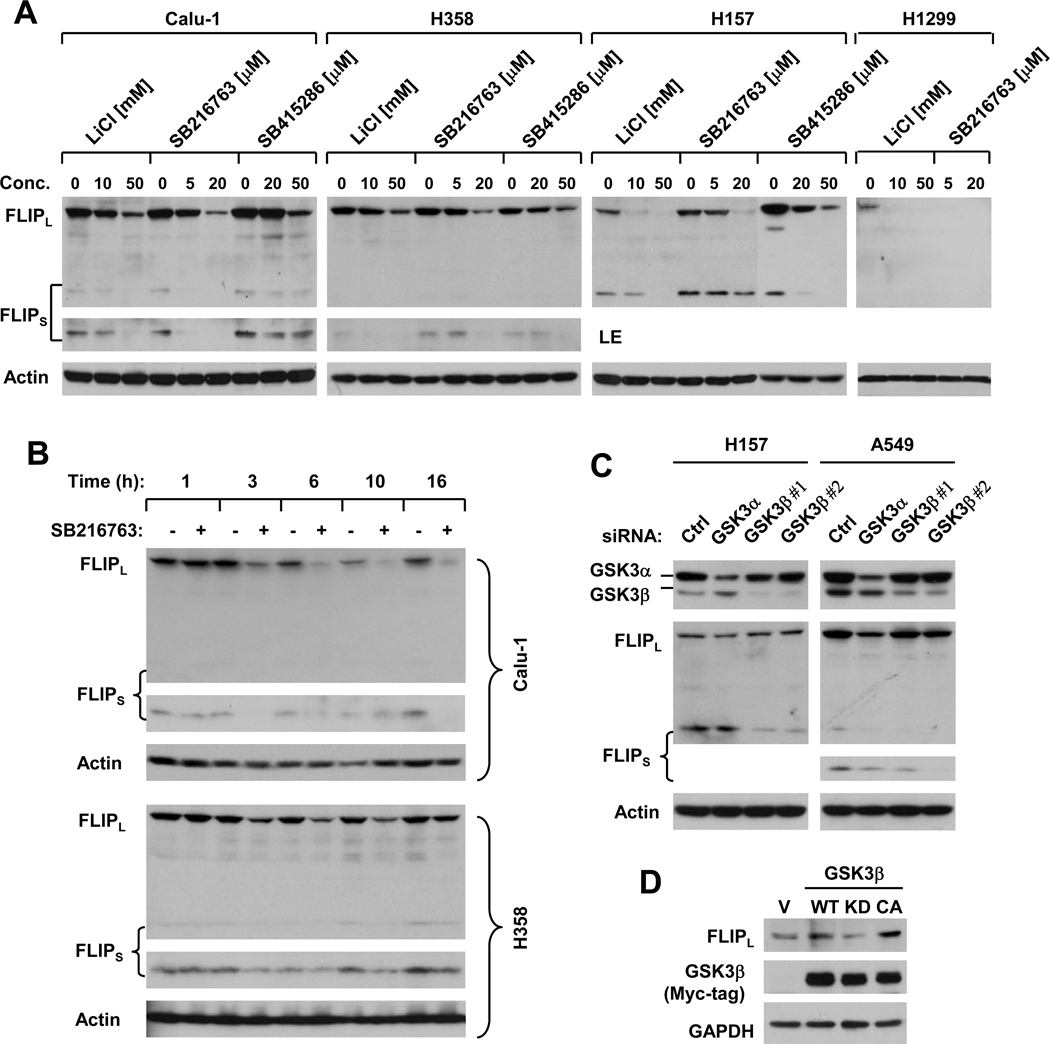
The above data on reduction of c-FLIP by GSK3 inhibition (Fig. 3) suggest that GSK3 positively regulates c-FLIP levels. Thus, we performed more detailed experiments to validate this finding. To this end, we first treated four human NSCLC cell lines with different pharmacological GSK3 inhibitors including LiCl, SB216763 and SB415286 and then detected c-FLIP levels in cells exposed to these treatments. As shown in Fig. 4A, all three GSK3 inhibitors exerted dose-dependent effects on reducing the levels of c-FLIP including FLIPL and FLIPS. Reduction of c-FLIP by GSK3 inhibition with a GSK3 inhibitor such as SB216763 occurred early, at 3 h post exposure to SB216763 in both Calu-1 and H358 cells (Fig. 4B), indicating that c-FLIP downregulation is an early event post GSK3 inhibition. Moreover, we further inhibited GSK3 by knocking down its expression using GSK3 siRNAs against the α and β forms, respectively, in two NSCLC cell lines. As presented in Fig. 4C, silencing of GSK3α minimally decreased the levels of FLIPL, but not FLIPS in H157 cells; however, it reduced the levels of both FLIPL and FLIPS in A549 cells. Silencing of GSK3β with two different siRNAs minimally decreased the levels of FLIPL in A549 cells; but reduced the levels of FLIPS to a greater extent in both H157 and A549 cells. Alternatively we enforced expression of WT, KD and CA GSK3β in H1299 cells and then examined their impact on c-FLIP levels. As presented in Fig. 4D, expression of WT, particularly CA GSK3β, but not KD GSK3β, increased the levels of c-FLIP. Thus, it appears that activation of GSK3β elevates c-FLIP levels. Collectively, these results clearly indicate that GSK3 positively regulates c-FLIP.
Fig. 4. Inhibition of GSK3 with either small molecule inhibitors (A and B) or GSK3 siRNAs (C) or activation of GSK3 (D) modulates c-FLIP levels.
A and B. The given cell lines were treated with the given concentration of the respective GSK3 inhibitors for 8 h (A) or 20 µM SB216763 for the indicated times (B). C, The given cell lines were transfected with the indicated siRNAs for 48 h; D, H1299 cells were transfected with empty vector (V) or expression plasmids carrying wild-type (WT), kinase-dead (KD) or constitutively activated (CA) GSK3β using Lipofectamine 2000 for 48 h. After the aforementioned treatment or transfection, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis for the indicated proteins. LE, longer exposure.
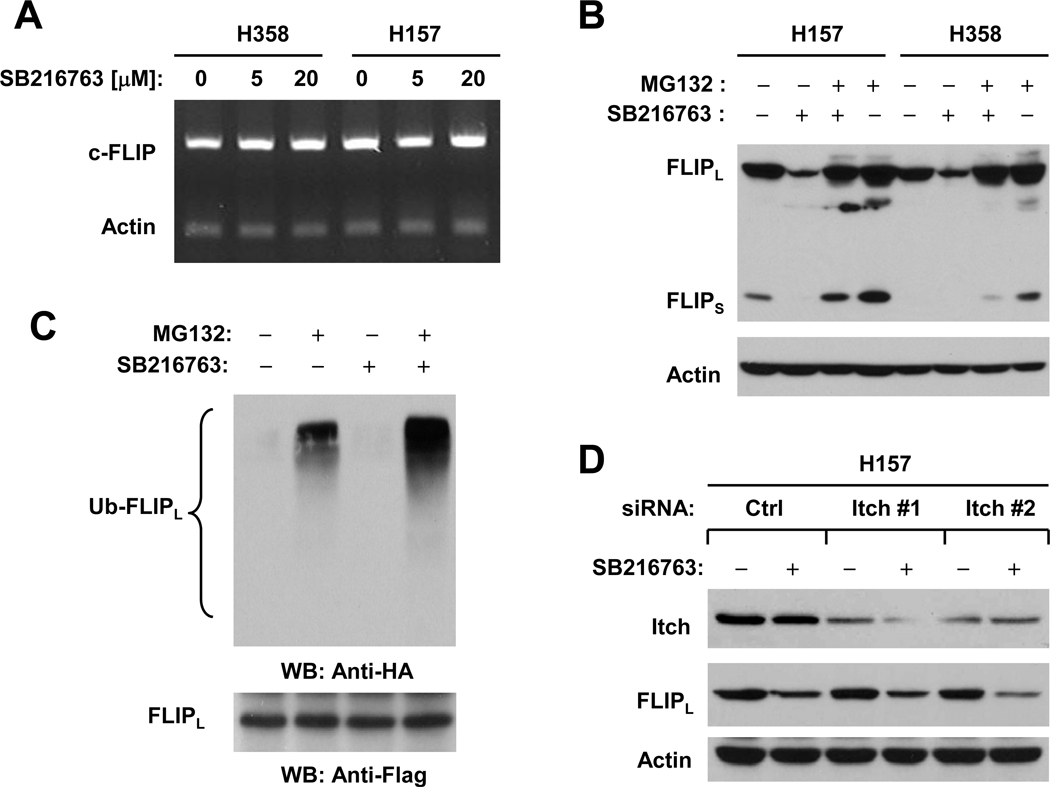
Inhibition of GSK3 Reduces c-FLIP Levels by Facilitating Its Ubiquitination and Proteasome-mediated Degradation
Given that c-FLIP protein is subjected to rapid turnover through ubiquitin/proteasome-dependent degradation (5, 6) and that celecoxib downregulates c-FLIP levels through this mechanism (18), we examined whether inhibition of GSK3 results in ubiquitin/proteasome-mediated c-FLIP degradation. Before these experiments, we determined whether inhibition of GSK3 affects c-FLIP at the mRNA level. Using RT-PCR, we did not detect any changes in c-FLIP mRNA levels in cells exposed to SB216763 (Fig. 5A), indicating that GSK3 inhibition-induced c-FLIP reduction does not occur at the transcriptional level. In the absence of the proteasome inhibitor MG132, SB216763 reduced c-FLIP levels; however, this effect was abolished by the presence of MG132 in both H157 and H358 cells (Fig. 5B). By immunoprecipitation/Western blotting, we detected the highest levels of ubiquitinated FLIPL in cells treated with SB216763 plus MG132 compared to cells exposed to SB216763 alone or MG132 alone (Fig. 5C), indicating that SB216763 increases c-FLIP ubiquitination. Collectively, we conclude that inhibition of GSK3 facilitates ubiquitin/proteasome-mediated c-FLIP degradation, leading to c-FLIP downregulation.
Fig. 5. Inhibition of GSK3 does not alter c-FLIP mRNA levels (A), rather facilitates ubiquitin/proteasome-mediated c-FLIP degradation (B and C) independent of Itch (D).
A, The indicated cell lines were treated with the given concentrations of SB216763 for 6 h and then subjected to preparation of total cellular RNA and subsequent detection of c-FLIP using RT-PCR. B, The given cell lines were pretreated with 20 µM MG132 for 30 minutes prior to the addition of 20 µM SB216763. After co-treatment for 4 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. C, H157-FLIPL-21 cells which stably express ectopic flag-FLIPL were transfected with HA-ubiquitin plasmid using FuGENE 6 transfection reagent for 24 h. The cells were then pretreated with 20 µM MG132 for 30 minutes and then co-treated with 20 µM SB216763 for 4 h. Whole-cell protein lysates were then prepared for immunoprecipitation using anti-Flag antibody followed by Western blotting (WB) using anti-HA antibody for detection of ubiquitinated FLIPL (Ub-FLIPL) and anti-Flag antibody for detection of ectopic FLIPL. D, H157 cells were transfected with the indicated siRNAs for 48 h and then treated with 20 µM SB216763 for additional 8 h. The cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis for detection of the indicated proteins.
Inhibition of GSK3 Induces c-FLIP Degradation Independent of the E3 Ligase Itch
The E3 ligase Itch has been suggested to be involved in TNFα-induced FLIPL degradation (7). We then asked whether Itch is involved in mediating ubiquitin/proteasome-dependent degradation of c-FLIP induced by GSK3 inhibition. Transfection of two different Itch siRNAs into H157 cells substantially reduced the levels of Itch, indicating successful knockdown of Itch (Fig. 5D). However, knockdown of Itch neither increased basal levels of c-FLIP nor prevented c-FLIP reduction induced by SB216763 (Fig. 5D). Similar results were also generated in cells exposed to celecoxib (Supplemental Fig. S3). These results clearly indicate that Itch is unlikely to be the E3 ligase that mediates GSK3 inhibition-induced ubiquitin/proteasome-dependent c-FLIP degradation.
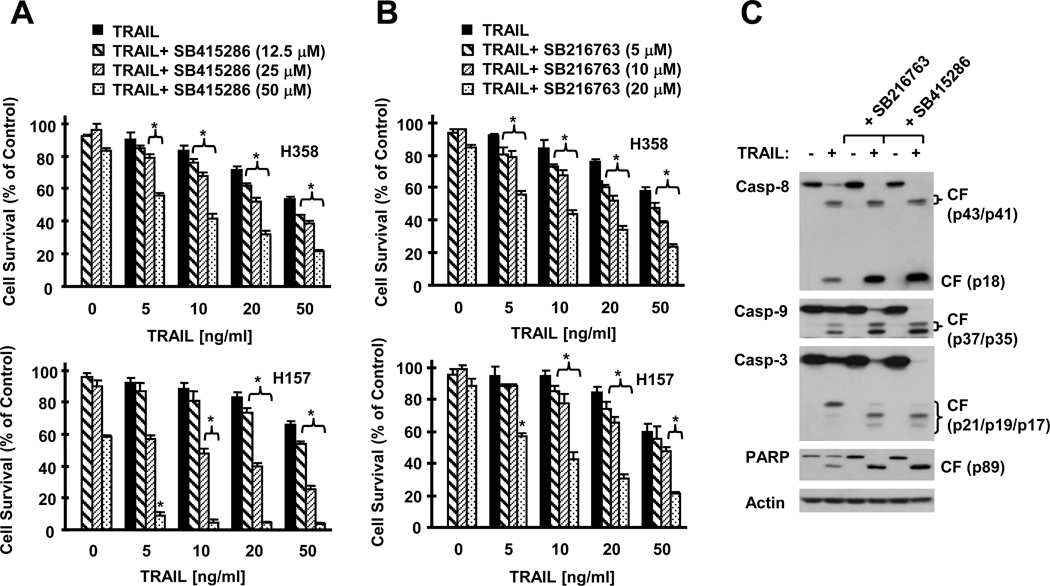
Inhibition of GSK3 Enhances TRAIL-induced Apoptosis
Given that c-FLIP is the major inhibitor of the extrinsic apoptotic pathway, it is plausible to speculate that downregulation of c-FLIP by inhibition of GSK3 will sensitize cancer cells to TRAIL-induced apoptosis as celecoxib does (17). Indeed, the combination of TRAIL with a GSK3 inhibitor such as SB415286 or SB216763 exerted much more potent effects than TRAIL or the inhibitors alone in decreasing the survival of human NSCLC cells (Figs. 6A and 6B). In agreement, the combinations were also more potent than each single agent alone in inducing cleavage of caspase-8, caspase-9, caspase-3 and PARP (Fig. 6C), i.e., activation of caspase cascades. Collectively, these results indicate that inhibition of GSK3 (e.g., with a small molecule inhibitor) augments TRAIL-induced apoptosis.
Fig. 6. Inhibition of GSK3 with a GSK3 inhibitor augments TRAIL-induced apoptosis.
A and B, The given cell lines were plated on 96-well plates and treated next day with the given doses of TRAIL alone, different concentrations of SB415286 (A) or SB216763 (B) alone, or their respective combinations. After 24 h, the cells were subjected to estimation of cell number using the SRB assay. Columns, means of triplicate determinations; Bars, ± SDs. *, P < 0.01 compared with both TRAIL alone and a GSK3 inhibitor alone with ANOVA test. C, H157 cells were treated with 50 ng/ml TRAIL alone, 20 µM SB216763 or 50 µM SB415286 alone, and their respective combinations. After 8 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. CF, cleaved form.
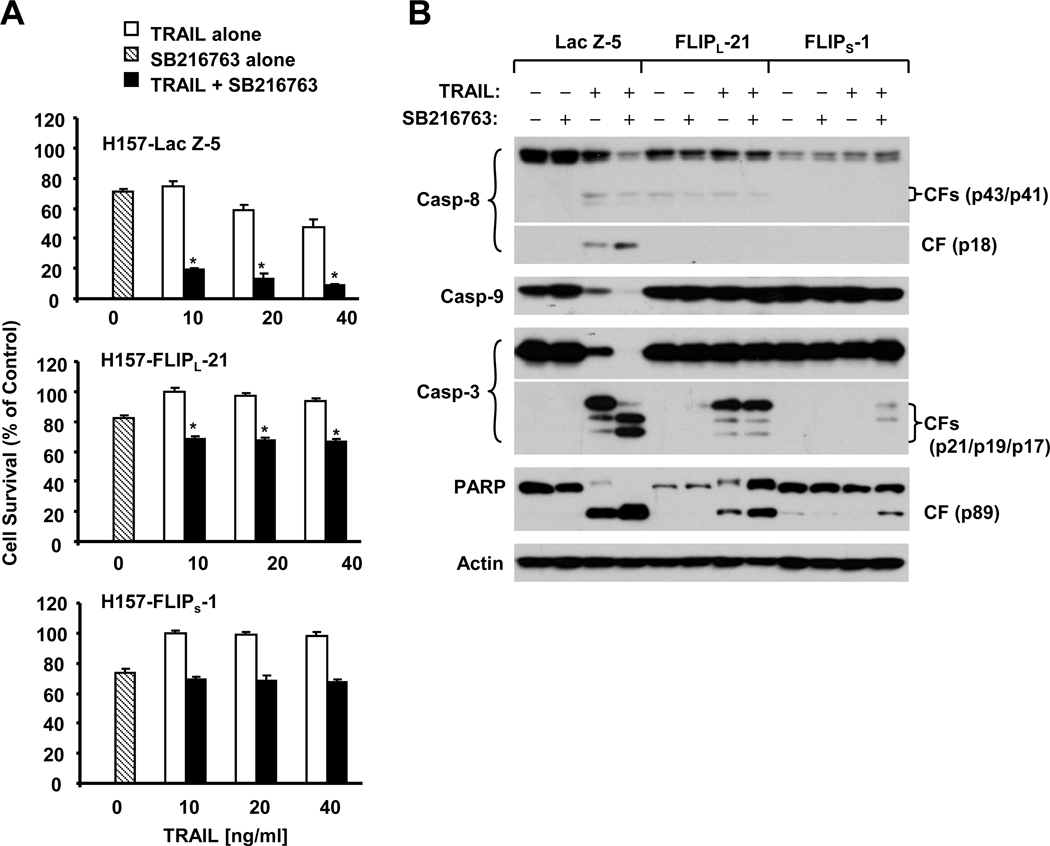
Moreover, we tested whether downregulation of c-FLIP by GSK3 inhibition indeed contributes to TRAIL-induced apoptosis. We further compared the effects of TRAIL combined with a GSK3 inhibitor, SB216763, on cell survival and caspase activation in H157 cell lines which express Lac Z (as a control), FLIPS and FLIPL. As presented in Fig. 7A, the combination effectively decreased the survival of H157-Lac Z-5 cells (e.g., by > 50% compared with SB216763 or TRAIL alone), but not the survival of H157-FLIPS-1 cells. The combination reduced the survival of H157-FLIPL-21 cells only by < 10% compared with SB216763 or TRAIL alone although the reduction was statistically significant. Consistently, the SB216763 and TRAIL combination was more effective than either agent alone in inducing cleavage of caspase-8, caspase-9, caspase-3 and PARP in H157-Lac Z-5 cells, but this effect was substantially attenuated in both H157-FLIPL-21 and H157-FLIPS-1 cells (Fig. 7B). Thus, enforced expression of ectopic FLIPS or FLIPL abolished or attenuated the ability of GSK3 inhibition to sensitize cancer cells to TRAIL-induced apoptosis.
Fig. 7. GSK3 inhibition-induced augmentation of TRAIL-induced apoptosis can be abrogated by enforced expression of ectopic c-FLIP.
A, The indicated transfectants were seeded in 96-well plates and treated next day with 20 µM SB216763 alone, the given concentrations of TRAIL alone and the respective combinations of SB216763 and TRAIL. After 24 h, the cells were subjected to estimation of cell number using the SRB assay. Columns, means of triplicate determinations; Bars, ± SDs. *, P < 0.001 compared with both TRAIL alone and SB216763 alone with ANOVA test. B, The indicated transfectants were treated with 20 µM SB216763 alone, 20 ng/ml TRAIL alone and their combination. After 18 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. CF, cleaved form.
Discussion
The mechanisms by which celecoxib and its analogues induce apoptosis have long been a subject of intensive research. One such mechanism seems to be the inhibition of PDK1/Akt signaling as documented in some studies (26–29). However, other studies have failed to demonstrate such a mechanism (30, 42, 43), thus, leaving this as a controversial issue (16). In our studies primarily involving human NSCLC cell lines, we have never observed inhibition of p-Akt levels by celecoxib or its analogues such as DMC when used at growth arrest and apoptosis-inducing concentration ranges (up to 50 µM). Instead, we detect increased p-Akt levels in some cell lines when exposed to celecoxib as presented in Fig. 1. Thus, our data do not support a role for Akt inhibition in mediating celecoxib-induced growth arrest and apoptosis, at least in NSCLC cells.
Interestingly, the phosphorylation of GSK3 including both α (at Ser 21) and β (at Ser 9) isoforms, which are well known to be phosphorylated and inhibited by Akt (31, 44), was increased by celecoxib in dose- and time-dependent manners in the tested NSCLC cells, even in those without an increase in Akt phosphorylation (e.g., H1792) (Fig. 1). Given that phosphorylation of GSK3 at Ser 21/Ser9 results in inactivation of GSK3 (9, 11), our findings thus imply that celecoxib actually inhibits GSK3 function. Although celecoxib increases the phosphorylation of both Akt and GSK3 in some of our tested cell lines (e.g., H358 and Calu-1), inhibition of celecoxib-induced Akt phosphorylation with the PI3K inhibitor LY294002 or wortmannin did not accordingly abrogate celecoxib-induced GSK3 phosphorylation (Fig. 2), suggesting that celecoxib induces Akt-independent GSK3 phosphorylation or inhibition. To the best of our knowledge, this is the first report of celecoxib inhibition of GSK3.
In addition to Akt, other kinases such as p70S6K and PKC can also phosphorylate GSK3 (9, 32, 33, 36–41). In our study, we did not demonstrate a role for mTOR/p70S6K in celecoxib-induced GSK phosphorylation because rapamycin effectively inhibited the basal levels of p-S6, but did not prevent the increase in Akt phosphorylation by celecoxib (Fig. 2). However, both Rö31-8220 and GF109203X, which are PKC pan inhibitors, abolished celecoxib-induced GSK3 phosphorylation, suggesting that celecoxib induces PKC-dependent GSK3 phosphorylation or inhibition. It is well known that PKC comprises multiple isoforms (45). Among these isoforms, PKCα, β or δ isoforms have been suggested to regulate GSK3 phosphorylation (36, 41). In our study, we found that both Gö6983, a specific PKC inhibitor lacking activity against the μ isoform, and Gö6979, a specific PKC α/β inhibitor, but not Rottlerin, a specific PKCδ inhibitor, were as effective as the PKC pan inhibitors in abolishing celecoxib-induced GSK3 phosphorylation (Fig. 2). Thus, we suggest that the PKC α/β isoforms may be important for mediating celecoxib-induced GSK3 phosphorylation. These findings warrant further study toward this direction. Our finding on celecoxib activation of PKC is novel although we have yet to define the mechanism by which celecoxib activates PKC, warranting the further investigation of this subject.
It has been shown that GSK3β inhibition with either small molecule inhibitors or siRNAs potentiates TRAIL-induced apoptosis in human prostate cancer cells (12). However, the underlying mechanisms are unknown. In our study, we could reproduce this biological phenomenon in human NSCLC cells (Fig. 6). Very importantly, we found that inhibition of GSK3 with either siRNAs or small molecule inhibitors downregulated c-FLIP levels, clearly indicating that GSK3 inhibition results in downregulation of c-FLIP levels. Complementarily, enforced expression of CA GSK3 increased c-FLIP levels (Fig. 4D). Thus, our findings clearly demonstrate that GSK3 regulates c-FLIP levels. To the best of our knowledge, this is the first study demonstrating GSK3-dependent regulation of c-FLIP. Given that enforced expression of ectopic c-FLIP expression protects cells from induction of apoptosis induced by GSK inhibition plus TRAIL (Fig. 7), it is plausible to conclude that c-FLIP downregulation should be a major event accounting for GSK3 inhibition-mediated enhancement of TRAIL-induced apoptosis. Thus, our findings on GSK3 regulation of c-FLIP provide a reasonable mechanism by which GSK inhibition potentiates TRAIL-induced apoptosis.
It is known that c-FLIP, including FLIPL and FLIPS, are proteins subjected to rapid turnover regulated through ubiquitin/proteasome-mediated protein degradation (5–7). However, the signaling event that triggers c-FLIP degradation has not been characterized. Our previous studies have shown that celecoxib and its analogue DMC downregulate c-FLIP levels through facilitating ubiquitination and proteasome-mediated degradation of c-FLIP (18, 19). In the current study, we found that the inhibition of GSK3 with SB216763 did not increase c-FLIP mRNA levels, and that the presence of the proteasome inhibitor MG132 prevented SB216763-induced c-FLIP downregulation. Moreover, SB216763 substantially increased c-FLIP ubiquitination (Fig. 5). Collectively, these results indicate that GSK3 inhibition-induced c-FLIP downregulation occurs at a post-translational level via promoting ubiquitin/proteasome-mediated protein degradation. Given that celecoxib inhibits GSK3, as discussed above, and reduces c-FLIP levels through the same mechanism as we previously demonstrated (18), we suggest that celecoxib inhibits GSK3, leading to facilitation of c-FLIP degradation. The E3 ligase Itch has been suggested to be involved in TNFα-induced c-FLIP (i.e., FLIPL) degradation (7). In our study, we found that silencing of Itch expression with Itch siRNAs neither increased basal levels of c-FLIP nor blocked c-FLIP downregulation induced by either SB216763 or celecoxib (Figs. 4 and S2), suggesting that Itch is unlikely to be involved in GSK3 inhibition-induced c-FLIP degradation.
Previous work has demonstrated that c-FLIP downregulation contributes to celecoxib-induced apoptosis and enhancement of TRAIL-induced apoptosis (18). In agreement, we found in this study that siRNA-mediated silencing of GSK3β enhanced the ability of celecoxib to downregulate c-FLIP (i.e., FLIPL). Similar results were also generated when cells were co-treated with celecoxib and a GSK3 inhibitor (e.g., SB216763) (Fig. 3). Thus, our results further support an important role of c-FLIP downregulation, which is mediated by GSK3 inhibition, in celecoxib-induced apoptosis.
We have previously shown that celecoxib downregulates c-FLIP independent of its COX-2 inhibitory activity by using COX-2 siRNA and DMC, which lacks COX-2 inhibitory activity (18, 19). In this study, we further showed that DMC also increased p-GSK3 levels; this effect could not be abrogated by LY294002 (Fig. S1). Thus, celecoxib-induced GSK3 phosphorylation and subsequent downregulation of c-FLIP is unlikely to be secondary to COX-2 inhibition.
In summary, the current study demonstrates a novel mechanism by which celecoxib induces c-FLIP degradation through Akt-independent phosphorylation or inhibition of GSK3. Through this study, we are able to show, for the first time, that inhibition of GSK3 is associated with induction of c-FLIP degradation, thus providing a reasonable explanation for how GSK3 inhibits the extrinsic death receptor-mediated apoptotic pathway.
Supplementary Material
Acknowledgement
We are grateful to Dr. B. P. Zhou for providing GSK3 expression constructs, Dr. R. Lotan for providing cell lines and Dr. A. Hammond for editing of the manuscript.
Grant Support: Georgia Cancer Coalition Distinguished Cancer Scholar award (to S-Y. Sun); Department of Defense VITAL grant W81XWH-04-1-0142 (to S-Y. Sun for Project 4) and NIH/NCI SPORE P50 grant CA128613 (to S-Y. Sun for Project 2).
References
- 1.Wajant H. Targeting the FLICE Inhibitory Protein (FLIP) in cancer therapy. Mol Interv. 2003;3:124–127. doi: 10.1124/mi.3.3.124. [DOI] [PubMed] [Google Scholar]
- 2.Kamarajan P, Sun NK, Chao CC. Up-regulation of FLIP in cisplatin-selected HeLa cells causes cross-resistance to CD95/Fas death signalling. Biochem J. 2003;376:253–260. doi: 10.1042/BJ20030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L, et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–848. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 4.Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23:6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Suh N, Sporn M, Reed JC. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem. 2002;277:22320–22329. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- 6.Poukkula M, Kaunisto A, Hietakangas V, Denessiouk K, Katajamaki T, Johnson MS, et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem. 2005;280:27345–27355. doi: 10.1074/jbc.M504019200. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 12.Liao X, Zhang L, Thrasher JB, Du J, Li B. Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol Cancer Ther. 2003;2:1215–1222. [PubMed] [Google Scholar]
- 13.Rottmann S, Wang Y, Nasoff M, Deveraux QL, Quon KC. A TRAIL receptor-dependent synthetic lethal relationship between MYC activation and GSK3beta/FBW7 loss of function. Proc Natl Acad Sci U S A. 2005;102:15195–15200. doi: 10.1073/pnas.0505114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M, Song L, Li Y, Zhou T, Jope RS. Identification of an antiapoptotic protein complex at death receptors. Cell Death Differ. 2008;15:1887–1900. doi: 10.1038/cdd.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 16.Schonthal AH. Antitumor properties of dimethyl-celecoxib, a derivative of celecoxib that does not inhibit cyclooxygenase-2: implications for glioma therapy. Neurosurg Focus. 2006;20:E21. doi: 10.3171/foc.2006.20.4.14. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–1780. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. Cellular FLICE-inhibitory protein downregulation contributes to celecoxib-induced apoptosis in human lung cancer cells. Cancer Res. 2006;66:11115–11119. doi: 10.1158/0008-5472.CAN-06-2471. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. CHOP-dependent DR5 induction and ubiquitin/proteasome-mediated c-FLIP downregulation contribute to enhancement of TRAIL-induced apoptosis by dimethyl-celecoxib in human non-small cell lung cancer cells. Mol Pharmacol. 2007;72:1269–1279. doi: 10.1124/mol.107.037465. [DOI] [PubMed] [Google Scholar]
- 20.Raja SM, Chen S, Yue P, Acker TM, Lefkove B, Arbiser JL, et al. The natural product honokiol preferentially inhibits cellular FLICE-inhibitory protein and augments death receptor-induced apoptosis. Mol Cancer Ther. 2008;7:2212–2223. doi: 10.1158/1535-7163.MCT-07-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot B, Hong WK, et al. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene. 1999;18:2357–2365. doi: 10.1038/sj.onc.1202543. [DOI] [PubMed] [Google Scholar]
- 22.Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–4939. [PubMed] [Google Scholar]
- 23.Chen C, Sun X, Ran Q, Wilkinson KD, Murphy TJ, Simons JW, et al. Ubiquitin-proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene. 2005;24:3319–3327. doi: 10.1038/sj.onc.1208497. [DOI] [PubMed] [Google Scholar]
- 24.Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, et al. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003;94:1066–1073. doi: 10.1111/j.1349-7006.2003.tb01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panka DJ, Mano T, Suhara T, Walsh K, Mier JW. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6896. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- 26.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 27.Kulp SK, Yang YT, Hung CC, Chen KF, Lai JP, Tseng PH, et al. 3-phosphoinositide-dependent protein kinase-1/Akt signaling represents a major cyclooxygenase-2-independent target for celecoxib in prostate cancer cells. Cancer Res. 2004;64:1444–1451. doi: 10.1158/0008-5472.can-03-2396. [DOI] [PubMed] [Google Scholar]
- 28.Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, et al. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 29.Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756–768. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- 30.Kardosh A, Wang W, Uddin J, Petasis NA, Hofman FM, Chen TC, et al. Dimethyl-celecoxib (DMC), a derivative of celecoxib that lacks cyclooxygenase-2-inhibitory function, potently mimics the anti-tumor effects of celecoxib on Burkitt's lymphoma in vitro and in vivo. Cancer Biol Ther. 2005;4:571–582. doi: 10.4161/cbt.4.5.1699. [DOI] [PubMed] [Google Scholar]
- 31.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong JL, Bonavaud SM, Toole BJ, Yeaman SJ. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. J Biol Chem. 2001;276:952–956. doi: 10.1074/jbc.M004812200. [DOI] [PubMed] [Google Scholar]
- 34.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 35.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E Survival Pathways by Rapamycin-Mediated Mammalian Target of Rapamycin Inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Zhou Y, Evers BM. Neurotensin phosphorylates GSK-3alpha/beta through the activation of PKC in human colon cancer cells. Neoplasia. 2006;8:781–787. doi: 10.1593/neo.06259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilimek D, Duronio V. Cytokine-stimulated phosphorylation of GSK-3 is primarily dependent upon PKCs, not PKB. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2006;84:20–29. doi: 10.1139/o05-154. [DOI] [PubMed] [Google Scholar]
- 38.Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. Embo J. 1996;15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 39.Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin SY, Yoon SC, Kim YH, Kim YS, Lee YH. Phosphorylation of glycogen synthase kinase-3beta at serine-9 by phospholipase Cgamma1 through protein kinase C in rat 3Y1 fibroblasts. Experimental & molecular medicine. 2002;34:444–450. doi: 10.1038/emm.2002.62. [DOI] [PubMed] [Google Scholar]
- 41.De Servi B, Hermani A, Medunjanin S, Mayer D. Impact of PKCdelta on estrogen receptor localization and activity in breast cancer cells. Oncogene. 2005;24:4946–4955. doi: 10.1038/sj.onc.1208676. [DOI] [PubMed] [Google Scholar]
- 42.Kardosh A, Soriano N, Liu YT, Uddin J, Petasis NA, Hofman FM, et al. Multitarget inhibition of drug-resistant multiple myeloma cell lines by dimethyl-celecoxib (DMC), a non-COX-2 inhibitory analog of celecoxib. Blood. 2005;106:4330–4338. doi: 10.1182/blood-2005-07-2819. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z, Zhang X, Li M, Wang Z, Wieand HS, Grandis JR, et al. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:5930–5939. doi: 10.1158/1078-0432.CCR-03-0677. [DOI] [PubMed] [Google Scholar]
- 44.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 45.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.